Abstract
Background
Gastric electrical stimulation synchronized to the refractory period of gastric electrical activity and applied during meals was evaluated for safety and for improvement of body weight and glycemic control in obese type 2 diabetes.
Methods
The study involved obese diabetic type 2 (ODM) patients in a multicenter open-label European feasibility trial. A total of 24 ODM (nine males, 15 females) treated with insulin and/or oral hyperglycemic agents and body mass index between 33.3 to 49.7 kg/m2 were implanted laparoscopically with a TANTALUS system.
Results
There were 18 adverse events related to the implant procedure or the device reported in 12 subjects. All were short lived and resolved with no sequelae. In the 21 subjects that reached the 1-year visit weight was reduced by 4.5 ± 2.7 kg (p < 0.05) and HbA1c by 0.5 ± 0.3% (p < 0.05). In a subgroup (n = 11) on stable or reduced oral medication, weight was reduced by 6.3 ± 3.4 kg (p < 0.05) and HbA1c by 0.9 ± 0.4% (p < 0.05). The group on insulin (n = 6) had no significant changes in weight and HbA1c.
Conclusions
The TANTALUS system is well tolerated in obese type 2 diabetic subjects. Gastric electrical stimulation can potentially improve glucose metabolism and induce weight loss in obese diabetic patients, who are not well controlled on oral antidiabetic therapy. Further evaluation is required to determine whether this effect is due to induced weight loss and/or to direct signal dependent mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes and obesity affect almost 200 million individuals worldwide and constitute a global epidemic which is spreading in increasing numbers even in the young ones. The impact on patients’ lives and the global economy are large and grow at an alarming rate [1, 2]. Treatment with oral antidiabetic drugs and insulin has been proven beneficial regarding morbidity and mortality. Several drugs, however, are characterized by limited efficacy and side effects, especially in obese diabetic patients. Therefore, the potential of surgical bariatric procedures, which sometimes even lead to complete remission [3, 4], is increasingly acknowledged.
Gastric electrical stimulation may present a novel approach for improving glycemic control for those obese diabetic patients, who are unwilling to undergo restrictive and anatomically irreversible bariatric procedures or those who do not meet the required level for obesity to justify these procedures. The TANTALUS system was tested in a small group of morbid obese patients in an open-label clinical protocol [5]. We reported an excess weight loss of 27% in 1 year. The treatment is intended to enhance gastric contractility, while maintaining natural gastric rhythmic contractions, by delivering electric signals in synchrony with sensed spontaneous smooth muscle electrical activity. These signals are thus referred to as gastric contractility modulation (GCM) signals. Moreover, the signals are delivered upon detection of the onset of food intake which may avoid development of tolerance that can occur with continual signal delivery. The proposed mechanism of weight loss is that increased gastric contractility affects mechanoreceptor discharges propagated by gastric nerves. This results in an increased afferent neural traffic to specific areas of the brain involved with the sensation of satiety. Preclinical studies have confirmed increased vagal afferent signaling secondary to enhanced gastric contractions upon application of GCM to the rat stomach [6]. Moreover, it was observed that antrum contractility is maximal at the end of a meal in dogs [7]. The hypothesis was further supported by patients’ reports of decreased hunger and increased cognitive control assessed with validated questionnaires [5]. In addition, multiple efferent signals active only during the digestive period may be reached by secondary effects in enteric nerves. Recent evidence shows that other underlying mechanisms of the metabolic syndrome and cardiovascular risk such as hepatic glucose production and inflammation can be regulated by hypothalamic vagal fiber efferent discharges [8, 9]. Thus, central modulation of vagal might affect inflammation and glucose homeostasis.
In preliminary clinical studies [10], it has been shown that metabolic parameters such as HbA1c, fasting blood glucose (FBG), and the lipid profile improved in a subgroup of diabetic patients enrolled with morbid obese patients. This has led us to the hypothesis that the same type of electrical treatment paradigm used in treating obesity may also improve glycemic control, perhaps beyond that achieved by weight loss. Therefore, the aim of this feasibility study was to evaluate the safety and functionality of the TANTALUS system in obese type 2 diabetic subjects on a variety of different diabetes treatments.
Research Design and Methods
This was a nonrandomized, open-label, safety, and feasibility study involving 24 subjects from two centers. The study included subjects aged 18 to 60 years with type II diabetes mellitus, HbA1c range was 6.5% to 9.7%, and body mass index (BMI) ranged 33.3 to 49.5 kg/m2. Obesity was present for five or more years. Out of the 24 initially implanted patients, three withdrew from the study before the 1-year visit (one lost to follow-up by week 13, two patients did not want to undergo a device replacement at weeks 28 and 32, respectively). Of the remaining 21 subjects, six were on adjustable doses of either basal, prandial insulin, or a combination of both (“insulin group”) and the rest were on oral agents. Regarding the analysis, one patient had increased the medication dose after 8 months and three were included with Hba1c <7%. In the remaining 11 subjects, medication remained unchanged or was reduced during the study period. Medications consisted of metformin alone (n = 3) or in combination with sulfonylureas (n = 5), rosiglitazone (n = 1), pioglitazone and glucosidase inhibitors (n = 1), or sulfonylureas alone (n = 1). This subset of patients was termed “oral group” for post hoc analysis of the effects of the system on glycemic control and changes in body weight. Table 1 shows the baseline characteristics of the patient population. Medications were lowered by 25% in two patients, after 6 and 8 months, due to marked HbA1c and FBG improvement.
Inclusion/Exclusion Criteria
Subjects were excluded if they had taken medications for weight loss or gastric motility within the last 3 months, if they had severe eating or motility disorders, prior bariatric surgery, and any other significant medical or psychiatric condition that may have impaired their ability to comply with the study procedures. The study was approved by the local ethics committee of each participating center and all patients provided informed consent prior to any protocol-related evaluations.
Study Design
Patients were evaluated during 4 weeks for weight stability (defined as a variation by less than 1 BMI unit). Blood was obtained for fasting glucose levels and HbA1c. Subjects completed a Three-Factor Eating Questionnaire (TFEQ). The implant date (week 0) was followed by a 6-week recovery and monitoring period, during which subjects were seen weekly and device parameters were adjusted for eating detection and GCM delivery. Subjects received information about the caloric content of foods and general advice about a healthy diet, although were not under a specific diet or behavioral control. The device was activated on week 6. Patients were followed weekly for the first month, biweekly for the next 3 months, and then monthly through the end of the study. Follow-up evaluations of baseline tests (weight, fasting glucose levels, hemoglobin A1c, and TFEQ) were performed at specific visits as detailed in “Results” section. Clinical follow-ups carefully assessed the presence of adverse events. Blood was collected by venipuncture for laboratory assessments of fasting blood glucose (hexokinase enzymatic method) [11] and HbA1c (ion-exchange high-performance liquid chromatography method) [12].
Implant Procedure
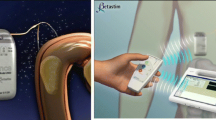
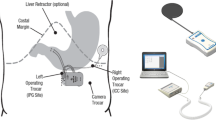
The implantation of the TANTALUS system has been described previously [5]. Briefly, three bipolar leads (TIZER, MetaCure Ltd.) were laparoscopically placed in the subserosa of the gastric wall. One lead was placed in the fundus to detect gastric distension, and the other two leads were placed in the antrum for slow wave detection and signal delivery. The device was placed in a subcutaneous pocket in the left side of the abdomen inside a Dacron pouch.
Eating Behavior
The TFEQ was used to assess the effect of the TANTALUS system on eating behavior [13]
Post Hoc Analysis of Effects of TANTALUS Treatment
Results of data analysis are described for the 21 patients who finished the study and for two subgroups, the insulin group (n = 6) and for the subset on stable or reduced oral medication or “oral group” (n = 11). Statistical analysis focuses on HbA1c and weight at 20 and 52 weeks.
Additional Tests
Ghrelin and adiponectin were sampled at baseline and at week 37 of the study. Blood samples for ghrelin measurements were collected in fasting patients in vacutainers with EDTA at the same time and under the same conditions as for adiponectin. Ghrelin was measured using a radioimmunoassay kit (Peninsula Laboratories, Inc., San Carlos, CA, USA). Likewise, adiponectin was measured using a kit by LINCO Research (St. Charles, MO, USA).
Statistical Analysis
Statistical analysis was performed using paired t test (two-sample test assuming equal variances) and linear regression analysis by using the “least squares” method. A p value ≤0.05 was considered significant. Data are presented as mean ± standard error of the mean.
Results
Safety
The mean duration of implantation procedures was 2:08 ± 0:07 h and the hospital stay was 2.3 ± 0.1 days. Adverse events occurring after implantation followed a pattern of symptoms often seen in laparoscopic procedures such as shoulder pain [14]. Table 2 describes the adverse events that were deemed related to the procedure or to the device for all patients (n = 24). These are distributed according to the study period. There were a total of 29 adverse events related to either the surgical procedure or possibly associated with the device reported in 15 patients. There were nine nonsevere adverse events associated with the implantation procedure. During treatment and depending on signal amplitude and eating detection, depletion of the device battery occurred in most patients. Device battery lasted an average of 8 ± 0.6 months (range 4.5 to 18.7 months). This led to device replacements in 17 patients, increasing the chances for device–pocket-related adverse events due to reopening of the pocket scar. Secondary procedures were performed in the outpatient clinic under local or general anesthesia. These, however, resulted in twelve additional procedure-related adverse events, four of them requiring further intervention. The Dacron pouch fixed to the pocket inner wall originated a second cause for adverse events during explant (see Table 2). During the study period, there were two hypoglycemic events (one in an insulin-treated patient and one in an oral patient), and one event of itching around the device occurring 4 months after implantation.
The type and frequency of adverse events on this population was compared with that of a previous study with the same system done previously in nondiabetic obese [5]. We found a very similar profile of postoperative events including abdominal and device implant site pain, bloating, left shoulder pain, and discomfort in that group, which was as well, associated with laparoscopic surgery. No further changes in gastrointestinal function were observed in both studies.
Effect of TANTALUS Treatment on Weight and Glycemic Control
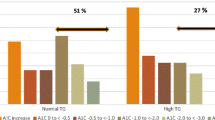
Figs. 1 and 2 present the effect of TANTALUS treatment on weight and HbA1c and the linear regression of the changes after 1 year for all subjects and for the group in stable or reduced oral medication and basal HbA1c between 7.5% and 9.5%, respectively. The treatment resulted in a significant weight loss for the entire group (n = 21) at week 20 (−5.8 ± 1.4 kg or −4.7% change, p < 0.05), a reduction that remained largely stable by week 52 (−4.5 ± 2.7 kg or −3.7% change, p < 0.05). Fig. 2 includes in the analysis only the patients with stable oral medication. This group (n = 11) shows considerable improvement, as most of the patients that experienced significant weight loss were on oral medication. In this group, the mean body weight decreased by −7.39 ± 1.59 kg or −5.6% of the initial body weight (p < 0.05) by week 20 and reached −6.25 ± 3.42 kg, or a reduction of 4.7% in weight (p < 0.05) by week 52. Patients on insulin (n = 6) did not show significant changes in weight.
Effect in body weight and glycemic control on all subjects. Body weight (black) and HbA1c (gray) after 20 and 52 weeks with the TANTALUS resulted in significant weight loss and HbA1c (upper panel). Correlation analysis (lower panel) shows a weak though statistically significant relation between weight and HbA1c. *p < 0.05 compared to baseline
Effect in body weight and glycemic control on oral subjects. A subgroup of patients on stable oral medication and basal HbA1c between 7.5% and 9.5% exhibited comparatively more improvement in weight (black) and glycemic control (grey, upper panel). *p < 0.05 compared to baseline. Correlation between changes in body weight and HbA1c (lower panel) show a greater effect of weight on HbA1c changes
Baseline HbA1c for the entire group (n = 21) decreased −0.6 ± 0.2% by week 20 (p < 0.05) and remained in −0.5 ± 0.2 % by week 52 (p < 0.05). For the oral group, HbA1c decreased 1.5 ± 0.2% (p < 0.05) after 14 weeks of treatment. By the year 1 visit, HbA1c increased slightly, although it remained significantly lower than at baseline (−0.9 ± 0.3%, p < 0.05). Six patients in this group experienced reductions of HbA1c of ≥1%, two returned to baseline values, and three showed increases in HbA1c. Overall, the insulin group showed no changes in HbA1c. In two patients, daily medication of metformin was reduced by 25% with no negative consequences.
The contribution of the weight change in the reduction of HbA1c was assessed by linear regression between the baseline and 1-year values. Whereas the correlation between changes in weight and in HbA1c is significant for the entire group, with weight loss explaining 23.5% (p < 0.05) of the HbA1c change, the weight loss explained 42.2% of the HbA1c change (p < 0.05) in the oral group.
Fasting Blood Glucose
When all patients are accounted for, FBG decreased from 183 ± 13 to 146 ± 10 mg/dL (p < 0.05) at week 20 and 148 ± 8 mg/dL (p < 0.05) by week 52. The mean fasting blood glucose followed previous trends on the oral group. FBG decreased from 187 ± 20 mg/dL, at baseline to 140 ± 12 mg/dL (p < 0.05) on week 20, and was 147 ± 13 mg/dL (p < 0.05) at week 52 (see Fig. 3). The insulin group showed a slight nonsignificant decrease from 175 ± 20 to 156 ± 21 mg/dL by week 20 reaching 145 ± 10 mg/dL by week 52 (n = 6).
Eating Behavior
TFEQ was assessed for the oral group, except one patient who did not speak German (n = 10). Improvements were observed in all the three dimensions of eating behavior (see Fig. 4). Cognitive control increased from 11.2 ± 1.7 at baseline (week 0) to 14.0 ± 1.2 (week 20, p < 0.05) and remained at that level by week 52 reaching 13.7 ± 1.3 (p < 0.05). Disinhibition, starting at 9.8 ± 1.3, decreased to 5.9 ± 1 (p < 0.05) by week 20 and remained stable throughout the study. Hunger was reduced from 6.5 ± 1.4 at baseline to 3.8 ± 1.2 (p < 0.05) by week 20 and reached 4.3 ± 1.5 (p < 0.05) on week 52. The slight increase (p = N.S.) in hunger from week 20 to week 52 follows trends observed for HbA1c and weight during that period. It appears that the three dimensions of eating behavior assessed by the test reveal the same pattern as improved glycemia and weight that was overall maintained at the 52-week visit (see Fig. 4).
Additional Tests
Complete sets of data for ghrelin and adiponectin were obtained for eight patients from the oral group. Measurement of ghrelin showed a significant decrease in the amount of circulating hormone during fasting, from 384 ± 84 to 232 ± 22 pg/mL (p < 0.05). Similarly, after 31 weeks of treatment, circulating adiponectin showed significant increases from 10.2 ± 2.6 to 12.1 ± 2.4 μg/mL (p < 0.05)
Blood lipids high- and low-density lipoproteins or their ratio did not show significant changes between baseline and the 1-year visit. Triglycerides, however, were significantly reduced in the oral group from 185 ± 23 to 146 ± 9 mg/dL (p < 0.05).
Discussion
This study demonstrates the safety and efficacy of the TANTALUS system in the treatment of obese diabetic patients over a period of 1 year. The TANTALUS systems induced favorable changes in eating behavior, which were accompanied by weight loss and improvement in glycemic control and triglyceride levels as well.
Due to its efficacy regarding glucose control, bariatric surgery is increasingly considered for the treatment of morbid obese subjects with type 2 diabetes [3, 4]. Its application, however, is limited due to its invasive nature, especially with malabsorptive procedures. The therapeutic potential of electrical stimulation follows a growing literature addressing this approach for a variety of clinical disorders [15, 16]. In particular, eating detection as used with the TANTALUS system as the basic trigger for gastric stimulation has been proven responsible for the observed effects seen with this technique [17]. Implantation of the TANTALUS system via laparoscopy requires general anesthesia; the procedure, however, does not alter gastric anatomy or absorption of nutrients unlike the commonly applied gastric bypass operation. We have recently reported beneficial results on the safety and efficacy of the TANTALUS system in nondiabetic obese subjects [5]. This study over 1 year in diabetic patients on a various antidiabetic medications including insulin confirms the high degree of safety also reported for nondiabetic patients [5] in our previous study. Adverse events following the system implantation were mild and are commonly seen in abdominal laparoscopic procedures. Especially those associated with reopening of the pocket scar in order to change the battery will be avoided in the future due to the recent availability of externally rechargeable devices. In order to postpone the battery change in some subjects, the length and intensity of the electrical stimulation was reduced. This might have led to suboptimal treatment in these patients which could lead to an underestimation of the efficacy of the TANTALUS system.
Several mechanisms could be operative to induce beneficial effects via enteric nervous system and its vagal connections. GCM signals could influence gut organs and tissue functions by modulating both afferent and efferent signaling leading to an early satiety [18] induced by enhancing vagal afferent signaling to specific centers in the brain [19]. This is supported by the observation that GCM signals increase vagal afferent signaling in animals [6]. Moreover, these central effects may be associated with entero-enteric signals secondary to changes in the gastric emptying. In one study with TANTALUS in obese patients, gastric emptying was significantly accelerated [10]. Apparent beneficial effects on glycemic control could be propagated by gastric neurohormonal mechanisms that mediate changes in metabolism in remote tissues [20, 21]. Both concepts involve neurohumoral mechanisms that interconnect the brain and various parts of the gut. This is in line with reports showing that cells within the gastric wall respond to mechanical and chemical properties of ingested nutrients. The response involves the production and secretion of hormones, whose chemical and electrical cascades can be transmitted through vagal afferents [22]. These signals may be integrated within the CNS and can manifest as behavioral modifications related to food intake and as well as help reduce the actual or perceived range of meal-induced blood glucose excursions. Moreover, it is possible that enteric and parasympathetic nerves may induce responses on other gut segments. Recent evidence shows that electrical signals given at one point in the gut may affect mechanical properties to induce secretion of lumen enzymes in the colon on remote gut segments [23]. Thus, these results support the concept of indirect and immediate beneficial effects by propagation of electrical impulses from the site of stimulation into the enteric nerves and entero- and neuroendocrine cells as well.
Regarding the effects of the TANTALUS system on body weight and metabolic parameters, it has to be kept in mind that this study was designed to assess safety and functionality as primary parameter. Thus, some of the patients, especially those on insulin treatment, adjusted the doses of their medication thereby limiting the estimation of the specific effect of the TANTALUS system on the respective parameters. Analyzing the entire group, we could find a significant reduction in HbA1c from baseline after 14 weeks of treatment. The efficacy was considerably greater in subjects on stable oral medication. The mechanisms underlying this reduction could include nonspecific effects, such as the implant procedure itself, perceived or reduced stress, or intensified consultations. However, reduction of HbA1c and fasting blood glucose values were largely maintained throughout the 52-week study period, which speaks against these nonspecific effects. The small nonsignificant loss of efficacy between weeks 20 and 52 can be attributed to loss of placebo effects and a reduction in therapy parameters programmed approximately after 6 months to reduce the frequency of device replacements. Weight loss was similarly maintained throughout the study. As responsible mechanisms for these effects, beneficial changes in eating behavior and a reduction in ghrelin levels could be identified. However, regression analysis between changes in weight with changes in HbA1c revealed that only 23.5% of weight loss in the entire group and 42% in patients on stable oral medication could explain the improvement in glycemic control. Therefore, treatment with the TANTALUS may involve partially unknown mechanism such as changes in vagal nerve stimulation or improvement of insulin sensitivity and inflammatory status by an increase in adiponectin as observed in our study. These explanations, however, must remain speculative unless results of mechanistic studies are available. Likewise, a randomized trial involving a patient group with nonactivated device is warranted to finally assess the efficacy of the TANTALUS system for obese diabetic patients.
In conclusion, the delivery of nonexcitatory GCM signals was well tolerated, showed a safe 1-year profile, and appeared to be suited for long-term therapeutic treatment of type 2 diabetes. The impact on weight loss and HbA1c in the subset of patients on stable or reduced oral medication encourages further studies treatment for type II diabetes. Overall, GCM treatment may induce behavioral modifications of intake through effects on the neural, hormonal and cellular functions of the gastrointestinal system. Along with further clinical investigations, studies aimed at understanding the mechanisms underlying these effects of GCM signals will be important.
Abbreviations
- EWL:
-
Excess weight loss
- TFEQ:
-
Three-Factor Eating Questionnaire
- BMI:
-
Body mass index
- GCM:
-
Gastric contractility modulation
- PP:
-
Postprandrial
- BT:
-
Bed time
- GE:
-
Gastric emptying
- FBG:
-
Fasting blood glucose
- BL:
-
Baseline
References
Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–50.
Kopelman PG, Hitman GA. Diabetes: exploding type II. Lancet. 1998;352(Suppl. 4):5.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37.
Moo TA, Rubino F. Gastrointestinal surgery as treatment for type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2008;2:153–8.
Bohdjalian A, Prager G, Aviv R, et al. One-year experience with Tantalus: a new surgical approach to treat morbid obesity. Obes Surg. 2006;5:627–34.
Peles S, Petersen J, Aviv R, et al. Enhancement of antral contractions and vagal afferent signaling with synchronized electrical stimulation. Am J Physiol Gastrointest Liver Physiol. 2003;285:G577–85.
Sanmiguel CP, Aviv R, Policker S, et al. Association between gastric electromechanical activity and satiation in dogs. Obesity. 2007;15(12):2958–63.
Pocai A, Lam TK, Gutierrez-Juarez R, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–31.
Libert C. Inflammation: a nervous connection. Nature. 2003;421:328–9.
Sanmiguel CP, Haddad W, Aviv R, et al. The TANTALUS trade mark system for obesity: effect on gastric emptying of solids and ghrelin plasma levels. Obes Surg. 2007;17:1503–9.
Neeley WE. Simply automated determination of serum or plasma glucose by a hexokinase/glucose-6-phosphate dehydrogenase method. Clin Chem. 1972;18:509–15.
Dunn PJ, Cole RA, Soeldner JS. Further development and automation of a high pressure liquid chromatography method for the determination of glycosylated hemoglobins. Metabolism. 1979;28:777–9.
Albert J, Stunkard AJ, Samuel M. The three factors eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83.
Lundberg GD. How to reduce laparoscopy-induced shoulder pain. Medscape J Med. 2008;10:173.
De Vries J, De Jongste MJ, Spincemaille G, Staal MJ. Spinal cord stimulation for ischemic heart disease and peripheral vascular disease. Adv Tech Stand Neurosurg. 2007;32:63–89.
Zhang J, Chen JD. Pacing the gut in motility disorders. Curr Treat Options Gastroenterol. 2006;9:351–60.
Policker S, Lu H, Haddad W, et al. Electrical stimulation of the gut for the treatment of type 2 diabetes: effect of automatic eating detection. J Diabetes Sci Technol. 2008;2:904–12.
Dockray GJ. The brain-gut axis. In: Yamada T, editor. Basic mechanisms of normal and abnormal gastrointestinal function. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2003. p. 77–91.
Sobocki J, Thor PJ, Uson J, et al. Microchip vagal pacing reduces food intake and body mass. Hepatogastroenterology. 2001;48:1783–7.
Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140:1687–94.
Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol. 2002;6:650–6.
Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–91.
Liu S, Lei Y, Chen JD. Inhibitory effects and mechanisms of colonic electric stimulation on gastric and rectal tone in conscious dogs. Dis Colon Rectum. 2006;49:1749–54.
Acknowledgments
This work was supported by a research grant from MetaCure (USA) Inc, Orangeburg, NY. TANTALUS™ system is a registered trademark of MetaCure NV, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bohdjalian, A., Prager, G., Rosak, C. et al. Improvement in Glycemic Control in Morbidly Obese Type 2 Diabetic Subjects by Gastric Stimulation. OBES SURG 19, 1221–1227 (2009). https://doi.org/10.1007/s11695-009-9901-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-009-9901-z