Abstract
Background
This study was performed to assess postoperative nausea and vomiting (PONV) with application of postoperative continuous positive airway pressure (CPAP) for patients undergoing Roux-en-Y gastric bypass (RYGB).
Methods
The anesthesia database was searched for patients who underwent RYGB for 5 years. Three hundred fifty-six patients met the inclusive criteria. Wilcoxon two-sample rank test, Fisher’s exact test, and multivariate logistic regression were used to analyze the data and identify the potential factors. A p value less than 0.05 was considered significant.
Results
The overall incidence of the PONV (nausea or emesis or both) was 42%during the first 24 h postoperatively. Thirty-six percent and 35% in CPAP and no-CPAP groups respectively had reported nausea in postanesthesia care unit (PACU). There was no difference between groups (p > 0.05). There was a less frequent occurrence of emesis in both groups. The incidence of emesis in PACU was 19% in CPAP group and 17% in no-CPAP group (p > 0.05). No statistically significant differences of PONV in postoperative 24 h could be shown between the groups (p > 0.05). The postoperative hypertension occurred more often and intravenous antihypertensive medications were required more in no-CPAP patients (p = 0.013). More patients in no-CPAP group developed oxygenation disturbances (p = 0.012).The mean length of PACU stay was significantly longer in this group (p = 0.029). Reintubation and intensive care unit admission occurred more frequently in no-CPAP patients; however, the difference did not reach statistical significance.
Conclusions
There was no significantly increased risk of PONV with the use of postoperative CPAP. We recommend the routine use of postoperative CPAP for patients with obstructive sleep apnea undergoing RYGB to optimize their respiratory function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is probably the most important risk factor for obstructive sleep apnea (OSA). Several studies have shown an association between increase in body mass index (BMI) and the risk of OSA [1]. Significant OSA is present in 40% of obese individuals and 70% of OSA patients are obese [2]. The incidence of obesity is increasing in the US, resulting in the performance of a greater number of bariatric operations. Roux-en-Y gastric bypass (RYGB) is commonly performed for the treatment of clinically severe obesity [3].
Morbidly obese patients are greatly at risk from postoperative respiratory insufficiency [4]. The combination of preexisting OSA and abdominal surgery significantly increases morbidity and mortality from respiratory complications in obese patients [5]. Continuous positive airway pressure (CPAP) is currently the most effective medical treatment for OSA [6]. CPAP improves respiratory function in morbidly obese patients and accelerates reestablishment of preoperative pulmonary function [7, 8]. Despite the theoretical risk of anastomotic injury from pressurized air delivered by CPAP, the use of CPAP has been increasingly accepted for patients with OSA following upper gastrointestinal surgery because some studies have confirmed that routine use of CPAP after gastric bypass does not convey added risk of anastomotic disruption to patients with significant OSA [9, 10].
The application of CPAP causes pressurized air to inflate the stomach and intestine, which may result in an increased risk of postoperative nausea and vomiting (PONV), potentially aspiration. This study was undertaken to assess the risk of PONV with application of postoperative CPAP for patients undergoing laparoscopic gastric bypass. We were also interested in noting the pattern of using of CPAP and its impact on the postoperative outcome.
Methods
With Institutional Review Board approval, the anesthesia database was searched for patients who underwent laparoscopic RYGB for 5 years, from January 2001 through December 2005. The search was limited to patients aged 18 to 65 years and the American Society of Anesthesiology physical status I to III. Patients with more than one abdominal surgery during the same admission, preexisting nausea or vomiting, history of motion sickness, and history of PONV were excluded. A retrospective chart review was then undertaken. The medical history, the anesthesia records, the recovery room records, the nursing records, the information of preoperative and postoperative CPAP utilization, and the medication administration records for the first 24 postoperative hours were reviewed and a case report form was filled out for each patient by two trained graduate students. Although the reviewers were aware that the study was about CPAP, they were not informed of the objective of this study.
The following patient data were collected: demographics, comorbidities, and presence of PONV in recovery room and in the postoperative 24 h. Anesthetic data collected included total intraoperative propofol dose, choice of inhalation agents, administration of neuromuscular blocking and reversal agents, total intraoperative narcotic dose, preoperative and intraoperative antiemetic administration, anesthesia time, postoperative opioid and antiemetic administration, and postoperative course. We focused particularly on recording the details of the diagnosis of OSA, preoperative and postoperative CPAP utilization, the type of postoperative complications, and the reason for intensive care unit transfers.
Nausea was considered present only if noted as such in the nursing records. Emesis was recorded if retching or vomitus were noted by nursing staff. Antiemetic administration alone was not taken to indicate PONV. No attempt was made to grade nausea or emesis.
Postoperative incidence rates of nausea and vomiting were calculated from the data.
We used binomial logistic regression to identify which covariates (gender, BMI, choice of inhalation agents, total intraoperative narcotic dose, preoperative and intraoperative antiemetic administration, preoperative and postoperative CPAP utilization, settings of CPAP, and postoperative narcotic dose) were associated with PONV. The variables “male,” “sevoflurane,” “no postoperative opioid administration,” and “no postoperative CPAP utilization” were used as the referent category.
Univariate regressions were run first, using one covariate at a time. Then, covariates that were significant predictors at a p < 0.15 level were considered together in the multivariate regression model.
Data were analyzed using a software package (SAS; Cary, NC). Wilcoxon two-sample rank test, Fisher’s exact test, and multivariate logistic regression were used to analyze the data. For all tests, a p value < 0.05 was considered significant.
Results
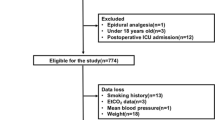
Three hundred fifty-six patients who underwent RYGB met the inclusion criteria. Table 1 summarized the patient demographic information. There was a predominance of females in the study. There were 281 (79%) women and 76 (21%) men. The male patients had higher BMI (p < 0.05). Of the 356 patients undergoing RYGB, 146 had OSA confirmed by polysomnography. All of the 102 patients who were CPAP dependent received postoperative CPAP using their preoperative settings (range 10 to 15 cm H2O at a rate of 12 to 15 cycles per minute).Use of CPAP was significantly more frequent in men than in women (p < 0.05).
There was no difference in intraoperative data of whether CPAP was used preoperatively during surgery (Table 2). The anesthetic times, anesthetic agents, propofol dose, and fentanyl dose did not differ. The antiemetics were administered intraoperatively to 353 patients (99%) for prophylaxis.
The overall incidence of the PONV (nausea or emesis or both) was 42% during the first 24 h postoperatively. The postoperative data are shown in Table 3. Of 102 patients, 37 (36%) and 89 of 254 patients (35%) in CPAP and no-CPAP groups, respectively, had reported nausea in PACU. There was no difference between groups (p > 0.05). There was a less frequent occurrence of emesis in both groups. The incidence of emesis in PACU was 19% (19/102) in CPAP group and 175% (42/254) in no-CPAP group (p > 0.05). No statistically significant differences of PONV in postoperative 24 h could be shown between the groups (p > 0.05).
The postoperative hypertension occurred more often and intravenous antihypertensive medications were required more in no-CPAP patients (p = 0.013). More patients in the no-CPAP group developed oxygenation disturbances (p = 0.012). The mean length of PACU stay was significantly longer in this group (211 ± 82 versus 159 ± 78 min, p = 0.029). Reintubation and intensive care unit admission occurred more frequently in no-CPAP patients; however, the difference did not reach statistical significance.
Factors identified for inclusion in multivariate regression model were gender, smoking history, and age. The multivariate regression model showed female gender (odds ration (OR) = 1.6, p < 0.001), age ≤ 39 years (OR = 1.4, p < 0.04), and nonsmoking (OR = 1.8 p < 0.001) as significant risk factors for PONV (Table 4).
Discussion
The incidence of OSA among morbidly obese patients is very high [6]. In our study, 146 (41%) patients were documented with OSA, of whom 102 patients were CPAP dependent. Gupta et al. reported a significantly higher percentage of patients with OSA who suffered serious complications or needed unplanned intensive care unit transfers compared with controlled patients. These complication included acute hypercapnia, with a PaCO2 > 45 mmHg; episodic desaturation reflected by SpO2 < 90% with by witnessed apneic events; arrhythmias; myocardial ischemia or infarction; and delirium The incidence of serious complications was higher in patients with OSA who were not using CPAP at home compared with OSA patients who were using CPAP at home [11]. Aside from OSA, obese patients have an increased risk for postoperative pulmonary complications such as sputum retention, atelectasis, and bronchopulmonary infection [12].The most effective and widely used treatment for OSA is the CPAP [13, 14]. CPAP treats apnea–hypopnea episodes by providing air under positive pressure through a nasal or facial mask, thus creating a pneumatic splint in the pharynx, which prevents collapse of the pharyngeal airway [13].
There are many side effects associated with CPAP treatment. This technique prevents airway collapse by providing high pressure during inspiration and low pressure during expiration. Aerophagia and bloating may occur [14]. The application of CPAP causes pressurized air to inflate the stomach and intestine, which may result in an increased risk of PONV. To our knowledge, the risk of PONV to the use of postoperative CPAP has not been cited previously. This study allowed us to evaluate this risk. Despite concerns that positive pressure ventilation may result in an increased risk of PONV, we did not identify a relationship between the use of CPAP and PONV. A possible limitation in our study is that the recorded PONV in a retrospective study may underestimate the extent of PONV. Patients may not volunteer that they are experiencing nausea; unless it is actively sought by nursing staff, it may not be recorded.
The length of PACU stay was significantly longer at a mean ± SD of 211 ± 82 min for patients in no-CPAP group compared with 159 ± 78 min for patients in the CPAP group. Many factors may contribute this prolonged PACU stay. Postoperative complications are more frequent in patients with OSA. These include airway obstruction, oxygen desaturation, and the need for reintubation as well as systemic hypertension, cardiac dysrhythmias, and need for admission [15]. Our data demonstrated a significant higher incidence of hypertension in the no-CPAP group patients postoperatively (p = 0.013). A number of studies assessed the effect of CPAP on systemic hypertension. The study of Becker and coworkers [16] found a reduction in mean arterial pressure in the region of 10 mmHg with therapeutic CPAP. Pepperell and coauthors [17] also found a greater fall in blood pressure levels among severe OSA patients with therapeutic CPAP use. A recent meta-analysis of the impact of CPAP therapy on blood pressure levels in OSA has confirmed an overall significant clinical benefit [18].
Our data have shown that a reduced number of patients in the CPAP group developed oxygenation disturbances in PACU. A few studies have investigated the role of noninvasive ventilation as a breathing support in preventing complications in general and cardiothoracic surgery [19, 20]. Böhner and coworkers reported that a 12-h prophylactic nasal CPAP treatment significantly reduces the number of postoperative severe oxygenation disturbances after major vascular surgery [21]. This study was able to show that using CPAP had a significant benefit of improving oxygenation and reducing oxygenation disturbances.
The nature of retrospective design of this study has all limitations associated with retrospective studies. We tried to avoid overinterpreting medical records by strictly defining nausea and vomiting. It is likely that routine medical charts may not capture processes and outcomes as accurately as more rigorous case report forms used in a prospective study.
In conclusion, we did not find the increased risk of PONV with the use of postoperative CPAP. Patients who were not using CPAP had a significantly higher incidence of postoperative hypertension and oxygenation disturbances. These are some of the contributing factors that cause prolonged PACU stay. This study provides the rational for the recommendation of CPAP treatment in this patient population. We recommend the routine use of postoperative CPAP for patients with OSA undergoing laparoscopic gastric bypass surgery to optimize their respiratory function.
References
Young T, Peppard PE, Taheri S. Excess weight and sleep disordered breathing. J Appl Physiol. 2005;99 4:1592–9.
Vgontzas AN, Tan TL, Bixler EO, et al. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154 15:1705–11.
Lara MD, Kothari AN, Sugerman HJ. Surgical management of obesity: a review of the evidence relating to the health benefits and risk. Treat Endocrinol. 2005;4 1:55–64.
Ogerg B, Poulsen TD. Obesity: an anaesthetic challenge. Acta Anaesthesiol Scand. 1996;40 2:191–200.
Rezaiguia S, Jayr C. Prevention of respiratory complications after abdominal surgery. Ann Fr Anesth Reanim. 1996;15:623–46.
Kyzer S, Lavie P, Ovnat A, et al. Obstructive sleep apnea in the obese. World J Surg 1998;22:998–1001.
Pelosi P, Ravagnan I, Giurati G, et al. Positive end-expiratory pressure improves respiratory function in obese but not in normal subjects during anesthesia and paralysis. Anesthesiology 1999;91:1221–31.
Joris JL, Sottiaux TM, Chiche JD, et al. Effect of bi-level positive airway pressure (biPAP) nasal ventilation on the postoperative pulmonary restrictive syndrome in obese patients undergoing gastroplasty. Chest 1997;111:665–70.
Huerta S, Deshields S, Shpiner R. Safety and efficacy of postoperative continuous positive airway pressure to prevent pulmonary complications after Roux-en-Y gastric bypass. J Gastrointest Surg 2002;6:354–8.
Nascimento J, Posner D, Rogers M, et al. Risks of noninvasive positive pressure ventilation postoperatively in laparoscopic gastric bypass patients. Crit Care Med 2006;33 12:a116.
Gupta R, Parvizi J, Hanseen A, et al. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76:897–905.
Clergue F, Whitelaw WA, Charles JC, et al. Inferences about respiratory muscle use after cardiac surgery from compartment volume and pressure measurements. Anesthesiology 1995;82:1318–27.
Moos DD, Prasch M, Cantral DE, et al. Are patients with obstructive sleep apnea syndrome appropriate candidates for the ambulatory surgical center? AANA J. 2005;73 3:197–204.
Kakkar RK. Positive airway pressure treatment for obstructive sleep apnea. Chest 2007;132:1057–72.
Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology 2006;104:1081–93.
Becker HF, Jerrentrup A, Ploch T, et al. Effect of nocturnal nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 2003;107:68–73.
Pepperell JC, Ramdasssing-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnea: a randomized parallel trial. Lancet 2002;359:204–10.
Bazzano LA, Khan Z, Renyolds K, et al. Effect of nocturnal nasal continuous positive pressure on blood pressure in obstructive sleep apnea. Hypertension 2007;50:417–23.
Carlsson C, Sonden B, Thylen U. Can postoperative continuous positive airway pressure (CPAP) prevent pulmonary complication after abdominal surgery? Intensive Care Med. 1981;7:225–9.
Thomas AN, Ryan JP, Pollard BJ. Nasal CPAP after coronary artery surgery. Anaesthesia 1992;47:316–9.
Böhner H, Kindgen-milles D, Crust A, et al. Prophylactic nasal continuous positive airway pressure after major vascular surgery: results of a prospective randomized trial. Langenbeck’s Arch Surg 2002;387:21–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meng, L. Postoperative Nausea and Vomiting with Application of Postoperative Continuous Positive Airway Pressure after Laparoscopic Gastric Bypass. OBES SURG 20, 876–880 (2010). https://doi.org/10.1007/s11695-008-9741-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-008-9741-2