Abstract
Background
Studies have reported significant improvement of obstructive sleep apnea (OSA) in obese patients after bariatric surgery (BS). Weight loss following BS is rapid in the first few months, but it can take at least 1 year to reach the final result. The aim of this study is to measure the effect of BS on various clinical, respiratory, and sleep parameters of OSA at two postoperative intervals.
Methods
Prospectively, all patients being evaluated for BS underwent a polysomnography (PSG). Patients diagnosed with OSA preoperatively were invited to undergo a PSG at least 6 months postoperatively and if OSA persisted, again at least 12 months postoperatively.
Results
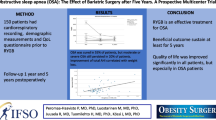
One hundred ten patients underwent a first postoperative PSG 7.7 months after surgery. The mean apnea–hypopnea index (AHI) significantly decreased from 39.5 to 15.6/h. In 58.2 %, the AHI was reduced to below 10 and in 25.5 % to below 5. Fifty patients underwent a first PSG 7.1 months and a second PSG 16.9 months after surgery. The mean AHI decreased from 49.1 to 22.7 to 17.4/h following BS.
Conclusions
BS initiates dramatic improvement and even remission of clinical and sleep parameters during the first 7 months, which continues at a slower rate over the next 10 months. We recommend a follow-up PSG after surgery to check for residual disease and if necessary retritration of continuous positive airway pressure, which may lead to higher treatment compliance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obesity is a significant risk factor for obstructive sleep apnea (OSA), the most prevalent sleep-disordered breathing problem. OSA affects 2–4 % of the general population [1]. Among patients suffering from morbid obesity, OSA is a lot more common. Not everyone with OSA is obese, but most people with obesity have OSA.
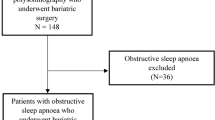
As reported previously by our group, there is a high prevalence of OSA in patients undergoing bariatric surgery (BS) [2]. Of 279 consecutive patients undergoing BS, 69.9 % were diagnosed with OSA preoperatively, specifically 40 % with severe OSA.
Small-scale studies have reported significant improvement of OSA in obese patients after BS (range, 3 months–12 years) [3–20]. Studies have shown that weight loss following BS is rapid in the first few months, but it can take at least 1 year or more to reach the final result [17]. Bearing this in mind, we report a large, prospective, follow-up study on a previously published paper, designed to assess the effect of significant weight loss after BS on various respiratory and sleep parameters of OSA measured by PSG 6 and 12 months after surgery [2].
Materials and Methods
Patients
We performed a prospective, multidisciplinary, single-center, observational study involving a consecutive series of patients who had been evaluated for BS in our clinic. Patients meeting the international IFSO criteria were eligible for BS (https://www.ifso.com). Specifically, patients aged 18–65, with a body mass index (BMI) > 40, or BMI > 35 with comorbidity (e.g. hypertension, diabetes, OSA, or joint problems). Secondly patients were required to have made sufficient attempts at weight loss using conservative measures and had to be motivated for dietary and behavior modification. Some patients with a BMI < 35 were also included if comorbid disease was present. A few exceptions were also made concerning the age restriction. Various BS methods were available in our clinic: laparoscopic adjustable gastric banding (LAGB), laparoscopic gastric bypass (LRYGB), and sleeve gastrectomy (SG) [2]. All patients underwent a mandatory preoperative screening for OSA in addition to our routine preoperative work-up. Apart from patients with OSA previously diagnosed elsewhere, preoperatively patients visited the ear, nose, and throat (ENT) outpatient department. Information was gained using patient history, ENT and general examination, and a full overnight PSG. Weight and length (BMI) were measured. The following BMI grading system was implemented: normal range (BMI, 18.5–24.9 kg/m2), overweight (BMI, 25–29.9 kg/m2), obese (BMI, 30–34.9 kg/m2), severely obese (BMI, 35–39.9 kg/m2), morbidly obese (BMI, 40–49.9 kg/m2), super obese (BMI, >50 kg/m2) (https://www.ifso.com).
If the apnea–hypopnea index (AHI) was >15/h, continuous positive airway pressure (CPAP) was prescribed. Patients were instructed to bring their CPAP appliance with them when being admitted for surgery. Postoperatively, CPAP was first applied in the recovery room. Patients were advised to use their CPAP while sleeping, both during the day and night. Patients with a preoperative AHI >30/h were routinely admitted to the intensive care ward for 24 h postoperatively. Morphinomimetic painkillers were not prescribed in patients with OSA.
All patients diagnosed with OSA preoperatively received an invitation for a PSG 6 months postoperatively (t 1). Patients with persistent OSA at t 1 were invited for a second PSG 12 months postoperatively (t 2).
Polysomnography
Besides patients with OSA previously diagnosed elsewhere, all patients underwent a full-night comprehensive sleep study using a digital Embla recorder (Flaga Medical devices, Reykjavik, Iceland). This records sleep architecture (derived from electroencephalogram, electrooculogram, and submental electromyogram), respiration (thoracic and abdominal measurement), movements of limbs, body position (trunk measurement), nasal airflow, and the intensity of the snoring (the last two measured by pressure sensor). Pulse oximetry was used to monitor oxygen saturation (SaO2) and heart rate [2]. Due to financial and capacity restrictions, 13 patients (26 %) who underwent a second postoperative PSG underwent a home polygraphy using a digital Embla titanium recorder (Flaga Medical devices, Reykjavik, Iceland). The same parameters are recorded except for the sleep architecture.
The severity of OSA is expressed by the AHI. Obstructive apneas were defined as cessation of airflow for at least 10 s. Hypopneas were defined as periods of reduction of >30 % oronasal airflow for at least 10 s and a ≥4 % decrease in oxygen saturation. Arousals were not scored as hypopneas. The AHI was calculated as the sum of total events (apneas and hypopneas) per hour of sleep. An AHI of 5–15/h is mild OSA, an AHI of 15–30/h is moderate, and AHI >30/h is severe OSA, as assessed by PSG [1, 2].
Statistical Analysis
Statistical analysis was performed using Microsoft Excel and SPSS statistical software (version 18, SPSS Inc., Chicago, USA). The distribution of recorded variables was characterized by calculating the mean and standard deviation. Since some parameters (especially the AHI) were expected to be non-normally distributed, also the median and range were calculated. Pre- and postoperative quantitative data were compared using the paired Student’s t test with additional application of the Wilcoxon signed-rank test. A p < .05 was considered statistically significant.
The prevalence of OSA and OSA severity was subdivided for obesity severity subgroups. To identify independent predictors of OSA cure (AHI < 5) or surgical success (Sher’s criteria, a postoperative reduction of the AHI by 50 % and to below 20), we used logistic multiple regression. We tested the variables age, preoperative BMI, and weight. To test the relationship between BS method and a postoperative AHI < 5 or surgical success according to Sher’s surgical criteria, the χ 2 test was applied.
Results
Two hundred seventy-nine patients who underwent a preoperative PSG were included in the study. Based on the PSG results, 195 (69.9 %) patients were diagnosed with OSA [2]. Of the 195 patients diagnosed with OSA preoperatively, 171 patients with OSA underwent surgery. The mean duration between the preoperative PSG and the date of surgery was 8.4 months. Patients lost to follow-up are depicted graphically in Fig. 1. The majority of patients refused a postoperative evaluation or did not show up for their scheduled sleep study. Ten patients underwent BS but are being treated for their sleep apnea in a different medical center; unfortunately, we were unable to gather postoperative PSG results for these patients. Additionally, one patient was pregnant in the postoperative period and two patients died, one from cardiac complications following surgery and the other from lung cancer.
Study Population with One Postoperative PSG (n = 110)
Preoperative Results
Of the 110 patients who underwent a first postoperative PSG, 73 (60.8 %) were women and 37 (30.8 %) men. Preoperatively, 1 (0.9 %) patient was obese, 30 (27.3 %) severely obese, 48 (43.6 %) morbidly obese, and 31 (28.2 %) super obese. Thirty-five (31.8 %) patients underwent LAGB [average BMI, 41.0 ± standard deviation (SD) 4.6 kg/m2], 70 (63.6 %) LRYGB (average BMI, 47 ± SD 7.1 kg/m2), and 5 (4.5 %) SG (average BMI, 53.4 ± SD 9.5 kg/m2). Patient baseline characteristics are shown in Table 1. Preoperatively, based on the PSG results, 28 (25.5 %) patients were diagnosed with mild OSA, 30 (27.3 %) with moderate OSA, and 52 (47.3 %) with severe OSA.
Postoperative Results
At the first postoperative visit, 7.7 ± 2.4 months after surgery, the mean AHI significantly decreased from 39.5 ± SD 31.7/h to 15.6 ± SD 17.4/h, the mean BMI significantly decreased from 45.4 ± SD 7.3 kg/m2 to 36.3 ± SD 6.1 kg/m2, and the mean weight significantly decreased from 132.5 ± SD 26.9 kg to 106.4 ± SD 23.8 kg. Pre- and postoperative clinical parameters are summarized in Table 1.
Postoperatively, 1 patient (0.9 %) had a normal weight, 12 (10.9 %) were overweight, 38 (34.5 %) obese, 31 (28.2 %) severely obese, 24 (21.8 %) morbidly obese, and 4 (3.6 %) super obese. Twenty-eight (25.5 %) were cured of their OSA. Forty-eight (43.6 %) still suffered from mild disease, 17 (15.5 %) moderate disease, and 17 (15.5 %) severe disease.
Patients with preoperative mild disease were more likely to be “cured” than those with a preoperative severe disease (53.6 vs. 17.9 %; Fig. 2). The mean AHI after BS in patients (n = 82) with residual disease was 20.2 ± SD 18.1/h. The improvement in mean AHI, stratified per OSA severity group, is shown in Fig. 3.
Improvement in mean AHI, stratified per OSA severity group. In the severe OSA group, the mean AHI decreased 66.1 ± SD 26.9 to 25.4 ± SD 20.8/h (p < .001), in the moderate OSA group from 21.2 ± SD 4.4 to 8.9 ± SD 6.5/h (p < .001) and in the mild OSA group from 9.6 ± SD 2.6 to 4.8 ± SD 3.2/h (p < .001)
Of the patients, 66.4 % could be considered successfully treated when applying Sher’s surgical success criteria. The mean percentage weight loss after treatment in the successfully treated group was higher than in the unsuccessfully treated group (22.4 ± SD 9.3 % vs. 15.4 ± SD 9.8 %, respectively, unpaired t test, p < .001).
Figure 4a shows a scatter plot of the preoperative and postoperative AHI data. On examination, the scatterplot shows two distinguishable clusters of subjects. Data points are either clustered around the x = y line (little to no effect of surgical weight loss on the AHI) or below the regression line (good effect of surgical weight loss on the AHI).
Despite a positive relation, the correlation between the percentage and absolute reduction in AHI and the percentage and absolute reduction in weight and BMI is poor (R 2 ranging between 0.095 and 0.211).
Study Group with Two Postoperative PSGs (n = 50)
Preoperative Results
Of the 50 patients who underwent two postoperative PSGs, 33 (66 %) were women and 17 (34 %) men. Preoperatively, 1 (2 %) patient was obese, 15 patients (19 %) severely obese, 19 (38 %) morbidly obese, and 15 (30 %) super obese. Eighteen (36 %) patients underwent LAGB (average BMI, 40.9 ± SD 4.9 kg/m2), 29 (58 %) LRYGB (average BMI, 46.7 ± SD 7.9 kg/m2), and 3 (6.0 %) SG (average BMI, 53.9 ± SD 12.9 kg/m2). Patient baseline characteristics are shown in Table 2. Preoperatively, based on the PSG results, 5 (10 %) patients were diagnosed with mild OSA, 15 (30 %) with moderate OSA, and 30 (60 %) with severe OSA.
Postoperative Results
Fifty patients underwent a first PSG 7.1 ± SD 1.3 months after surgery (t 1) and a second PSG 16.9 ± SD 4.3 months after surgery (t 2). The results are summarized in Table 2. The mean AHI decreased statistically significantly from 49.1/h (t 0) to 22.7/h (t 1) to 17.4/h (t 2) following BS (p < .001, p < .001, and p = .003), the mean BMI from 45.0 kg/m2 (t 0) to 36.7 kg/m2 (t 1) to 35.0 kg/m2 (t 2) (p < .001, p < .001, and p = .001), and the mean weight from 130.2 kg (t 0) to 106.6 kg (t 1) to 102.1 kg (t 2) (p < .001, p < .001, and p = .004; Table 3).
Postoperatively at t2, 2 patients (4 %) had a normal weight, 7 (14 %) were overweight, 18 (26 %) obese, 12 (24 %) severely obese, 10 (20 %) morbidly obese, and 1 (2 %) super obese. Twelve (24 %) were cured of their OSA. Twenty-one (42 %) still suffered from mild disease, 6 (12 %) moderate disease, and 11 (22 %) severe disease. The mean AHI after BS in patients (n = 38) with residual disease was 22.1 ± SD 17.5/h. The mean AHI in the severe OSA group decreased from 69.7 ± SD 28.3/h to 24.1 ± SD 19.3/h (p < .001), in the moderate OSA group from 21.3 ± SD 4.1 to 8.6 ± SD 6.3/h (p < .001), and in the mild OSA group from 8.6 ± SD 2.4 to 3.5 ± SD 3.1/h (p = .091).
Of the patients, 58 % could be considered successfully treated when applying Sher’s surgical success criteria (50 % reduction in AHI and/or ≤20). The mean percentage weight loss after treatment in the successfully treated group was higher than in the unsuccessfully treated group (21.7 ± SD 10.5 % vs. 19.0 ± SD 12.3 %, respectively, unpaired t test, p = .417).
Figure 4b shows a scatter plot of the preoperative and postoperative AHI at t 1 and t 2 (with separate linear regression lines for each measurement interval). The difference in average AHI between the first and second postoperative recording was not explained by a difference in percentage of sleeping time in supine position.
Despite a positive relation, the correlation between the percentage and absolute reduction in AHI and the percentage and absolute reduction in weight and BMI is poor (R 2 ranging between 0.057 and 0.253).
Even though the mean pre- and postoperative AHI was higher in the study population who underwent two postoperative PSGs (mean = 42.75 ± SD 36.4 %) than in the study population who underwent one postoperative PSG (mean = 55.6 ± SD 33.7 %), the difference in mean percentage AHI reduction was not statistically significant t (158) = 2.18, p = .031 (unpaired t test).
Identification of Predictors
In logistic regression models, age, preoperative BMI, and weight in all combinations were inadequate predictors (p values for the regression coefficient, >.05) of cure and surgical success (Sher’s criteria) at both measurement intervals (t 1 and t 2).
When testing the relationship between BS method and a postoperative AHI < 5 or surgical success according to Sher’s criteria, a significant association was found between gastric bypass surgery and Sher’s surgical success χ 2 (1, n = 105) = 6,300, p = .012. The odds of patients undergoing gastric bypass surgery reaching surgical success is 2.8 times higher than patients undergoing gastric banding (95 % confidence interval, 1.24–6.64). There was no significant association between operation type and an AHI < 5 in both study groups. The test could not be applied to the study group, which underwent two postoperative PSGs as the group was too small.
Discussion
To our best knowledge, this is the first paper studying the effect of BS on OSA at two intervals postoperatively. The effect of BS on weight is gradual. Weight loss in the first few months is rapid, but it can take at least 1 year or more to reach the final result [17]. The same pattern can be observed when studying the effect of BS on OSA. In this series of patients with OSA, BS initiates dramatic improvement of clinical and sleep parameters during the first 7 months; thereafter, improvement continues albeit at a slower rate. Physicians should bear in mind that sleep and clinical parameters will most probably have improved significantly 6 months after surgery, but a continuation of reduction in the severity of OSA and improvement of the success rate can be expected thereafter, supporting the recommendation to perform a second postoperative PSG, which may provide new insights.
In line with previous studies, the gross majority of patients reported a significant decrease in AHI and other OSA parameters following BS, but the mean AHI after BS is consistent with moderately severe OSA [20]. Nevertheless, it is important to bear in mind that the effect on OSA by BS is comparable to other OSA treatment modalities. Studies have shown that a mean AHI < 5, for both sleep surgery and CPAP therapy, is rarely achievable [21]. Despite being very efficacious, it is a clinical reality that the use of CPAP is often cumbersome and that CPAP compliance rates are poor. Weaver and Grunstein report in their review that 29–83 % of patients are nonadherent and use their CPAP <4 h per night [22]. Sleep surgery, on the other hand, has a continuous effect, but success rates are poor. When the traditional surgical definition (50 % reduction in AHI and/or ≤20) is applied, the pooled success rate for phase I procedures (soft palate surgery with or without adjuvant treatment targeting base of tongue obstruction) is 55 %, but with AHI ≤ 10 as a cut-off point, success rate decreases to 31.5 %; and at AHI ≤ 5, success is reduced to 13 % [23]. Studies have shown that the success rates of sleep surgery are lower in patients with an increased BMI [24, 25]. In this current study, the success rates of bariatric surgery decreases from 66.4 to 54.5 to 26.4 %, respectively, in the study population who underwent one postoperative PSG (n = 110).
The treatment of OSA is a stepwise approach. In patients with OSA and obesity, BS is an important treatment option, resulting in a decrease in AHI and if indicated, consequent less aggressive future OSA treatment plans. For example, as the AHI drops, so does the CPAP pressure needed, potentially improving tolerance and compliance [26]. Patients with moderate to severe OSA should continue to lose weight if necessary and pursue CPAP treatment to alleviate symptoms and decrease the risk of cardiovascular disease.
Comparable to previous studies, we did not find a linear relationship between the extent of weight loss and improvement in OSA. In a randomized controlled trial, the effect of conventional vs. surgical weight loss with LAGB on OSA in obese patients was compared [27]. Despite greater weight loss in the surgical weight loss group, there was no statistically significant difference in AHI between the two groups. There was substantial inter-individual variability in AHI changes. In our current study, we confirm that weight loss and reduction in BMI is associated with a decrease in AHI, but the correlation between the percentage and absolute reduction in AHI and the percentage and absolute reduction in weight and BMI is poor. Furthermore, age, preoperative weight, and BMI were inadequate predictors of cure and surgical success at both measurement intervals. One can conclude that the effect of BS on the AHI is difficult to predict. Furthermore, these results support the hypothesis that the pathogenesis of OSA in obese patients is not just due to local fatty deposition in the neck resulting in reduction of the lumen of the upper airway thereby reducing airflow and inducing airway collapse, but is of a more complex nature, including factors such as age, sex, skeletal structures, neuromuscular, and metabolic function [27–29]. Thus, the pathogenesis and relationship of the extent of weight loss with improvement of OSA needs further investigation.
Several limitations of this study need to be recognized. Ideally all patients who underwent a preoperative PSG and BS would have been invited for a PSG 6 months postoperatively regardless of the presence or absence of OSA preoperatively. Likewise, regardless of the outcome of the first postoperative PSG, ideally, all patients would have been invited for a second PSG 12 months postoperatively. It can be questioned whether patients with a preoperative AHI below 5, continue to maintain an AHI below 5 postoperatively. As for the patients who underwent a second PSG, the chance is greater that a patient will maintain an AHI >5 at the first postoperative PSG if the preoperative AHI was high. Therefore, the mean AHI is relatively high at the three measurement intervals, and the chance is greater that these patients will not be cured at the second postoperative PSG. Even though the mean pre- and postoperative AHI was higher in the study population who underwent two postoperative PSGs than in the study population who underwent one postoperative PSG, the difference in mean percentage AHI reduction (42.75 ± SD 36.4 % and 55.6 ± SD 33.7 %, respectively) was not statistically significant t(158) = 2.18, p = .031 (unpaired t test).
The second main limitation of this study was the loss of patients to follow-up. Despite conscientiously performing a preoperative PSG on all patients undergoing BS irrespective of history or clinical findings, 31.3 % did not show up or refused the first postoperative evaluation. Patients undergoing BS have a reputation of noncompliance; our data support this observation [29, 30]. Patients received a written invitation. If not accepted, patients were contacted by telephone and encouraged to accept the follow-up evaluation. Most patients reported that their symptoms had subsided; therefore, they deemed a second evaluation unnecessary. Others, despite our best efforts, simply refused. We may, therefore, be underestimating the success rate, as patients with residual symptoms were keener for re-evaluation. Of the tested patients, 64.5 % maintained residual disease. Patients are blinded by the benefits of weight loss and note an allover improvement of their general health, while they may still suffer from OSA albeit less severe. On the contrary, patients who have not measured significant weight loss are often reluctant to continue follow-up. This is particularly true for patients undergoing gastric bypass or gastric sleeve surgery as nothing further can be done to induce weight loss besides dietary modification [28]. Plus, studies have shown that attendance of follow-up appointments is associated with better weight loss outcomes [31].
The third study limitation is absent data. Due to financial and capacity restrictions, a few of the patients invited for a second postoperative PSG underwent a home respiratory polygraphy, which does not record sleep efficiency or the arousal index.
As we did not take an arterial blood gas in all patients, to measure the carbon dioxide level in the blood, we were not able to identify presence of obesity hypoventilation syndrome (OHS). It can be questioned whether some patients not only suffer from OSA but also from OHS.
Lastly, we did not perform a CPAP washout period prior to the postoperative PSG. CPAP is thought to play a role in reducing edema resulting from snoring-associated vibration and apnea-induced suction of the upper airway. The baseline AHI may be reduced by a fraction in chronic CPAP use. One could argue that we are overestimating the effect of BS on OSA, even though research suggests that the effect is minimal [32].
Advising patients not to use CPAP, leaving patients untreated for a period of time prior to a follow-up PSG, raised ethical concerns (traffic or professional accidents, cardiovascular complications) within our research group, especially since so many patients were diagnosed with extremely severe OSA preoperatively.
Conclusion
These data clearly demonstrate the significant, marked improvement and even remission of OSA following BS in obese patients, as measured by PSG. BS initiates dramatic improvement of clinical and sleep parameters during the first 7 months, which continues at a slower rate over the next 10 months. It is of key importance to educate patients prior to BS on OSA: the high prevalence of OSA in the BS population, the risks of untreated OSA, and need for appropriate treatment, especially since the effect of BS on the AHI is not predictable. One hopes that patients will recognize the importance of preoperative PSG to ensure appropriate treatment and a follow-up PSG to check for residual disease and if necessary retritration of CPAP, which may lead to higher treatment compliance.
Abbreviations
- AI:
-
Apnea index
- AHI:
-
Apnea–hypopnea index
- BMI:
-
Body mass index
- BS:
-
Bariatric surgery
- CPAP:
-
Continuous positive airway pressure
- DI:
-
Desaturation index
- ENT:
-
Ear nose and throat
- ESS:
-
Epworth sleepiness scale
- IFSO:
-
International Federation for the Surgery of Obesity
- LAGB:
-
Laparoscopic gastric banding
- LRYGB:
-
Laparoscopic gastric bypass
- OHS:
-
Obesity hypoventilation syndrome
- OSA:
-
Obstructive sleep apnea
- PSG:
-
Polysomnography
- SaO2 :
-
Oxygen saturation
- SG:
-
Sleeve gastrectomy
- WHO:
-
World Health Organization
References
Young T, Palta M, Dempsey J, et al. The occurrence of sleep disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–5.
Ravesloot MJL, van Maanen JP, Hilgevoord AAJ, et al. Obstructive sleep apnea is underrecognized and underdiagnosed in patients undergoing bariatric surgery. Eur Arch Otorhinolaryngol. 2012;269(7):1865–71.
Summers CL, Stradling JR, Baddeley RM. Treatment of sleep apnoea by vertical gastroplasty. Br J Surg. 1990;77(11):1271–2.
Rasheid S. Gastric bypass is an effective treatment for OSA in patients with clinically significant obesity. Obes Surg. 2003;13(1):58–61.
Sugerman HJ, Fairman RP, Sood RK, et al. Long-term effects of gastric surgery for treating respiratory insufficiency of obesity. Am J Clin Nutr. 1992;55(2 Suppl):597S–601S.
Haines KL, Nelson LG, Gonzalez R, et al. Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea. Surgery. 2007;141(3):354–8.
Varela JE, Hinojosa MW, Nguyen NT. Resolution of obstructive sleep apnea after laparoscopic gastric bypass. Obes Surg. 2007;17(10):1279–82.
Rao A, Tey BH, Ramalingam G, et al. Obstructive sleep apnoea (OSA) patterns in bariatric surgical practice and response of OSA to weight loss after laparoscopic adjustable gastric banding (LAGB). Ann Acad Med Singap. 2009;38(7):587–93.
Dixon JB, Schachter LM, O’Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond). 2005;29(9):1048–54.
Valencia-Flores M, Orea A, Herrera M, et al. Effect of bariatric surgery on obstructive sleep apnea and hypopnea syndrome, electrocardiogram, and pulmonary arterial pressure. Obes Surg. 2004;14(6):755–62.
Guardiano S, Scott JA, Ware JC, et al. The longterm results of gastric bypass on indexes of sleep apnea. Chest. 2003;124(4):1615–19.
Scheuller M, Weider D. Bariatric surgery for treatment of sleep apnea syndrome in 15 morbidly obese patients: long-term results. Otolaryngol Head Neck Surg. 2001;125(4):299–302.
Charuzi I, Fraser D, Peiser J, et al. Sleep apnea syndrome in the morbidly obese undergoing bariatric surgery. Gastroenterol Clin North Am. 1987;16(3):517–9.
Charuzi I, Ovnat A, Peiser J, et al. The effect of surgical weight reduction on sleep quality in obesity-related sleep apnea syndrome. Surgery. 1985;97(5):535–8.
Peiser J, Lavie P, Ovnat A, et al. Sleep apnea syndrome in the morbidly obese as an indication for weight reduction surgery. Ann Surg. 1984;199(1):112–5.
Pillar G, Peled R, Lavie P. Recurrence of sleep apnea without concomitant weight reduction increase 7.5 years after weight reduction surgery. Chest. 1994;106(6):1702–4.
Fritscher LG, Canani S, Mottin CC, et al. Bariatric surgery in the treatment of obstructive sleep apnea in morbidly obese patients. Respiration. 2007;74(6):647–52.
Busetto L, Enzi G, Inelmen EM, et al. Obstructive sleep apnea syndrome in morbid obesity: effects of intragastric balloon. Chest. 2005;128(2):618–23.
Lettieri CJ, Eliasson AH, Greenburg DL. Persistence of OSA after surgical weight loss. J Clin Sleep Med. 2008;4(4):333–8.
Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122(6):535–42.
Ravesloot MJ, de Vries N. Reliable calculation of the efficacy of non-surgical and surgical treatment of obstructive sleep apnea revisited. Sleep. 2011;34(1):105–10.
Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–8.
Elshaug AG, Moss JR, Southcott AM, et al. Redefining success in airway surgery for obstructive sleep apnea: a meta analysis and synthesis of the evidence. Sleep. 2007;30(4):461–7.
Kezirian EJ, Malhotra A, Goldberg AN, et al. Changes in obstructive sleep apnea severity, biomarkers, and quality of life after multilevel surgery. Laryngoscope. 2010;120(7):1481–8.
Kezirian EJ, Goldberg AN. Hypopharyngeal surgery in obstructive sleep apnea: an evidence-based review. Arch Otolaryngol Head Neck Surg. 2006;132(2):206–13.
Lankford AD, Proctor CD, Richard R. Continuous positive airway pressure changes in bariatric surgery patients undergoing rapid weight loss. Obes Surg. 2005;15(3):336–41.
Dixon JB, Schachter LM, O’Brien PE, et al. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;208(11):1142–9.
Maciel Santos ME, Rocha NS, Laureano Filho JR, et al. Obstructive sleep apnea–hypopnea syndrome—the role of bariatric and maxillofacial surgeries. Obes Surg. 2009;19:796–801.
Hallowell PT, Stellato TA, Schuster M, et al. Potentially life-treatment sleep apnea is unrecognized without aggressive evaluation. Am J Surg. 2007;193:364–7.
O’Brien P. Is weight loss more successful after gastric bypass than gastric banding for obese patients? Nat Clin Pract Gastroenterol Hepatol. 2009;6(3):136–7.
Compher CW, Hanlon A, Kang Y, et al. Attendance at clinical visits predicts weight loss after gastric bypass surgery. Obes Surg. 2012;22(6):927–34.
Ryan CF, Lowe A, Li D, et al. Magnetic resonance imaging of the upper airway in obstructive sleep apnea before and after chronic nasal continuous positive airway pressure therapy. Am Rev Respir Dis. 1991;144(4):939–44.
Acknowledgments
Many thanks to the contribution of P.M.M. Sickinger, project manager at the Amsterdam Obesity Centre of the Sint Lucas Andreas Ziekenhuis.
Statements
No external funding was used for this study.
Conflict of Interest
The authors do not have any commercial associations that might be a conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravesloot, M.J.L., Hilgevoord, A.A.J., van Wagensveld, B.A. et al. Assessment of the Effect of Bariatric Surgery on Obstructive Sleep Apnea at Two Postoperative Intervals. OBES SURG 24, 22–31 (2014). https://doi.org/10.1007/s11695-013-1023-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-013-1023-y