Abstract
Background
The storage capacity of adipose tissue may be an important factor linking obesity, insulin resistance (IR), and associated morbidities. The aim of this study was to analyze the expression of lipogenic and lipolytic genes in adipose tissue and the influence of IR.
Methods
We studied the mRNA expression of peroxisome proliferator-activated receptor-γ (PPARγ) and lipogenic and lipolytic enzymes in the visceral (VAT) and subcutaneous adipose tissue (SAT) from 23 morbidly obese patients (MO; 13 with low IR and ten with high IR) and from 15 healthy, lean controls.
Results
In the VAT and SAT from the MO, we found an increased expression of PPARγ (p = 0.001 and p = 0.022, respectively), acyl-coenzyme A (CoA)/cholesterol acyltransferase (p < 0.001 and p < 0.001), aquaporin 7 (p < 0.001 and p = 0.003), and adipose triglyceride lipase (p < 0.001 and p < 0.001) and a reduced expression of acetyl-coenzyme A carboxylase (p = 0.004 and p < 0.001), independently of the state of IR. The expression of phosphoenolpyruvate carboxykinase and acyl-CoA synthetase, however, was significantly lower in the MO with high IR (p < 0.05). Glycerol kinase (p = 0.010), hormone-sensitive lipase (p < 0.001), and perilipin (p = 0.006) were only significantly increased in VAT. Acyl-CoA synthetase (p = 0.012) and fatty acid binding protein-4 (p = 0.003) were only significantly decreased in SAT. The expression of the genes studied was only greater in the SAT than the VAT in the controls.
Conclusion
Our results show an upregulation of genes facilitating triglyceride/fatty acid cycling and a reduction in the genes involved in de novo synthesis of fatty acids in morbid obesity. The expression of some of the genes studied seems to be related with the state of IR. VAT and SAT differ metabolically and also between controls and MO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The importance of adipose tissue in maintaining metabolic equilibrium is well known from studies in both animal models and humans. Triglyceride synthesis is a determining factor for adipose tissues mass. The fatty acids (FA) from adipose tissue are primarily an important energy substrate for a number of organs. In addition, triglyceride synthesis in cells may protect them from the potentially toxic effects of excess FA. These FA alter insulin action and glucose metabolism, as well as altering gene function like transcription factors and regulating insulin production [1, 2]. The efficient energy store is influenced by the supply of fatty acids–coenzyme A (CoA), of glycerol phosphate precursors, or by the activity of enzymes at later stages of triglyceride synthesis. Availability of glycerol phosphate is a determinant of triglyceride synthesis and lipolysis. Another key enzyme in triglyceride synthesis is acyl-coenzyme A/cholesterol acyltransferase (DGAT1), which catalyzes the final step of lipogenesis. Inactivation of DGAT1 results in lean mice [3]. Although lipogenesis accounts for only a small fraction of total triglyceride flux, its importance in the determination of adipose tissue mass has not been resolved [4].
The regulation of lipolysis is also essential to ensure an adequate supply of lipids to tissues utilizing FA. During intracellular lipolysis, adipose triglyceride lipase (ATGL) is rate limiting for the first step in triglyceride hydrolysis, generating diglycerides (DG) and FA [5]. Hormone-sensitive lipase (HSL) hydrolyzes both triglycerides and DG substrate. Perilipin is essential for the recruitment of HSL to lipid droplets and full enzyme activation [6]. The function of perilipins is to prevent lipolysis in basal conditions, favoring lipid deposition. Half of the hydrolyzed FA are recycled into triglyceride synthesis [7]. The combination of an excess calorie intake, physical inactivity, and genetic factors contributes to an alteration of the function of normal adipose tissue as an energy store and lipid buffer. Lipolysis in normal subjects is extremely sensitive to the action of insulin. However, hormone regulation of lipolysis in obesity is under debate. Regarding insulin, increased, normal, or even decreased antilipolytic effects have been reported [8].
Peroxisome proliferator-activated receptor-γ (PPARγ) are ligand-activated transcription factors strongly expressed in adipocytes, and they play a critical role in glucose and lipid homeostasis [9]. The PPARγ regulate the transcription of genes involved in lipid synthesis and facilitate the entrance of free fatty acids (FFA) into adipocytes, activate triglyceride synthesis, and stimulate the esterification of FFA by acyl-CoA synthase. They also seem to stimulate the glycerol cycle [10] and to regulate the transcription of genes involved in lipolysis [11]. Several studies have helped in understanding the function of PPARγ [12].
Morbid obesity represents an extreme phenotype involving a very important increase of adipose tissue. Most morbidly obese patients have alterations in lipid and carbohydrate metabolism [13–15], which is regulated by PPARγ [16]. To better understand the mechanisms underlying the adipose tissue mass, the aim of this study was twofold: firstly, to examine in adipose tissue from morbidly obese patients with low and with high insulin resistance the expression of PPARγ and genes involved in lipogenesis and lipolysis and secondly, to explore the expression of these genes in visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT). To date, only a few studies have been undertaken simultaneously in the two tissues. We hypothesized that the expression of lipogenic and lipolytic genes varies according to the degree of insulin resistance and the particular adipose tissue studied.
Materials and Methods
Subjects
The study included 23 morbidly obese patients (BMI 57.4 ± 5.2 kg/m2), 13 with low insulin resistance (homeostasis model assessment of insulin resistance index (HOMA-IR) <4.7) and ten with high insulin resistance (HOMA-IR >8), and 15 healthy, lean persons (BMI 22.7 ± 1.5 kg/m2). The cutoff point for the HOMA-IR was taken from previous studies carried out in a healthy population with no carbohydrate metabolism disorders (HOMA-IR <4.7) [17]. Patients were excluded if they had cardiovascular disease, arthritis, acute inflammatory disease, infectious disease, or were receiving drugs that could alter the lipid profile or the metabolic parameters at the time of inclusion in the study. None of the morbidly obese patients was being treated with insulin, oral antidiabetic agents, or diet. The weight of all the persons had been stable for at least 1 month, and none had renal involvement. All participants gave their informed consent and the study was reviewed and approved by the Ethics and Research Committee of Virgen de la Victoria Clinical University Hospital, Malaga, Spain.
Laboratory Measurements
Blood samples were collected after a 12-h fast. The serum was separated and immediately frozen at −80°C. Serum biochemical parameters were measured in duplicate. Serum glucose, cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides (Randox Laboratories Ltd., Antrium, UK) and FFA (WAKO Chemicals, Richmond, VA, USA) were measured by standard enzymatic methods. Low-density lipoprotein cholesterol was calculated by the Friedewald formula. The insulin was analyzed by an immunoradiometric assay (BioSource International, Camarillo, CA, USA). Leptin was analyzed by enzyme immunoassay (ELISA) kits (DSL, Webster, TX, USA). Adiponectin was analyzed by enzyme immunoassay (ELISA) kits (DRG Diagnostics GmbH, Germany). The HOMA-IR was calculated from fasting insulin and glucose with the following equation: HOMA-IR = fasting insulin (μIU/mL) × fasting glucose (mmol/L)/22.5.
Visceral and Subcutaneous Adipose Tissue mRNA
VAT and abdominal SAT were obtained during bariatric surgery in the morbidly obese patients or during elective abdominal surgery procedures in lean subjects [18]. The biopsy samples were washed in physiological saline and immediately frozen in liquid nitrogen. Biopsy samples were maintained at −80°C until analysis. Total RNA isolation from adipose tissues was obtained using RNeasy Lipid Tissue Mini Kit (Qiagen GmbH, Germany). The purity of the RNA was determined by the absorbance260/absorbance280 ratio on the Nanodrop. The integrity of total purified RNA was checked by denaturing agarose gel electrophoresis and ethidium bromide staining. Quantitative real-time PCR was performed in a Mastercycler gradient (Eppendorf AG, Hamburg, Germany). The cDNAs obtained were used as the template for quantitative real-time PCR by a Light Cycler Instrument (Roche Diagnostics, Basel, Switzerland), using the SYBR green fluorescent method. Primers for the PCR reaction were designed based on NCBI database sequences of the human genes PPARγ, DGAT1, phosphoenolpyruvate carboxykinase (PEPCK), glycerol kinase (GK), aquaporin 7 (AQP7), acetyl-coenzyme A carboxylase alpha (ACC1), acyl-CoA synthetase short-chain family member 2 (ACSS2), fatty acid binding protein 4 (FABP4), ATGL, HSL, phosphodiesterase type 3 (PDE3), and perilipin. Adipose tissue expression levels for each gene were normalized using β-actin. Statistical analysis showed no differences in gene expression for this housekeeping gene in the groups studied.
Statistical Analysis
The statistical analysis was done with SPSS (Version 11.5 for Windows; SPSS, Chicago, IL, USA). Comparison between the results of the controls and morbidly obese patients was made with the Mann–Whitney test. Comparison between the results of the different groups of morbidly obese patients and the controls was made with the Kruskal–Wallis test. Comparison between the results of the different adipose tissues was made with the Wilcoxon test. The Spearman correlation coefficients were calculated to estimate the correlations between variables. Values were considered to be statistically significant when the p ≤ 0.05. The results are given as the mean ± SD.
Results
Anthropometric and Biochemical Characteristics
Table 1 summarizes the characteristics of the controls and the morbidly obese patients, with low and high insulin resistance. The weight and the anthropometric data were, as expected, significantly greater in the morbidly obese patients, though no significant differences were seen between the two groups of obese patients.
VAT mRNA Expression
Compared with the morbidly obese patients, the mRNA expression in the VAT from the controls was significantly lower: PPARγ (1.12 ± 0.65 vs. 2.57 ± 1.52, p = 0.001), GK (0.98 ± 0.45 vs. 2.39 ± 2.36, p = 0.010), AQP7 (1.35 ± 0.65 vs. 3.10 ± 1.34, p < 0.001), DGAT1 (1.06 ± 0.37 vs. 2.00 ± 0.86, p < 0.001), ATGL (1.33 ± 0.61 vs. 5.54 ± 2.71, p < 0.001), HSL (1.58 ± 1.12 vs. 4.48 ± 3.25, p < 0.001), and perilipin (1.15 ± 0.54 vs. 1.83 ± 0.82, p = 0.006). The mRNA expression of ACC1 (1.80 ± 0.52 vs. 1.08 ± 0.78, p = 0.004) and PEPCK (3.22 ± 2.67 vs. 1.93 ± 1.16, p = 0.049), however, was significantly greater in the controls than the morbidly obese patients.
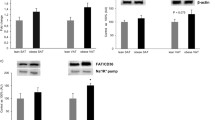
When the morbidly obese patients were classified according to their level of insulin resistance, the differences remained significant as compared with the controls for the expression of PPARγ, DGAT1, ACC1, AQP7, ATGL, and HSL (Figs. 1 and 2). The expression of PEPCK in the controls was only significantly higher than those morbidly obese patients with high insulin resistance (Fig. 1). The expression of GK in the controls was only significantly lower than those morbidly obese patients with low insulin resistance (Fig. 1). The expression of perilipin in the morbidly obese patients with low insulin resistance was significantly higher than those morbidly obese patients with high insulin resistance and the controls (Fig. 2).
mRNA expression levels of lipogenic genes in subcutaneous (black bar) and visceral adipose tissue (white bar) in the controls (n = 15) and the morbidly obese patients, classified according to their insulin resistance. MO low IR morbidly obese persons with low insulin resistance (n = 13), MO high IR morbidly obese persons with high insulin resistance (n = 10). Adipose tissue expression levels for each gene were normalized using β-actin. The results are given as the mean ± SD. Different letters indicate significant differences between the means of the three groups in VAT or in SAT (p < 0.05). *p < 0.05; † p < 0.005; ‡ p < 0.001: significant differences within the same group of patients between subcutaneous and visceral adipose tissues
mRNA expression levels of lipolytic genes in subcutaneous (black bar) and visceral adipose tissue (white bar) in the controls (n = 15) and the morbidly obese patients, classified according to their insulin resistance. MO low IR morbidly obese persons with low insulin resistance (n = 13), MO high IR morbidly obese persons with high insulin resistance (n = 10). Adipose tissue expression levels for each gene were normalized using β-actin. The results are given as the mean ± SD. Different letters indicate significant differences between the means of the three groups in VAT or in SAT (p < 0.05). *p < 0.05; † p < 0.005; ‡ p < 0.001: significant differences within the same group of patients between subcutaneous and visceral adipose tissues
SAT mRNA Expression
Compared with the morbidly obese patients, the mRNA expression in the SAT from the controls was significantly lower: PPARγ (1.65 ± 0.51 vs. 2.50 ± 1.50, p = 0.022), AQP7 (1.87 ± 0.57 vs. 2.92 ± 1.36, p = 0.003), DGAT1 (1.89 ± 0.53 vs. 2.81 ± 0.76, p < 0.001), and ATGL (2.18 ± 0.59 vs. 5.47 ± 2.76, p < 0.001). The mRNA expression of ACC1 (2.52 ± 1.00 vs. 1.09 ± 0.63, p < 0.001), ACSS2 (1.65 ± 0.61 vs. 1.07 ± 0.68, p = 0.012), PEPCK (3.88 ± 4.02 vs. 1.88 ± 1.19, p = 0.034), FABP4 (1.96 ± 0.80 vs. 1.14 ± 0.73, p = 0.003), and PDE3 (2.14 ± 0.61 vs. 1.57 ± 0.86, p = 0.005) was significantly greater in the controls than the morbidly obese patients.
When the morbidly obese patients were classified according to their level of insulin resistance, the differences only remained significant as compared with the controls for the expression of PPARγ, DGAT1, FABP4, ACC1, and ATGL (Figs. 1 and 2). The expression of ACSS2, PEPCK, and PDE3 in the controls was only significantly higher than those morbidly obese patients with high insulin resistance (Figs. 1 and 2), and the expression of AQP7 in the controls was only significantly higher than those morbidly obese patients with low insulin resistance (Fig. 1).
Differences Between VAT and SAT mRNA Expression
Comparison in the controls of the different expression levels in the two adipose tissues showed that the expression of PPARγ, DGAT1, AQP7, ACC1, ACSS2, FABP4, GK, ATGL, HSL, PDE3, and perilipin was significantly higher in the SAT than the VAT (Figs. 1 and 2). In the morbidly obese patients, the expression of GK in the SAT was lower than in the VAT only in those with high insulin resistance (Fig. 1). No significant differences between the VAT and the SAT were seen in the expression of the other genes studied in the morbidly obese patients.
Correlations of the mRNA Gene Expression
A positive correlation was found between the expression of a group of genes (PPARγ, DGAT1, AQP7, GK, ATGL, HSL, and perilipin) and the BMI, insulin, and HOMA-IR in all the persons studied, in SAT (Table 2), and in VAT (Table 3). However, another group of genes (ACC1, PEPCK, ACSS2, FABP4, and PDE3) correlated negatively with the BMI, insulin, and HOMA-IR (Tables 2 and 3).
The expression of PPARγ correlated positively in the VAT with the expression of DGAT1 (r = 0.635, p < 0.001), AQP7 (r = 0.636, p < 0.001), GK (r = 0.449, p = 0.005), ACSS2 (r = 0.336, p = 0.039), FABP4 (r = 0.589, p < 0.001), ATGL (r = 0.849, p < 0.001), HSL (r = 0.497, p = 0.002), PDE3 (r = 0.675, p < 0.001), and perilipin (r = 0.819, p < 0.001). The expression of PPARγ correlated positively in the SAT with the expression of DGAT1 (r = 0.504, p < 0.001), AQP7 (r = 0.504, p = 0.001), FABP4 (r = 0.398, p = 0.015), ATGL (r = 0.534, p = 0.001), HSL (r = 0.405, p = 0.013), PDE3 (r = 0.541, p = 0.001), and perilipin (r = 0.535, p = 0.001).
Discussion
Our results show an upregulation of genes involved in facilitating adipocyte lipid storage and lipolysis in morbidly obese patients, though ACC1 and PEPCK were downregulated, especially in the morbidly obese patients with high insulin resistance. They also show that VAT and SAT behave differently in lean controls compared with morbidly obese patients.
PPARs are nuclear receptors that play a key role in the entraining of adipose tissue lipid metabolism to the nutritional state [19]. As we determined in our study, the expression of PPARγ, in both VAT and SAT, was significantly increased in the morbidly obese patients. Additionally, the expression of PPARγ correlated with the expression of most genes involved in the metabolism of fatty acids in adipose tissue. Our results suggest that this favors the storage of fatty acids in adipose tissue in order to ensure the storage and relocalization of the excess triglycerides [19]. However, PPARγ also correlated with the expression of genes involved in lipolysis. Previous studies have shown that PPARγ agonism also stimulates lipolysis by increasing the lipolytic potential, including the gene expression levels, an effect that is compensated by lipid-retaining pathways [11]. Our findings also suggest that increased expression may contribute to the positive action of PPARγ agonism on adipose lipolysis.
The expression of several genes involved in lipogenesis and lipolysis (DGAT1, ACC1, AQP7, and ATGL) was significantly altered in the morbidly obese patients, regardless of the state of insulin resistance. DGAT1 catalyzes the final step of lipogenesis. Transgenic mice overexpressing DGAT1 in adipose tissue have increased body weight and adiposity [20]. Our results show that morbidly obese patients have a greater expression of DGAT1, although with no correlation with PPARγ, as suggested in previous studies [21]. However, transcription factors regulating DGAT1 genes have not been studied in detail.
The expression of ACC1 is reduced in morbidly obese persons. ACC1 is highly expressed in adipose tissue [22] and plays an important role in energy balance by controlling malonyl-CoA synthesis. The ACC1 gene has an inducible expression in response to a variety of factors, including insulin [23]. Although our results show a negative correlation with plasma insulin levels and the HOMA-IR, the relation between ACC1 and insulin resistance is unclear [22, 23], with less extensive studies in adipocytes [24].
Glycerol is a key metabolite in adipose tissue. Under normal conditions, glycerol released from the triglycerides is not recycled intracellularly. AQP7 has been identified as the transporter of glycerol in the adipocytes [25] and is increased in obesity [26]. The increased expression of AQP7 in morbidly obese patients seen in our study may represent a mechanism that supplies the necessary glycerol to the adipose tissue for the increase in triglyceride synthesis. Changes in glycerol transporter expression could also be associated with alterations in the rate of glycerol efflux (as observed during lipolysis). As in other studies [26], our data show a positive association with insulin resistance in the VAT, but not in the SAT. This may agree with studies showing that AQP7 in SAT is not affected in persons with type 2 diabetes [27].
Lipolysis and FFA release from adipose tissue are commonly increased in obesity [14]. As we determined in our study, the expression of both ATGL and HSL is mainly increased in VAT of morbidly obese patients, independently of insulin resistance. However, in SAT, only ATGL is increased. The antilipolytic effect of insulin may vary between normal and impaired in the obese [28]. However, previous studies investigating this issue have produced conflicting results [29]. In states of insulin resistance, insulin cannot exert the antilipolytic effect through stimulation of PDE3. A decrease in PDE3 activity and mRNA seems to be associated with insulin resistance [30]. Our results show that the morbidly obese subjects with high insulin resistance have a lower expression of PDE3 in SAT, the adipose tissue that is more sensitive to the action of insulin. High levels of PDE3 inhibition decrease glucose uptake by adipocytes and may lead to glucose intolerance [31].
As with PDE3 and perilipin, the expression of some of the enzymes studied, such as PEPCK and GK, depends on the state of insulin resistance. These enzymes are involved in the synthesis of glycerol-3-phosphate, key for the synthesis of triglycerides. PEPCK is the enzyme that catalyzes the rate-limiting step in glyceroneogenesis. Glyceroneogenesis is about three- to fourfold more efficient than GK, as previously observed in 3T3-F442A rodent adipocytes [32]. Our results suggest something similar. PEPCK expression was greater than that of GK in the controls, though this was different in the morbidly obese patients. This change depended on the state of insulin resistance in the patients. The reduction in PEPCK was greater in the morbidly obese patients with high insulin resistance. The transcription of PEPCK in adipose tissue is known to be inhibited by insulin [33]. Something similar happens to the VAT ACSS2. The expression of GK in the VAT from the morbidly obese patients with greater insulin resistance, however, was similar to that of the controls. This suggests that these patients have a lower capacity to synthesize glycerol-3-phosphate in VAT and a lower triglyceride storage capacity in this tissue. Recent data suggest that it may not be the absolute amount of fat accumulated but the mismatch between energy surplus and storage capacity that may be an important factor linking obesity, insulin resistance, and associated morbidities [34].
As we see in our study, visceral and subcutaneous adipose tissues are metabolically different [35]. In states of low insulin resistance, as for instance in the controls, the expression of most of the enzymes studied was greater in the SAT. This tissue is more sensitive than VAT to the action of insulin and has high avidity for FFA and triglyceride uptake, preventing their deposition in nonadipose tissue [36]. Even so, the differences between the tissues disappeared almost completely in the morbidly obese patients, in whom we found a significant increase in insulin resistance. In these patients, we noted a relatively increased expression in the VAT as compared with the SAT. Our results may be in agreement with the hypothesis suggesting that the insulin-sensitizing effects of the thiazolidinediones are explained by a selective accumulation of subcutaneous adipose tissue, with concomitant lack of change or reduction in the adiposity of visceral depots [37].
The expression of several lipogenic and lipolytic genes changes simultaneously in VAT and SAT from morbidly obese patients, regardless of the insulin resistance. However, when the expression is only modified in VAT or SAT, the behavior of these tissues is different. VAT from morbidly obese patients shows a significant increase in the expression of other genes involved in lipolysis (GK, HSL, and perilipin). These findings reinforce the notion that VAT has a greater lipolytic activity than SAT [36] and that fewer fatty acids are produced from SAT [28]. Moreover, SAT shows a decrease in the expression of genes involved in de novo lipid synthesis and energy generation (ACSS2 and FABP4). The differences between VAT and SAT may partly be explained by the regional variations in the action of catecholamines and the variations in insulin sensitivity. These actions are markedly altered in the adipose tissue of the obese [38]: A higher catecholamine-induced lipolysis has been observed in VAT than in SAT [28, 38]. This suggests that altered expression of genes might play a role in adipose tissue distribution and expansion. This relative change may also be related with the concept that VAT contributes to the morbidity associated with the metabolic syndrome features. A relatively increased expression in VAT may be associated with an increased metabolic and cardiovascular risk.
This study provides new information regarding adipose tissue physiology in obesity. In the face of increased lipolysis, the net release of FFA into the bloodstream is counteracted by increased reuptake and cycling. In adipocytes, there is constant fatty acid turnover and also enormous enzyme activity to convert large quantities of free fatty acids to neutral lipids. From these results, we can suggest the presence of an association in the upregulation of PPARγ and genes facilitating adipocyte lipid storage in vivo and lipolysis in morbidly obese patients. On the other hand, the expression of enzymes synthesizing de novo fatty acids and glycerol was reduced. The expression of some enzymes studied in the morbidly obese patients depended on the state of insulin resistance. Our results also suggest that the degree of involvement of VAT and SAT in obesity and insulin resistance differs. However, a limitation of this study is that separation of the adipocytes from the stroma vascular fraction was not made. These results all facilitate our understanding of the coordinated regulation of the different genes and serve to enhance our understanding of the molecular mechanisms that underlie the regulation of fatty acid metabolism in obesity and insulin resistance.
References
Wyne KL. Free fatty acids and type 2 diabetes mellitus. Am J Med. 2003;115:29S–36.
Frayn KN. The glucose–fatty acid cycle: a physiological perspective. Biochem Soc Trans. 2003;31:1115–9.
Wang SJ, Cornick C, O'Dowd J, et al. Improved glucose tolerance in acyl CoA:diacylglycerol acyltransferase 1-null mice is dependent on diet. Lipids Health Dis. 2007;6:2.
Wang S, Soni KG, Semache M, et al. Lipolysis and the integrated physiology of lipid energy metabolism. Mol Genet Metab. 2008;95:117–26.
Zimmermann R, Strauss JG, Haemmerle G, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–6.
Sztalryd C, Xu G, Dorward H, et al. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol. 2003;161:1093–103.
Kalderon B, Mayorek N, Berry E, et al. Fatty acid cycling in the fasting rat. Am J Physiol Endocrinol Metab. 2000;279:E221–7.
Arner P. Control of lipolysis and its relevance to development of obesity in man. Diab Metab Rev. 1988;4:507–15.
Picard F, Auwerx J. PPAR(gamma) and glucose homeostasis. Annu Rev Nutr. 2002;22:167–97.
Semple RK, Chatterjee VK, O'Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116:581–9.
Festuccia WT, Laplante M, Berthiaume M, et al. PPARgamma agonism increases rat adipose tissue lipolysis, expression of glyceride lipases, and the response of lipolysis to hormonal control. Diabetologia. 2006;49:2427–36.
Zieleniak A, Wójcik M, Woźniak LA. Structure and physiological functions of the human peroxisome proliferator-activated receptor gamma. Arch Immunol Ther Exp (Warsz). 2008;56:331–45.
García-Fuentes E, García-Almeida JM, García-Arnés J, et al. Morbidly obese individuals with impaired fasting glucose have a specific pattern of insulin secretion and sensitivity: effect of weight loss after bariatric surgery. Obes Surg. 2006;16:1179–88.
Soriguer F, García-Serrano S, García-Almeida JM, et al. Changes in the serum composition of free-fatty acids during an intravenous glucose tolerance test. Obesity (Silver Spring). 2009;17:10–5.
Garrido-Sánchez L, García-Almeida JM, García-Serrano S, et al. Improved carbohydrate metabolism after bariatric surgery raises antioxidized LDL antibody levels in morbidly obese patients. Diab Care. 2008;31:2258–64.
Kintscher U, Law RE. PPARgamma-mediated insulin sensitization: the importance of fat versus muscle. Am J Physiol Endocrinol Metab. 2005;288:E287–91.
Garcia-Fuentes E, Murri M, Garrido-Sanchez L, et al. PPARγ expression after a high-fat meal is associated with plasma superoxide dismutase activity in morbidly obese persons. Obesity (Silver Spring). 2010;18:952–8.
Macias-Gonzalez M, Moreno-Santos M, Garcia-Almeida JM, et al. PPARγ2 protects against obesity by means of a mechanism that mediates insulin resistance. Eur J Clin Invest. 2009;39:972–9.
Medina-Gomez G, Gray SL, Yetukuri L, et al. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3:e64.
Chen HC, Stone SJ, Zhou P, et al. Dissociation of obesity and impaired glucose disposal in mice overexpressing acyl coenzyme a:diacylglycerol acyltransferase 1 in white adipose tissue. Diabetes. 2002;51:3189–95.
Yen CL, Stone SJ, Koliwad S, et al. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283–301.
Zang Y, Wang T, Xie W, et al. Regulation of acetyl CoA carboxylase and carnitine palmitoyl transferase-1 in rat adipocytes. Obes Res. 2005;13:1530–9.
Brownsey RW, Boone AN, Elliott JE, et al. Regulation of acetyl-CoA carboxylase. Biochem Soc Trans. 2006;34:223–7.
Ortega FJ, Mayas D, Moreno-Navarrete JM, et al. The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjects. Obesity (Silver Spring). 2010;18:13–20.
Kishida K, Kuriyama H, Funahashi T, et al. Aquaporin adipose, a putative glycerol channel in adipocytes. J Biol Chem. 2000;275:20896–902.
Catalán V, Gómez-Ambrosi J, Pastor C, et al. Influence of morbid obesity and insulin resistance on gene expression levels of AQP7 in visceral adipose tissue and AQP9 in liver. Obes Surg. 2008;18:695–701.
Ceperuelo-Mallafré V, Miranda M, Chacón MR, et al. Adipose tissue expression of the glycerol channel aquaporin-7 gene is altered in severe obesity but not in type 2 diabetes. J Clin Endocrinol Metab. 2007;92:3640–5.
Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005;19:471–82.
Mairal A, Langin D, Arner P, et al. Human adipose triglyceride lipase (PNPLA2) is not regulated by obesity and exhibits low in vitro triglyceride hydrolase activity. Diabetologia. 2006;49:1629–36.
Tang Y, Osawa H, Onuma H, et al. Improvement in insulin resistance and the restoration of reduced phosphodiesterase 3B gene expression by pioglitazone in adipose tissue of obese diabetic KKAy mice. Diabetes. 1999;48:1830–5.
Flechtner-Mors M, Jenkinson CP, Alt A, et al. Studies of phosphodiesterase effects on adipose tissue metabolism in obese subjects by the microdialysis technique. J Physiol Pharmacol. 2005;56:355–68.
Tordjman J, Chauvet G, Quette J, et al. Thiazolidinediones block fatty acid release by inducing glyceroneogenesis in fat cells. J Biol Chem. 2003;278:18785–90.
Sasaki K, Cripe TP, Koch SR, et al. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem. 1984;259:15242–51.
Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–62.
Misra A, Vikram NK. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition. 2003;19:457–66.
Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2009;11:11–8.
Larsen TM, Toubro S, Astrup A. PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? Int J Obes Relat Metab Disord. 2003;27:147–61.
Large V, Arner P. Regulation of lipolysis in humans. Pathophysiological modulation in obesity, diabetes, and hyperlipidaemia. Diab Metab. 1998;24:409–18.
Acknowledgments
We thank Ian Johnstone for help with the English language version of the text. We thank Juan Alcaide for the technical assistance. This work was supported in part by grants from the Instituto de Salud Carlos III (CP04/00133, CP04/0039, PS09/01060, PS09/00997), Programa Juan de la Cierva (JCI-2009-04086), and Servicio Andaluz de Salud (PI 0255/2007 and 0438/2006). CIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN) and CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM) are ISCIII projects.
Conflict of interest disclosure
None of the authors has anything to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
García-Fuentes E and Garrido-Sanchez L contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tinahones, F.J., Garrido-Sanchez, L., Miranda, M. et al. Obesity and Insulin Resistance-Related Changes in the Expression of Lipogenic and Lipolytic Genes in Morbidly Obese Subjects. OBES SURG 20, 1559–1567 (2010). https://doi.org/10.1007/s11695-010-0194-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-010-0194-z