Abstract
Background
The Roux-en-Y gastric bypass (RYGB) performed laparoscopically (LRYGB) is the most frequently performed bariatric procedure in Belgium. However, late results in terms of weight loss or weight regain are inconsistent and may warrant a second procedure. This retrospective study analyzes the laparoscopic options for revisional surgery after LRYGB.
Methods
Between January 1, 2001 and December 31, 2009, 70 patients underwent a new laparoscopic procedure for poor weight loss or weight regain after LRYGB. The revisional procedure was performed a median of 2.6 years after the initial bypass operation. Fifty-eight patients were available for follow-up (82.9 %); 19 underwent distalization; and 39 a new restrictive procedure.
Results
The mean mass index (BMI) before the revisional procedure was 39.1 + 11.3 kg/m2 (30.8–51.8), down from 42.7 + 19.7 kg/m2 (33.0–56.6) initially, which corresponded to a percentage of excess weight loss (EWL) of 12.4 + 9.3 % (−1.0–29.1). After the corrective procedure, with a follow-up of approximately 4 years, mean BMI was 29.6 + 12.4 kg/m2 (18.0–45.5), for a significant additional percentage of EWL of 53.7 + 9.8 % (2.0–65.8). The overall complication rate was 20.7 %, and the reoperation rate was 7.3 %. The overall leak rate was 12.1 %. Patients suffering from leaks could consistently be treated conservatively or by stent placement. Two patients needed reconversion after distal bypass. The satisfaction index was good in just over 50 % of the patients.
Conclusion
Revisional laparoscopic surgery after RYGB performed for weight issues provides good additional weight loss but carries significant morbidity. Leaks can usually be handled non-surgically. Patient satisfaction is only fair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The laparoscopic Roux-en-Y gastric bypass (LRYGB) has become the most frequently performed bariatric procedure in Belgium (Fig. 1), while for adjustable band gastroplasty (LAGB), the numbers are declining rapidly. Unfortunately, even the LRYGB is fraught with a significant number of failures in terms of weight loss. Despite the fact that many patients can be helped by counseling to correct their weight problems, a substantial number of patients need corrective procedures, often for weight issues. Some procedures aim at increasing restriction, whereas others aim at inducing malabsorption. Both endoscopic and surgical techniques are available.
Material and Methods
Patients
Nine hundred sixty-one patients were treated by LRYGB in our department of obesity surgery between January 1, 2000 and December 31, 2009. Among these patients, 623 underwent a gastric bypass as their first weight loss operation, but 338 (35.2 %) had undergone at least one bariatric procedure beforehand. In a sample of 77 patients followed over 9 years, we recently found that the final weight loss was not significantly different between the primary and the secondary LRYGB patients and averaged a loss of excess body mass index (EBMIL) of some 56 % (data not shown, submitted for another publication in the Journal of Obesity Surgery)
Some of the LRYGB patients in our practice, however, did not experience satisfactory weight loss, or presented significant weight regain after an initial acceptable weight loss. According to Reinhold, weight loss is deemed insufficient when it does not reach 50 % of the excess weight, or when the residual body mass index (BMI) remains higher than 35 kg/m2 [1].
The decision to reintervene, either laparoscopically or endoscopically, was made by a multidisciplinary team, consisting of a dietitian, a psychologist, a gastro-enterologist, a radiologist, an endocrinologist, and a bariatric surgeon. Whereas the psychologist evaluated the patients’ psychological power to bear yet another operation, the dietitian merely analyzed the subjects’ dietary adjustment mode to the bypass construction [2, 3]. The gastro-enterologist and the radiologist evaluated the size of the gastric pouch and of the gastro-enteral anastomosis (GE) and documented possible aberrations such as gastro-gastric fistula (GGF) or marginal ulcers. The endocrinologist investigated the need for additional measures to address the possible lack of response or the resurgence of comorbidities amenable to improve with additional weight loss. Finally, the surgeon decided on the type of corrective procedure compatible with the local anatomical conditions that could have been severely altered as after revisional LRYGB or in patients who had suffered from a leak after the LRYGB procedure.
Procedures
The following six corrective procedures were carried out:
-
Endoscopic refashioning of the stomach pouch and of the GE.
-
Laparoscopic distalization of the bypass, in which the alimentary limb was sectioned flush with the anastomosis with the biliopancreatic limb and moved distally some 150 cm proximal to the ileocecal valve.
-
Laparoscopic placement of a non-adjustable, custom-made 6.5-cm long “Fobi” ring adjusted around the gastric pouch some 2 cm cephalad of the GE.
-
Laparoscopic refashioning of the gastric pouch and the GE. The procedure consisted when indicated of resection of the “candy cane” (the blind end of the alimentary limb at the GE), and in all cases of refashioning of the GE, and of a sleeve resection of the greater curvature side of the gastric pouch, around an orogastric 34-French tube kept in close contact with the lesser curvature side. As a rule, at the same time the upper pole of the gastric remnant was resected.
-
Laparoscopic reconversion of the bypass into a normal anatomy and transformation into a sleeve gastrectomy (LSG) in one session
-
Laparoscopic plication of the pouch, the GE and the alimentary limb, around a 34-French orogastric tube.
The indications for each of the aforementioned procedures can be found in Table 1.
-
Endoscopic procedure: patients with weight loss issues after revisional LRYGB, especially with evidence of loss of restriction at endoscopy and X-ray.
-
Distalization procedure: patients after primary or revisional LRYGB, who did not experience sufficient weight loss OR who presented weight regain and increased caloric intake.
-
Placement of a “Fobi” ring: patients who maintained a rather normal aspect of the bypass but developed a hyperphagia behavior after primary LRYGB.
-
Reconstruction of the bypass: patients who after primary or secondary presented with an objective anatomical flaw (gastro-gastric fistula, anastomotic ulcer, candy cane deformation of the alimentary loop concomitant with size increase of the gastric pouch).
-
LSG construction at reconversion into normal anatomy: patients who never lost acceptable weight after primary LRYGB.
-
Plication of the gastric pouch as well as the alimentary loop: patients who regained weight after secondary bypass or after bypass fraught with anastomotic complication.
Study Design
All patients operated on for insufficient weight loss or for weight regain after a LRYGB procedure performed at our institution were included in this retrospective study, that covered the period of time between January 1, 2000 and December 31, 2009.
The charts were reviewed and the data collected by two of the authors (AV, LC).
The primary outcome measurements were the weight loss parameters BMI and percentage of excess weight loss (EWL%) recorded in October 2011, as well as the postoperative complications. The secondary outcome analyzed was patient satisfaction, which was evaluated in accordance with the Bariatric Analysis and Reporting Outcome System (BAROS). In brief, with this evaluation tool, three psychomedical aspects are estimated: weight loss, changes in comorbidities, and quality of life. Up to 3 points are allotted for each category, and points are deducted for complications and reoperations [3].
Statistical Analysis
Data were collected from a prospectively kept database. When normally distributed, the results were reported as mean + standard deviation and range. When the distribution was not normal, the results were expressed as median plus range. The t test for paired variables was used for significance analysis of the evolution of the variables weight, BMI, and %EWL. Statistical significance was reached at p < 0.05.
Results
Between January 1, 2001 and December 31, 2009, 88 of the patients (9.2 %) who had undergone LRYGB in our department underwent revision for weight issues: either insufficient weight loss or weight regain. The revisional procedure took place 3.0 years (range 1.5–8.0) after the LRYGB. Eighteen patients underwent endoscopic reshaping of the gastric pouch and anastomosis by use of the StomaphyX® device (Endogastric Solutions, Redmond, WA, USA). They were part of a feasibility study protocol and are not further discussed here.
Seventy patients were treated laparoscopically. Of the latter, complete data was available in 58 (82.9 %) and follow-up extended over a median of 48 months (18–122).
Forty-eight patients (82.8 %) were women, and the patients’ age averaged 50 + 2.5 years old (18–78).
Laparoscopic Procedures (Tables 2 and 4)
-
Nineteen patients were treated by laparoscopic distalization of their bypass. Three patients in this group had undergone LAGB before the LRYGB procedure, followed by poor weight loss. One additional patient had had little benefit from her primary bypass in terms of weight loss and diabetes control. Fifteen additional patients had presented weight regain after primary LRYGB.
-
In ten patients, presenting weight regain after primary bypass, a non-adjustable, custom-made 6.5-cm “Fobi” ring was placed laparoscopically around the gastric pouch that had essentially remained unchanged.
-
In 12 patients, the gastric pouch and the GE were revised and refashioned laparoscopically. Three of the latter patients, all of whom had undergone bariatric procedures previously (LAGB in one and vertical banded gastroplasty (VBG) in two) suffered from poor weight loss and presented with medical therapy-resistant concomitant anastomotic ulcers. Five additional patients of this group, all of whom had undergone LAGB previously, suffered a gastro-gastric fistula. In four patients after primary LRYGB, gastroscopy and upper GI series had demonstrated a significant candy cane deformation of the alimentary loop.
-
In nine other patients who presented remarkably poor weight loss (EWL < 10 %) after primary LRYGB, the bypass was reversed and transformed into an LSG in one laparoscopic session, followed at a later stage by a laparoscopic duodenal switch in three.
-
Eight patients underwent plication of the pouch, the GE and the alimentary limb for weight regain. Four of the patients had undergone LAGB before the bypass; three had had a VBG; the last patient had suffered an anastomotic leak at the time of the bypass carried out as a primary procedure.
Main Outcome
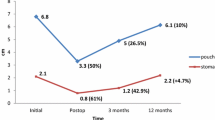
The mean BMI before the revisional procedure was 39.1 + 11.3 kg/m2 (30.8–51.8), down from 42.7 + 19.7 kg/m2 (33.0–56.6) initially, which corresponded to a pre-procedural percentage of excess weight loss of 12.4 + 9.3 % (−1.0–29.1).
The mean hospital stay for the revisional procedure was 5.9 + 10 days (1–78). There were no mortalities.
At the time of this study, after a median of 48 (18–122) months after the revisional procedure, the overall BMI was 29.6 + 12.4 kg/m2 (18.0–45.5). Compared to the initial BMI value, the difference was statistically significant (p = 0.001). Compared to the preprocedural value, this number reached statistical significance as well (p = 0.001). The additional percentage of EWL, with reference to preprocedural weight loss, was significant: 53.7 + 9.8 % (2.0–65.8) (p < 0.001).
The progression of BMI with time can be found in Figs. 2 (for the distalization procedure), 3 (for reconstruction of the bypass), and 4 (for all procedures combined).
Reoperations and Complications (Table 3)
The overall reoperation rate was 7.3 %, and the overall severe complication rate was 20.7 %.
-
In the distalization group only, the morbidity rate was severe, at 21.1 %. One of the patients in this latter group developed a Douglas abscess, probably on a hidden leak. She was treated conservatively and eventually healed but stayed in the hospital for 2 months. Two other distalization patients (10.5 %) needed reversal of the bypass into its initial form because of cachexia and anorexia appearing some 2 years after the revisional procedure. A fourth patient required laparoscopic reoperation for an internal hernia.
-
One (12.5 %) of the patients who underwent plication of the pouch, GE, and alimentary limb developed overwhelming sepsis from an infected intrauterine device 6 weeks after the procedure and underwent exploratory laparotomy that could exclude a leak at the operative site.
-
There were two (20 %) postoperative intragastric erosions of Fobi rings. Both cases were reoperated on, and the bands were removed; they developed a leak and were successfully treated by stents.
-
Three patients developed a leak after refashioning of the bypass construction. This group included two (40 %) of the five GGF patients; they were managed successfully by stents. The third patient who had presented with an anastomotic ulcer was treated conservatively by antibiotics and parenteral nutrition.
-
Two of the patients (28.6 %) who were submitted to reversal of the bypass together with LSG suffered leaks at the gastro-gastric anastomosis: one was treated successfully by a stent; the other suffered a short-lived fistula that healed with conservative measures.
Thus, the overall leak rate in the group of 58 patients was 12.1 % (n = 7). All the leaks encountered were successfully managed endoscopically or conservatively.
Patient Satisfaction
After the corrective laparoscopic procedures, the BAROS score evaluation for quality of life was 3.02 (1–7.5), which is considered a “fair” outcome [1]; of the 58 patients who could be evaluated, 30 (51.7 % of the patients) were satisfied, seven (12.1 %) were neutral, and 21 (36.2 %) were dissatisfied.
Discussion
Not all patients do well after a bariatric procedure, and the LRYGB is no exception. Some patients do not lose sufficient weight; others regain weight after an initial good weight loss [5]; still others do well in terms of weight loss but suffer from side effects the procedure induced. Common invalidating side effects after LRYGB include: persisting gastro-esophageal reflux (GERD)[6], exaggerated dumping [7], and hypoglycemic syndrome [8]. In this study, we focused solely on the roughly 9 % of patients who required reoperation after LRYGB because of weight issues.
The decision to revise a patient surgically for a bypass that failed in terms of weight loss is critical [9]. If at all possible, conservative means should be preferred [10]. Hence, a multidisciplinary team should be consulted for a unanimous decision before proceeding to reoperation [11].
The revisional procedures can be classified into two groups: endoscopic techniques (n = 18) and laparoscopic procedures (n = 70).
Because the incidence of complications after revisional surgery increases with the number of previous operations [12], the primary or secondary character of the initial bypass plays an important role in the decision-making. According to the literature [13], an endoscopic procedure should be the first choice to improve weight loss in previously operated cases, to limit morbidity.
Consequently, we usually elected for laparoscopic revisional surgery in relatively simple primary bypass patients. In addition, we chose for this approach in more complex cases where, according to the multidisciplinary consultation, the potential benefit from additional weight loss clearly outweighed the surgical risk. The choice as to the type of corrective procedure should limit the risks for complications. In patients in whom the bypass had been constructed in previously dissected tissues, or in whom tissues were altered by a postoperative fistula, we therefore tried to avoid stapling in that area and elected either non-resective techniques including plication of the gastric pouch and the alimentary loop, or distalization of the Y anastomosis.
Other factors influencing the choice of procedure, besides local anatomical conditions included: did the patient experience weight regain versus insufficient weight loss? (the latter inviting for a different philosophy, as a distal bypass or a sleeve gastrectomy); how did the comorbidities (especially DMII) evolve? (lack of response inviting for a more radical procedure, i.e., a distal bypass); how did the patient adjust his/her eating behavior? [14]. Patients who according to the dietitian’s diagnosis had become grazers were preferably treated with a malabsorptive procedure, whereas volume eaters were treated by adding new restrictions.
Despite the diversity of procedures and their indications, the surgical procedures overall provided significant additional weight loss (Fig. 3).
The fact that the mechanism of action of the distal bypass, being malabsorptive, substantially differs from the classic RYGB makes it (theoretically) a logical choice in case of outright failure of the latter. However, while good weight loss indeed was obtained by distalizing the bypass [14] (Fig. 1), in the long term, protein malnutrition and cachexia developed in a number of patients. This latter evolution has been described extensively in the literature [15]. The team around Sugerman [16] demonstrated that converting a primary into a distal bypass could carry severe metabolic consequences, even resulting in mortality in some patients. Brolin [17] found that only half of patients had clear benefits from distalization of the bypass. In a recent paper, Rawlins et al. [9] described a series of 29 patients undergoing open distalization of a RYGB. In this construction, with a 100-cm-long common limb and a 250-cm alimentary-common limb, six patients developed protein malnutrition, and one patient required reversal. We agree with these authors that, when distalization of the bypass is contemplated, it can only be performed in compliant follow-up patients. Despite these precautions, two (10.5 %) of our patients treated by this technique developed protein malnutrition. Fortunately, distal bypass construction proved to be just as reversible as conventional LRYGB [3], and protein malnutrition could be halted in the two patients who underwent reversal of their distalization. More gentle attempts at distalization (by lengthening the alimentary loop to 200 cm, for example), however, have proved useless in the long term [18].
Leading authors have stated that restriction corrected by restriction is fraught with poor results in terms of weight loss [19]. Repeating a restrictive measure can be questioned, because weight loss failure after bypass, in fact, implies a failure to change old eating behaviors [20]. Indeed, a bypass itself very seldom succeeds in changing a patient’s eating habits [21]. Conversely, it appears that volume increases after bypass surgery, as demonstrated by endoscopic or X-ray evidence, do not necessarily imply weight regain, therefore making new restrictive efforts theoretically all but futile [22]. However, in very carefully selected cases, especially when patient compliance is questionable, additional restriction, rather than malabsorption, can be salutary. Additionally, the presence of a new restricting factor can be useful in patients who besides weight regain suffer from exaggerated dumping [23].
-
One option is the placement of a band, whether adjustable [24] or not [25]. The banded bypass has been popularized by Fobi [26]. Implanting an adjustable band reportedly carries better results, but this finding is not uniformly found [27]. A significant drawback with the banding technique is the incidence of intragastric migration (erosion) as demonstrated in our series [28].
-
Another option for additional restriction is to laparoscopically reshape the bypass at the gastric pouch, the GE anastomosis, and the proximal alimentary loop. This technique is technically demanding, and weight loss results are usually modest [29]. It represents, however, a valuable option when the patient presents a clear anatomical flaw at endoscopy or barium swallow. These aberrations include a gastrogastric fistula, a candy cane deformation of the alimentary loop, or an anastomotic ulcer, a condition that is very hard to treat by techniques other than surgery [5, 12]. The downside of the “bypass reshaping” procedure is the high incidence of postoperative leaks, especially when the procedure aimed at the correction of a GGF. To reduce complications, treatment of GGF has been attempted endoscopically, unfortunately with poor results [30].
-
Still another possibility is reversing the bypass [31] and to complement this procedure with a sleeve gastrectomy [32]. In our department, the conversion of a LRYGB into a LSG was carried out with the intention of eventually performing a DS, a clearly malabsorptive procedure, at a later stage. This technique was described earlier by our team [32]. The numbers were too small and the follow-up too short to draw final conclusions about this technique, but the transformation into LSG could constitute a valuable option for patients who presented only modest weight loss after LRYGB and in whom a different “operative philosophy” was pursued (Table 4).
-
An interesting last option is to address the pouch, the anastomosis, and the alimentary limb all at once and to reduce their size. Parikh and Gagner have demonstrated that resecting the pouch and alimentary loop in a longitudinal fashion is feasible but not very effective [33]. We believe that, in light of the risk of complications, especially because of significant tissue alteration as after redo-LRYGB or in cases who suffered a postoperative leak, it is preferable to choose a procedure with limited risks. The plication we performed, while probably safer, mimicked the longitudinal resection carried out by Gagner but was inspired as well by the greater curvature plication techniques developed in similarity with LSG [34]. As in this latter procedure, we performed the plication with non-absorbable material around a 34-French orogastric tutor tube. The results of these latter attempts appeared promising, but longer-term results must again be awaited.
Obviously, the beneficial results of revisional surgery should be weighed against the morbidity of this second procedure. Usually, secondary procedures are characterized by more severe side effects and complications [12]. Our reoperation rate was comparable to the reoperation rate in open surgery [35]. In our series, roughly one out of five patients developed a severe surgical complication. Whereas the mortality was zero, the severity of the complications translated into long hospital stays, approaching 6 days, versus the usual 3 days for a “normal” bypass [36]. In our group, morbidity was quite high, especially when foreign material was involved. This result paralleled our finding with LAGB, in which approximately 30 % of the patients developed intragastric migration of the band [37]. As an analogy, it is not surprising to find high numbers of erosion when a band is placed around a staple line [38] or in poorly vascularized tissues [39], as typically encountered in revisional surgery. We elected to remove the eroding “Fobi” rings laparoscopically, but a better option would have been to remove them endoscopically [40], as demonstrated by two patients developing leaks after laparoscopic ablation of the band.
Overall, the leaks that our patients encountered were successfully treated with conservative methods. As a rule, if at all possible, we preferred to treat leaks in bariatric patients by conservative means, as described by others [41], or endoscopically by stents, if the leaks appeared too large or the tissues too atonic [42]. Self-expanding, partially covered metallic stents were successfully used in patients who developed leaks after GGFs, after band erosion, or after anastomotic revisions, as well as in one patient who leaked after transformation into LSG. The only patient we had to treat with a laparotomy was a patient in whom we had performed a plication of the gastric pouch and the alimentary loop and in whom we suspected a leak. Some 2 months postoperatively, she developed a fulminating sepsis that, with hindsight, appeared to be gynecological in origin.
When we analyzed patient satisfaction, the results were only fair. Reperformed operations, as a rule, have poorer results than initial operations [43], and this rule goes for patient satisfaction as well. This finding most likely has to do with the complication rate, which is responsible for lower BAROS scores [4] and/or with relatively poor patient acceptance, especially after new restrictive procedures [44]. Patients should therefore be notified that despite anticipated, acceptable, additional weight loss, long-term satisfaction might become an issue after revisional surgery for failed gastric bypass.
Conclusions
With the increasing numbers of LRYGB, long-term weight issues have become a problem for some patients. Several types of actions are possible, depending on the history (primary bypass or not) and on the type of weight problem (insufficient weight loss or weight regain). We focused here on laparoscopic revisions. Laparoscopic reoperations aimed at increasing malabsorption were effective, but at the cost of severe morbidity and symptoms of malnutrition, and they should therefore be performed only in very well-selected cases. Adding a restrictive aspect can be performed by different means. The use of a constricting band, as well as reshaping the bypass construction, had a significant morbidity. Transforming the bypass into a sleeve, possibly as the first step of a DS, as well as plicating the pouch and the alimentary loop, deserves further consideration. Patient satisfaction was relatively disappointing, which may have had to do with the high morbidity figures.
References
Reinhold RB. Critical analysis of long term weight loss following gastric bypass. Surg Gynecol Obstet. 1982;155(3):385–94.
Kruseman M, Leimgruber A, Zumbach F, et al. Dietary, weight, and psychological changes among patients with obesity, 8 years after gastric bypass. J Am Diet Assoc. 2010;110(4):527–34.
Dapri G, Cadière GB, Himpens J. Laparoscopic reconversion of Roux-en-Y gastric bypass to original anatomy: technique and preliminary outcomes. Obes Surg. 2011;21(8):1289–95.
Oria HE, Moorehead MK. Bariatric analysis and outcome reporting system (BAROS). Obes Surg. 1998;8:487–99.
Higa K, Ho T, Tercero F, et al. Laparoscopic Roux-en-Y gastric bypass: 10 year follow-up. Surg Obes Relat Dis. 2011;7(4):516–25.
Merrouche M, Sabaté JM, Jouet P, et al. Gastro-esophageal motility disorders in morbidly obese patients before and after bariatric surgery. Obes Surg. 2007;17(7):894–900.
Tack J, Arts J, Caenepeel P, et al. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol. 2009;6(10):583–90.
Cui Y, Elahi D, Andersen DK. Advances in the etiology and management of hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass. J Gastrointest Surg. 2011;15(10):1879–88.
Rawlins ML, Teel II D, Hedgcorth K, et al. Revision of Roux-en-Y gastric bypass to distal bypass for failed weight loss. Surg Obes Relat Dis. 2011;7(1):45–9.
Faria SL, de Oliveira Kelly E, Lins RD, et al. Nutritional management of weight regain after bariatric surgery. Obes Surg. 2010;20(2):135–9.
Runkel N, Colombo-Benkmann M, Huettl TP, et al. Evidence-based German guidelines for surgery for obesity. Int J Color Dis. 2011;26(4):397–404.
Patel S, Szomstein S, Rosenthal RJ. Reasons and Outcomes of reoperative bariatric surgery for failed and complicated procedures (excluding adjustable gastric banding). Obes Surg. 2011;21:1209–19.
Ryou M, Ryan MB, Thompson CC. Current status of endoluminal bariatric procedures for primary and revision indications. Gastrointest Endosc Clin N Am. 2011;21(2):315–33.
Dapri G, Cadière GB, Himpens J. Laparoscopic conversion of Roux-en-Y gastric bypass to distal gastric bypass for weight regain. L Laparoendosc Adv Surg Tech A. 2011;21(1):19–23.
Fobi MA, Lee H, Igwe Jr D, et al. Revision of failed gastric bypass to distal Roux-en-Y gastric bypass: review of 65 cases. Obes Surg. 2001;11(2):190–5.
Sugerman HJ, Kellum JM, DeMaria EJ. Conversion of proximal to distal gastric bypass for failed gastric bypass for superobesity. J Gastrointest Surg. 1997;1(6):517–24.
Brolin RE, Cody RP. Adding malabsorption for weight loss failure after gastric bypass. Surg Endosc. 2007;21(11):1924–6.
Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244(5):734–40.
Brolin RE, Cody RP. Weight loss outcome of revisional bariatric operations varies according to the primary procedure. Ann Surg. 2008;248(2):227–32.
Cook CM, Edwards C. Success habits of long-term gastric bypass patients. Obes Surg. 1999;9(1):80–2.
Guerdjikova AI, West-Smith L, Mc Elroy SL, et al. Emotional eating and emotional eating alternatives in subjects undergoing bariatric surgery. Obes Surg. 2007;17(8):1091–6.
Roslin M. Comment on: Long-term follow-up in patients undergoing open gastric bypass as a revisional operation for previous failed restrictive procedures. Surg Obes Relat Dis 2011:7(5)572–4.
Z’graggen K, Guweidhi A, Steffen R, et al. Severe recurrent hypoglycemia after gastric bypass surgery. Obes Surg. 2008;18(8):981–8.
Bessler M, Daud A, DiGiorgi MF, et al. Adjustable gastric banding as revisional bariatric procedure after failed gastric bypass—intermediate results. Surg Obes Relat Dis. 2010;6(1):31–5.
Dapri G, Cadière GB, Himpens J. Laparoscopic placement of non-adjustable silicone ring for weight regain after Roux-en-Y gastric bypass. Obes Surg. 2009;19(5):650–4.
Fobi M. Banded gastric bypass: combining two principles.2004 ABS Consensus Conference. Surg Obes Relat Dis. 2005;1(3):304–9.
Irani K, Youn HA, Ren-Fielding GA, et al. Midterm results for gastric banding as salvage procedure for patients with weight loss failure after Roux-en-Y gastric bypass. SOARD. 2011;7(2):219–24.
Júnior WS, Pitanga CK, Borges CN, et al. Treatment of gastrogastric fistula after Roux-en-Y gastric bypass: surgery combined with gastroscopy. Obes Surg. 2007;17(6):836–8.
Mueller MK, Wildi S, Schlz T, et al. Laparoscopic pouch resizing and redo of gastro-jejunal anastomosis for pouch dilatation following gastric bypass. Obes Surg. 2005;15(8):1089–95.
Fernandez-Esparrach G, Lautz DB, Thompson CC. Endoscopic repair of gastrogastric fistula after Roux-en-Y gastric bypass: a less-invasive approach. SOARD. 2010;6(3):282–8.
Himpens J, Dapri G, Cadière GB. Laparoscopic conversion of the gastric bypass into a normal anatomy. Obes Surg. 2006;16(7):908–12.
Dapri G, Cadière GB, Himpens J. Laparoscopic conversion of Roux-en-Y gastric bypass to sleeve gastrectomy as first step of duodenal switch: technique and preliminary outcomes. Obes Surg. 2011;21(4):517–23.
Parikh M, Heacock L, Gagner M. Laparoscopic “gastrojejunal sleeve reduction” as a revision procedure for weight loss failure after Roux-en-Y gastric bypass. Obes Surg. 2011;21(5):650–4.
Menchaca HJ, Harris JL, Thompson SE, et al. Gastric plication: preclinical study of durability of serosa-to-serosa apposition. SOARD. 2011;7(1):8–14.
Hedberg J, Gustavsson S, Sundbom M. Long-term follow-up in patients undergoing open gastric bypass as a revisional operation for failed previous restrictive procedures. Surg Obes Relat Dis 2011 Jun 30
Tiwari MM, Goede MR, Reynoso JF, et al. Differences in outcomes of laparoscopic gastric bypass. SOARD. 2011;7(3):277–82.
Himpens J, Cadière GB, Bazi M, et al. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg. 2011;146(7):802–7.
Msika S. Surgery for morbid obesity: 2. Complications. Results of a technologic evaluation by the ANAES. J Chir (Paris). 2003;140(1):4–21.
Kurian M, Sultan S, Garg K, et al. Evaluating gastric erosion in band management: an algorithm for stratification of risk. SOARD. 2010;6(4):386–9.
Evans JA, Williams NN, Chan EP, et al. Endoscopic removal of eroded bands in vertical banded gastroplasty: a novel use of endoscopic scissors (with video). Gastrointest Endosc. 2006;64(5):801–4.
Casella G, Soricelli E, Rizzello M, et al. Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg. 2009;19(7):821–6.
Eisendrath P, Cremer M, Himpens J, et al. Endotherapy including temporary stenting of fistulas of the upper gastrointestinal tract after laparoscopic bariatric surgery. Endoscopy. 2007;39(7):625–30.
Cadière GB, Himpens J, Bazi M, et al. Are laparoscopic gastric bypass after gastroplasty and primary laparoscopic gastric bypass similar in terms of results? Obes Surg. 2011;21(6):692–8.
Al Harakeh AB, Larson CJ, Mathiason MA, et al. BAROS results in 700 patients after laparoscopic Roux-en-Y gastric bypass with subset analysis of age, gender, and initial body mass index. SOARD. 2011;7(1):94.
Disclosures
The authors have no conflict of interest in the material presented in this study.
Dr. Himpens is a consultant with Covidien and Ethicon Endosurgery; he performs workshops and receives a honorary from Gore. Dr. Cadière is a consultant with Ethicon Endosurgery and Storz; he performs workshops and receives an honorary from Covidien. Dr. Coromina and Dr. Verbrugghe have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Himpens, J., Coromina, L., Verbrugghe, A. et al. Outcomes of Revisional Procedures for Insufficient Weight Loss or Weight Regain After Roux-En-Y Gastric Bypass. OBES SURG 22, 1746–1754 (2012). https://doi.org/10.1007/s11695-012-0728-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-012-0728-7