Abstract
Background
Obesity is often associated with fatty liver (FL). In most cases, bright liver at ultrasound (US) and increased alanine aminotransferase (ALT) and gamma-glutamyltranspeptidase (GGT) levels are considered the hallmarks of nonalcoholic fatty liver disease (NAFLD). Insulin resistance (IR) is the main link between obesity and NAFLD. The use of the Bioenterics® intragastric balloon (BIB) is a safe procedure either for inducing a sustained weight loss with diet support or for preparing those patients who are candidates for bariatric surgery. The aim of the study was to investigate whether the weight loss induced by intragastric balloon might improve IR and liver enzymes. The presence or absence of FL at US and the influence of a body mass index (BMI) decrease ≥10% after BIB (ΔBMI ≥ 10%) were also considered.
Methods
One hundred and three consecutive obese (BMI > 30 kg/m2) patients (38 males/65 females; mean age 41.3, range 20–63 years) underwent BIB insertion under endoscopic control. The BIB was removed 6 months later. US, clinical, and routine laboratory investigations were performed before and after BIB. IR was calculated by the homeostasis model assessment (HOMA-IR > 2.5). Exclusion criteria were hepatitis B virus positive, hepatitis C virus positive, alcohol consumption >30 g/day, history of hepato-steatogenic drugs, and type 1 diabetes.
Results
Ninety-three patients were eligible for the study. The BMI significantly decreased in all investigated patients, and it was ≥10% in 59% of the patients. FL was seen at US in 70%, impaired fasting blood glucose was present in 13%, ALT exceeded the normal limit in 30.1%, GGT exceeded the normal limit in 15%, and HOMA-IR was >2.5 in 85%. Median HOMA-IR decreased significantly in FL (4.71 vs 3.10; p < 0.05) and non-FL (3.72 vs 2.81; p < 0.01) groups. Median ALT decreased significantly in the FL group (31.5 vs 24; p < 0.001) and GGT significantly decreased in the FL group (31 vs 23.5; p < 0.05). In the FL group with ΔBMI ≥ 10%, the median values of HOMA-IR (4.95 vs 2.69; p < 0.05), ALT (30 vs 23; p < 0.01), and GGT (28 vs 20; p < 0.001) significantly decreased after BIB. In the non-FL group, HOMA-IR values significantly decreased (4.07 vs 2.36; p < 0.01) in patients with a ΔBMI ≥ 10%; ALT and GGT did not significantly decrease.
Conclusions
Weight loss induced by intragrastric balloon reduces IR. The ALT and GGT decrease suggests an improvement in hepatic damage. The benefit depends on the decrease of BMI higher than 10%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing prevalence of obesity, mostly in western countries, has prompted intensive research on associated morbidities, as well as on the treatments for achieving weight reduction. In obesity, visceral fat accumulation, above all in the liver, namely, nonalcoholic fatty liver disease (NAFLD), is often associated with a cluster of metabolic alterations, i.e., type 2 diabetes, hypertension, and dyslipidemia, namely, the metabolic syndrome [1]. The main link among obesity, fatty liver (FL), and the metabolic syndrome, is represented by insulin resistance (IR) [2]. Previous reports have suggested that IR may be an intrinsic defect in NAFLD due to the impaired ability of insulin to suppress lipolysis, leading to increased delivery of free fatty acids to the liver. Insulin sensitivity is positively related to adiponectin, an adipose tissue hormone that promotes fatty acid oxidation in the liver [2–4]. Conversely, IR reduces adiponenctin with the consequent increase of the visceral fat, especially in the liver. In subjects with NAFLD, significantly lower levels of adiponectin were found when they were compared with body mass index (BMI)-matched controls [5]. The inflammatory cytokines are also increased in NAFLD and are positively related to IR [5]. In obese patients with NAFLD, IR is significantly associated with increased levels of liver enzymes, confirming the key role of IR in hepatic injury, throughout liver fat accumulation [6]. However, the pathogenesis of elevated liver enzymes in NAFLD has not yet been completely clarified [7]. In addition, the modest elevations in aminotransferase (ALT) and gamma-glutamyltranspeptidase (GGT), even near the upper half of the normal range, have been reported to predict liver damage in obese patients [8].
Until now, no treatment has been established for reducing the risk of progressive liver disease associated with NAFLD, even though weight loss and a low-fat diet are strongly recommended. Many approaches have been proposed to induce weight loss. They encompass diet, lifestyle modifications, medications (metformin, thiazolidinediones), and bariatric treatment [9, 10]. Temporary placement of an intragastric balloon is now widely used either for those patients who can maintain the weight loss with diet support or to prepare patients who are candidates for bariatric surgery or other surgical procedures [11].
The aim of our study was to investigate whether the weight loss induced by temporary bariatric treatment with intragastric balloon might lead to modifications of IR and improvement of liver enzymes. In addition, the presence or absence of FL at ultrasound (US) and the influence of BMI decrease ≥10% after Bioenterics® Intragastric Balloon (BIB) (ΔBMI ≥ 10%) were also considered.
Material and Methods
Patients
From March 2003 through November 2007, 103 consecutive obese (BMI > 30 kg/m2) patients (38 males/65 females; mean age 41.3 ± 10.4, range 20–63 years) were admitted to our digestive endoscopy service for bariatric treatment of obesity by means of BIB® (Bioenterics, Santa Barbara, CA, USA) insertion. The BIB was positioned under endoscopic control and removed 6 months later. Clinical, laboratory and metabolic determinations were assessed for each patient before and after BIB insertion. Exclusion criteria were positivity for hepatitis B virus or hepatitis C virus, previous or current alcohol consumption >30 g/day, use of medications with reported hepato-steatogenic effect (amiodarone, tamoxifene, estrogens), and type 1 diabetes. A personalized low-calorie diet was provided to each patient during BIB placement.
Laboratory and Instrumental Investigations
BMI (kg/m2) was determined before and after the BIB placement. Blood samples were obtained from each patient after an overnight fast. The determinations of blood glucose, liver enzymes (ALT, GGT), insulin, triglycerides, and HDL cholesterol were performed by standardized methods at time 0 (t0) and after 6 months (t6), when BIB was removed. IR was calculated by the homeostasis model assessment (HOMA-IR), as fasting serum insulin (μU/ml) × fasting plasma glucose (mmol/l)/22.5; values >2.5 indicate a state of IR [12]. The presence of FL was demonstrated by abdominal US, as “bright liver” [13] by two experienced investigators.
Statistical Analysis
Statistical analyses were performed with SPSS 15.0 software (SPSS, Chicago, IL, USA). For comparisons, both parametric (T Student) and nonparametric (Wilcoxon) tests were used for values normally or not-normally distributed. Statistical significance was defined as p < 0.05.
Results
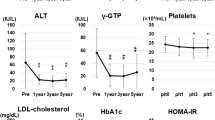
Ten patients met exclusion criteria. Ninety-three patients were eligible for the retrospective study. BMI decreased significantly after BIB in all patients (42.1 ± 5.8 vs 37.8 ± 5.5; p < 0.001). The BMI decrease was higher than 10% pre-BIB value (ΔBMI ≥ 10%) in 59% of the patients. Before BIB placement, “bright liver” echo pattern at US investigation, consistent with FL (FL group), was observed in 70%; impaired fasting blood glucose (≥125 mg/dL; ≥6.9 mmol/L) was observed in 13%; ALT exceeded normal limits (40 U/L) in 30.1%; GGT exceeded normal limits (50 U/L) in 15%; and HOMA-IR was >2.5 in 85% of patients. The values of hypertrigliceridemia (≥150 mg/dL) present in 33.3%, HDL cholesterol levels ≤40 mg/dL in 32%, and arterial hypertension in 22.2% did not significantly change after 6 months of BIB placement. HOMA-IR, ALT, and GGT values (not normally distributed) in obese patients with FL or without FL (non-FL) at US investigation are expressed in Table 1 and by box plot representation as medians and interquartile ranges (Fig. 1).
Table 1 shows medians and ranges of investigated values. Median HOMA-IR decreased significantly in the FL (4.71 vs 3.10; p < 0.05) and non-FL (3.72 vs 2.81; p < 0.01) groups of obese patients after 6 months of BIB placement. Median ALT decreased significantly in the FL group (31.5 vs 24; p < 0.001) and GGT significantly decreased in the FL group (31 vs 23.5; p < 0.05). By means of box plot representation, Fig. 2 shows the changes between the groups with distinction of FL or non-FL and ΔBMI ≥ 10% or <10%. In the FL group with ΔBMI ≥ 10%, median values of HOMA-IR (4.95 vs 2.69; p < 0.05), ALT (30 vs 23; p < 0.01), and GGT (28 vs 20; p < 0.001) significantly decreased after BIB. In the non-FL group with ΔBMI ≥ 10%, HOMA-IR values significantly decreased (4.07 vs 2.36; p < 0.01); ALT and GGT did not significantly decrease.
Discussion
In obese and severely obese subjects, liver fat accumulation commonly occurs, increasing the risk of hepatic disease progression from NAFLD to cirrhosis [1–5]. In such patients, especially when other comorbidities are also present, weight loss is strongly recommended. However, diet restrictions often fail to induce a sustained body weight reduction. In subjects with BMI higher than 35, or 30 with comorbidities, bariatric therapy is widely applied to obtain weight loss [10, 11].
Temporary intragastric balloon placement is considered a safe procedure for those obese patients who do not meet the requirements for a prompt surgical approach [14, 15]. In our study, we confirmed that BIB could induce a significant BMI reduction in all patients. More than half of the patients showed an appreciable BMI decrease ≥10%. Nevertheless, US investigation showed “bright liver,” consistent with FL, in the majority of our patients (70%). In addition, IR was demonstrated by HOMA index >2.5 in 85% of all cases. After BIB, HOMA-IR was significantly reduced in all patients, and the decrease was independent of the presence or absence of FL at US. Basal (t0) ALT and GGT values were higher than normal in less than one third of all patients. The patients with a decrease of BMI ≥10% showed an improvement of insulin impairment by a significant decrease of HOMA-IR in both the FL and non-FL groups. Only in FL patients, the improvement of liver dysfunction was shown by a significant decrease of both enzymes only with a reduction of BMI ≥10% (Fig. 2).
As previously reported, enzyme levels, even within the upper half of the normal range, may predict the early stages of FL disease in obesity [6–8]. After 6 months of BIB placement, we observed a significant decrease of both enzymes. In accordance with other studies [16, 17], we confirm that weight loss may reduce the risk of liver injury progression by normalizing the liver enzymes with lowered spread of values from medians (Fig. 1). ALT values better than GGT showed this behavior after BIB. It has been reported that ALT is the biomarker of liver dysfunction, more sensitive than other liver enzymes [4–6]. On the basis of our data, it could be suggested that a high BMI generally induced IR, as demonstrated by high HOMA values in the majority of obese patients. Visceral adiposity, namely, FL, throughout insulin sensitivity impairment, worsens hepatic function in which ALT should be considered a reliable biomarker. ALT elevations, even in the upper half of the normal range, must be monitored in obesity because they predict the liver disease progression [6–8]. Weight loss is strongly recommended to reduce liver injury due to visceral adiposity, as well as to reduce the impairment of insulin sensitivity [17, 18]. Such an end-point would be better achieved in those patients with BMI decrease ≥10%. In this way, even temporary bariatric treatment by means of BIB, associated with diet restriction, may provide a sustained benefit on liver function and on insulin sensitivity.
References
Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and metabolic syndrome. Hepatology. 2003;37:917–23.
Bugianesi E, McCullogh AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000.
Utzschneider KM, Kahn SE. The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–61.
Wallace TM, Utzschneider KM, Tong J, et al. Relationship of liver enzymes to insulin sensitivity and intra-abdominal fat. Diabetes Care. 2007;30:2673–8.
Hui JM, Hodge, A, Farrell GC, et al. Beyond insulin resistance in NASH: TNF-α or adiponectin? Hepatology. 2004;40:46–54.
Marchesini G, Avagnina S, Barantani EG, et al. Aminotrasferase and gamma-glutamyltranspeptidase levels in obesity are associated with insulin resistance and the metabolic syndrome. J Endocrinol Invest. 2005;28:333–9.
Burgert TS, Taksaly SE, Dziura J, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91:4287–94.
Chang Y, Ryu S, Sung E, et al. Higher concentrations of alanine aminotrasferase within the reference interval predict nonalcoholic fatty liver disease. Clin Chem. 2007;53:686–92.
Bellentani S, Delle Grave R, Suppini A, Marchesini G, and the Fatty Liver Italian Network (FLIN). Behavior therapy for nonalcoholic fatty liver disease: the need for a multidisciplinary approach. Hepatology. 2008;47:746–54.
Wolf AM, Beisiegel U. The effect of loss of excess weight on the metabolic risk factors after bariatric surgery in morbidly and super-obese patients. Obes Surg. 2007;17:910–9.
Mathus-Vligen EM, Tytgat GN. Intragastric balloon for treatment-resistant obesity: safety, tolerance and efficacy of 1-year balloon treatment followed by 1-year balloon-free follow-up. Gastrointest Endosc. 2005;61:19–27.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from plasma fasting glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Palmentieri B, de Sio I, La Mura V, et al. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis. 2006;38:485–9.
Genco A, Cipriano M, Bacci V, et al. Bioenterics® Intragastric Ballon (BIB®): a short-term double-bind, randomised, controlled, crossover study on weight reduction in morbidly obese patients. Int J Obes. 2006;30:129–33.
Rossi A, Bersani G, Ricci G, Petrini C, De Fabritiis G, Alvisi V. Intrgastric balloon insertion increases the frequency of erosive esophagitis in obese patients. Obes Surg. 2007;17:1346–9.
Hickam IJ, Johnsson JR, Prins JB, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminostransferase, fasting insulin, and quality of life. Gut. 2004;53:413–9.
Al-Momen A, El-Mogy I. Intragastric balloon for obesity: a retrospective evaluation of tolerance and efficacy. Obes Surg. 2005;15:101–5
Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl trasferase concentrations are associated with histologic improvement. Obes Surg. 2006;16:1278–86.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ricci, G., Bersani, G., Rossi, A. et al. Bariatric Therapy with Intragastric Balloon Improves Liver Dysfunction and Insulin Resistance in Obese Patients. OBES SURG 18, 1438–1442 (2008). https://doi.org/10.1007/s11695-008-9487-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-008-9487-x