Abstract
Closely related species in nature often show similarities in suites of direct and indirect traits that reveal aspects of their phylogenetic history. Here we tested how common descent affects trait evolution in several closely related parasitoid species in the genera Cotesia and Microplitis (Hymenoptera: Braconidae: Microgastrinae) by comparing development, resource use and allocation into reproduction and maintenance. Parasitoids in these genera exhibit traits, like haemolymph feeding as larvae and external pupation that are rare in most parasitoid lineages. The growth of parasitized hosts was reduced by 90 % compared with healthy hosts, and maximum host size depended to a large extent on adult parasitoid size. Development time was longer in the more generalist parasitoids than in the specialists. Adult body mass was sexually dimorphic in all Cotesia species, with females being larger, but not in Microplitis spp. In contrast, in one of the Microplitis species males were found to be the larger sex. Egg load dynamics during the first 6 days after emergence were highly variable but egg number was typically higher in Cotesia spp. compared to Microplitis spp. Longevity in the various species was only greater in female than in male wasps in two Microplitis sp. There was a clear inverse relationship between resource use and allocation, e.g. maximum egg load and longevity, in these parasitoids. Our results reveal that adaptation to constraints imposed by host quality and availability has resulted in trait convergence and divergence at the species, genus and subfamily level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The evolutionary history that species share can play an important role in shaping trait evolution. A classic, but much debated example comes from Ehrlich and Raven’s study on co-evolution between host plants and herbivores. One key message they conveyed was that closer taxonomic groups of butterflies tended to feed on more closely related plant species (Ehrlich and Raven 1964; Janz 2011); hence when habitat is a common denominator, traits vary less, species tend to be more alike and phylogenetic congruence is reflected by the species assemblages associated with one another in a community. Recent studies highlight the importance of phylogenetic diversity for promoting trait divergence and diversity in co-dependent or evolved species, also at higher trophic levels (Dinnage et al. 2012). However, trait diversification may be limited by phylogenetic constraints on certain evolutionary trajectories. For instance, Kellermann et al. (2012) found that phylogenetic inertia rather than directional selection pressures leads to interspecific similarities in upper thermal resistance levels in Drosophila. Similarly, phylogenetic divergence in host species can lead to trait diversification in co-evolved partners, but trait evolution might still be limited by phylogenetic effects.
Life history traits are elemental for optimizing fitness. In any organism, these traits include growth, development, body mass, maintenance and reproduction, as well as defence, morphology, and behaviour. Indirectly related to traits such as body size are other parameters including vigor and lifespan. In organisms with discrete developmental stages, like holometabolous insects that go through complete metamorphosis, a large proportion of metabolic resources are accrued during immature development and are thereafter allocated to different (and perhaps conflicting) functions such as reproduction and maintenance. The use of capital resources obtained during the larval stages may be partially offset by income resources that are obtained by the adult stage, although the extent to which this is true depends critically on a range of ecophysiological factors. The expression of life-history strategies in any organism often represents a series of trade-offs between different fitness functions. These in turn are determined to a large extent by a range of biotic and abiotic forces (Stearns 1992). Parasitoids comprise a highly diverse group of insects that occur within several insect orders, but which are by far the most species-rich in Hymenoptera. They are insects whose larvae feed on, or inside the bodies of other insects, whereas the adults are free living (Godfray 1994). The development of a parasitoid is usually dependent on a limited amount of resources contained within a single host that is often not much larger than the adult parasitoid itself (Harvey 2005; Pennacchio and Strand 2006). In contrast, most other insect consumers—including herbivores and predators—are much less constrained by the amount of resources necessary to achieve optimal growth and survival. In effect, these insects may need to ingest food that is ultimately many times their own body mass in order to successfully complete development to the adult stage. Parasitoids, on the other hand, are under extremely strong selection for optimal resource utilization and allocation to potentially competing fitness functions such as reproduction and survival (Slansky 1986; Ellers and van Alphen 1997; Jervis et al. 2008).
Many parasitoids attack early instar hosts that are a fraction of the size of the female at oviposition, and which therefore need to feed and grow considerably in order to attain the minimal nutritional requirements of the parasitoid progeny (Harvey 2005). Termed ‘koinobionts’ (Askew and Shaw 1986), these parasitoids develop in hosts that represent dynamic resources where the host may differ in mass by many factors between oviposition and host death (Sequeira and Mackauer 1992; Mackauer and Sequeira 1993; Harvey et al. 1994). One of the most important constraints on koinobiont development is how the immature stages respond to differences in host quality. Based on such factors as host size or stage at oviposition, diet, the presence of competing organisms, and abiotic conditions, the nutritional status (and subsequent growth rate) of the host might be affected (Harvey 2005), which in turn could affect parasitoid fitness in terms of survival, size and development time. Body size is considered to be a major component of fitness because it is often positively correlated with demographic traits such as egg production, longevity, and dispersal as well as competition for access to mates and hosts. On the other hand, selection for rapid development time may be intense early in the season, when populations (and competition for host access) are increasing, or else when there is an increased risk or precocious mortality from predators, pathogens or inclement weather conditions in slow-growing hosts (Benrey and Denno 1997).
However, adaptive radiation in parasitoids to host-related constraints is not solely based on biological or ecological characteristics of the host. Instead, constraints imposed by phylogenetic history can also play a significant role in determining the extent to which parasitoid clades can respond to host-related constraints. McKitrick (1993) defined a phylogenetic constraint as “any result or component of the phylogenetic history of a lineage that prevents an anticipated course of evolution in that lineage”. In other words, based on a purely evolutionary trajectory, we might expect a certain species or species-group of parasitoids to exhibit certain traits or suites of traits in response to various aspects of the biology and ecology of their host(s). On the other hand, in the absence of these traits, we may be able to assume that phylogenetic history has ‘held back’ the parasitoid from fully adapting to these characteristics of the host. In spite of this, most comparative studies exploring ecological and physiological traits in parasitoids have not considered the importance of phylogenetic constraints from the perspective of the parasitoid. For instance, Price (1972, 1973) compared morphological and reproductive traits in several parasitoid species attacking different stages of the same host species. He found that parasitoid guilds exhibited trait values in egg size, production and wing structure that closely reflected host abundance (recently challenged by Jervis et al. 2012). Harvey and Strand (2002) examined development patterns in a number of koinobiont parasitoids attacking lepidopteran hosts and found that trade-offs between adult size and development time were closely tailored with the feeding profile (exposed or concealed) of host caterpillars. However, these studies did not evaluate relatedness of the parasitoids and thus ignored the role of phylogeny in moulding this suite of traits. Studies by Blackburn (1991a, b) and Mayhew and Blackburn (1999) compared a range of life-history related traits in parasitoids, such as body size and longevity, but these comparisons encompassed a very wide breadth of taxa from distantly related clades. They found, perhaps unsurprisingly, that at such a broad taxonomic perspective most relationships were either weak or absent altogether.
Behaviour, morphology, reproduction and ontogeny are well described in many parasitoids, but at the same time only few studies have examined suites of traits amongst closely related species within the context of host use and resource allocation. Here, we compare life-history traits in parasitoid species with a recent evolutionary history, but that develop on phylogenetically distinct hosts. We are specifically interested in trade-offs between life history traits in relation to parasitoid phylogeny. To achieve this goal, we compared host utilization, regulation, development, longevity and egg production in seven closely related koinobiont endoparasitoids of lepidopteran larvae (Shaw and Huddleston 1991; Murphy et al. 2008). These seven species belong to two recently diverged genera (but see Quicke et al. 2004), Cotesia and Microplitis, and are classified in the same family and subfamily (Braconidae: Microgastrinae).
The seven species of parasitoids studies here exhibit similarities in morphological traits (Fig. 1) as well as in reproductive traits such as ovarian structure and egg shape (Fig. 2). In particular, the parasitoids all possess short ovipositors, adapted to stinging exposed feeding lepidopteran hosts, and the dominant abdominal pigments are orange in colour. The oviducts are well developed and are capable of storing fairly large numbers of small, ellipsoid microtype (or ‘hydropic’) eggs that expand greatly during embryogenesis in host tissues (Flanders 1950; Jervis and Kidd 1986). All of these parasitoids also enjoy symbiotic associations with polydnaviruses (Whitfield 1997) that are species-specific and function by abrogating the host’s immune system and/or by regulating host growth and development in accordance with the nutritional requirements of the parasitoid offspring. Host range in Microplitis species appears to be largely confined to caterpillars of moths in the family Noctuidae, whereas Cotesia species attack caterpillars of many different lepidopteran families.
By comparing different biological traits amongst a range of closely related species, we determine which adaptive syndromes are significantly retained and which may have been lost at the species level due to constraints imposed by eco-physiological characteristics of the host species. We further determine whether the various traits examined are retained at the level of genus or sub-family, reflecting phylogenetic conservation or convergence.
Methods and Materials
Insects
In total four Microplitis species and three Cotesia species were reared on six different hosts (see Table 1). Phylogenetic relationships of parasitoids in the Braconidae, including the Microgastrinae, are described in Murphy et al. (2008). Individuals of different Microplitis and Cotesia species (and their hosts) were obtained from cultures maintained at the following institutions: Microplitis mediator Haliday (and Mamestra brassicae L. Lepidoptera: Noctuidae), Cotesia rubecula Marshall (and Pieris rapae L. Lepidoptera: Pieridae) and Cotesia vestalis Haliday (and Plutella xylostella L. Lepidoptera: Plutellidae) were obtained from cultures maintained for several years at Wageningen University, The Netherlands; cultures of Microplitis rufiventris Kok and Cotesia marginiventris Cresson (and their shared host Spodoptera littoralis Boisduval Lepidoptera: Noctuidae) were obtained from Neuchâtel University, Switzerland; the culture of M. demolitor was obtained from University of Georgia, USA (and its natural host, Helicoverpa armigera Hübner (Lepidoptera: Noctuidae), from the Max Planck Institute, Jena, Germany); the culture of the Japanese (Asian) Microplitis sp.(under taxonomic revision) (and its host, Mythimna separata Walker (Lepidoptera: Noctuidae), from Nagoya University, Japan. All parasitoids were kept as adults in groups of 20–30 in large Petri dishes (18 cm dia.) and were constantly supplied with honey and water.
Cotesia rubecula and C. vestalis are highly specialized in nature on P. rapae and P. xylostella respectively; their hosts in turn are specialist feeders on plants in the Capparales that includes important agricultural crops such as mustards and cabbages. The remaining parasitoids are all specialized on hosts in the Noctuidae (the precise host range of each parasitoid is, however, unknown), whereas their noctuid hosts are broadly polyphagous generalists.
The noctuid hosts (M. brassicae, S. littoralis, H. armigera and M. separata) were reared in group of ~30 caterpillars in plastic boxes (18 × 10 × 10 cm) on an artificial diet as described in Shorey and Hale (1965). Late during the final instar, vermiculite was added along with diet, serving as a substrate for pupation. Upon emergence, adult moths were placed in groups of 15–20 (equal numbers of males and females) into plastic beakers (12 cm dia) containing 10 % sugar solution absorbed into cotton wool as a source of energy for the moths. For oviposition, white blotting paper was placed around the inside of the beakers as well as the lid that was secured with a rubber band.
Pieris rapae and P. xylostella hosts were reared on Brussels sprout (Brassica oleracea var. gemmifera cv. Cyrus) plants. Adult butterflies and moths were allowed to oviposit directly onto plants in rearing cages and their caterpillars developed on these plants. Each species was reared in a separate cage and newly formed pupae were kept in new cages until adult eclosion. The newly emerged butterflies/moths were allowed to mate and oviposit directly onto fresh plants. Until pupation, new plants were provided as necessary.
For parasitoid cultures, the method for all noctuid hosts and their parasitoids was the same: ~30 second instar (hereafter L2) caterpillars were placed into Petri dishes with 5-10 day old mated parasitoids along with small amounts of honey, water, and artificial diet. Female wasps parasitized the caterpillars directly, and after 24 h the caterpillars were removed from these dishes and placed in other dishes with diet until parasitoid cocoons were formed. The method was similar for P. rapae and Pl. xylostella, except that the wasps were allowed to parasitize their hosts directly on the food plants, also for 24 h. Here, ~20 female wasps were added to each cage. Parasitoid cocoons were later collected.
All insects were maintained in a climate room at 25° ± 2 °C with a 16:8 h light:dark regime.
Measuring Direct and Indirect Traits in Parasitoids
The different traits that were measured are described in Fig. 3. Mated adult female parasitoids ~5 d-old were taken from the cultures in groups of 20–30 and individually placed in glass vials (6 × 2 cm). Females were allowed to individually parasitize L2 instars of their respective hosts, which were presented to them on the end of a moistened brush. Parasitized hosts were then either reared in groups of 10 in Petri dishes (noctuid hosts) or were placed in groups of 10 caterpillars on B. oleracea plants in zipped nylon cages (30 × 60 × 30 cm) containing 3–4 plants per cage. At egression from the host, parasitoid cocoons were pooled in large Petri dishes (18 cm dia.) until adult eclosion. Adult parasitoid fresh mass was determined by weighing the wasp on a Mettler Microbalance (accuracy ± 1 μg). Development time was determined as the number of days between oviposition (stinging) and adult wasp emergence.
To measure host growth rates, 10–12 larvae of 5 of the host-parasitoid combinations (see Table 1) were reared as described above, and their growth monitored both in parasitized and unparasitized individuals. When the caterpillars reached their maximum size (i.e. 24 h before either parasitoid egression or pupation) they were weighed on a microbalance.
To determine longevity, wasps were parasitized and allowed to develop as described above. At eclosion wasps were sexed and maintained individually in 9.5-cm Petri dishes (n = 10 per sex per species for C. marginiventris and M. rufiventris, n = 20 per sex for all other species) with 5 small droplets of honey and a ball of moist cotton wool. Mortality was recorded daily and honey and cotton wool refreshed every other day until all wasps had died.
Egg load counts were made by carefully dissecting unmated adult female wasps at two day intervals over the course of 6 days post-eclosion (beginning at the day of eclosion, ‘day 0’). Wasps were placed on glass slides in drops of water and the ovaries were carefully teased from the wasps by pulling the end of the abdomen distally using a pair of rigid forceps and a dissecting needle. Only mature ovulated eggs were counted.
Statistical Analyses
ANOVAs were conducted with species as main factor to analyse the data on maximum larval masses of parasitized and healthy hosts. To investigate the effect of genus on the various life history variables (mass, development time, and adult longevity) of the parasitoids, a mixed model approach was used with genus and sex as fixed factors and species as a random factor. Estimation of effects in the model was based on restricted likelihood maximisation (REML). In a second analysis we compared these variables at the species level using a general linear model ANOVA. Post-hoc Tukey–Kramer multiple comparisons were conducted when the ANOVAs were significant. Mass, development time and longevity of the parasitoids were log-transformed to meet assumptions of normality and homoscedasticity. Similarly, we investigated differences in genus- and species-specific relationships between female age and egg loads using a mixed model for the effects at the genus and a GLM at the species level. All analyses were performed in the statistical package SAS version 9.2.
Results
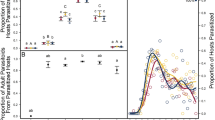
Maximum larval masses in the various host species differed significantly (Welch’s ANOVA F4,19.8 = 822.0, P < 0.001). The two largest host species were S. littoralis and M. brassicae, followed by H. armigera and P. rapae, whereas Pl. xylostella was by far the smallest species. The latter attained only about 1 % of the mass of S. littoralis (Fig. 4a). M. separata was not weighed, but grows approximately as large as M. brassicae. Parasitized caterpillars grew to only a fraction of the mass of healthy caterpillars (Fig. 4a). Moreover, although there were significant differences in the maximum mass of the different hosts when parasitized by their parasitoids (Welch’s ANOVA F5,21.6 = 375.0, P < 0.001), the magnitude of these differences was much less than those exhibited by healthy hosts (Fig. 4b). In four of associations tested the maximum size of the host-parasitoid complex was quite similar. C. marginiventris arrested growth when the hosts were smaller (Fig. 4b). C. vestalis allowed parasitized hosts to grow as large as healthy hosts (Fig. 4a, b). However, these hosts grow only a fraction of the size of the other lepidopteran caterpillars studied here.
a Maximum fresh larval masses (mg) of healthy (unparasitized) hosts (closed bars) and the mass of the parasitized host-parasitoid complex (open bars). The two open bars next to S. littoralis are the maximum larval masses of Microplitis rufiventis (left bar) and C. marginiventris (right bar); for M. brassicae the parasitoid is M. mediator; for H. armigera the parasitoid is M. demolitor and for P. rapae the parasitoid is C. rubecula. b Maximum fresh larval masses (mg) of the parasitized caterpillars alone. Line bars represent standared error of the mean. Bars with different letters are significantly different (Tukey’s tests, P < 0.05). Initial sample sizes = 10 for all bars except for P. rapae and M. brassicae controls for which n = 12
Egg-to-adult development time in the parasitoids differed significantly amongst the various species (GLM: F 6,1055 = 316, P < 0.001), but not between the two genera (REML: F 1,5 = 0.77, P = 0.42). At the species level, the interaction with offspring sex was also significant (F6, 1055 = 4.09, P < 0.001). All of the wasps were protandrous, with males developing faster than females (Fig. 5a). The specialized parasitoids of the specialist-feeding herbivores typically developed more rapidly than the parasitoids developing on the generalist herbivores (Fig. 5a). Interestingly, development time was longest in the two species (C. marginiventris and M. rufiventris) that shared the same host species (S. littoralis).
Developmental parameters and longevity of the different Cotesia and Microplitis species a Egg-to-adult development time; b Adult body mass in mg; c Longevity (in days) of male and female adult parasitoids. Line bars represent standard error of the mean. Bars with different letters are significantly different (Tukey–Kramer tests, P < 0.05). Sample sizes for body mass and development time: C. marginiventris (females/males), 85/235; C. rubecula, 45/76; C. vestalis, 92/66; M. sp., 27/112, M. mediator, 58/68, M. rufiventris, 44/100, and M. demolitor, 42/19. Sample sizes for longevity were n = 10 per sex for C. marginiventris and M. rufiventris and n = 20 for the other species
Adult body mass also varied significantly amongst the parasitoid species (GLM: F 6, 1055 = 1134, P < 0.001) but not between the two genera (REML: F 1,5 = 1.17, P = 0.33). However, the interaction between mass and sex was highly significant at both the genus (REML: F 1,5 = 22.2, P = 0.005) and the species level (GLM: F 6, 1055 = 4.09, P < 0.001). Importantly, sexual-size dimorphism in which females were larger was highly pronounced in all three Cotesia species, but not in any of the Microplitis species (Fig. 5b). In fact, in this genus there was actually a tendency for males to be larger, and this was significant in M. mediator (Fig. 5b).
As was found for development time and adult mass, longevity of the wasps only depended on the species (GLM: F 6, 222 = 19.4, P < 0.001), and not on the genus (REML: F 1,5 = 0.27, P = 0.63). Longevity varied considerably among the species in both genera. Moreover, the effect of parasitoid sex on longevity depended on the species (species-sex interaction GLM: F 6, 222 = 4.04, P < 0.001) and appeared more pronounced in Microplitis than in Cotesia species, however this was not statistically significant (REML: F 1,5.1 = 3.23, P = 0.13, Fig. 5c); female Microplitis species tended to live longer than male conspecifics. Moreover, the three longest lived species were among the smallest in terms of adult body mass in the study (C. marginiventris, M. mediator and Microplitis sp.).
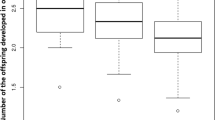
Egg loads in the different parasitoids increased from eclosion to their maximum at approximately 4–6 days of age (Fig 6). There was a significant interaction between egg loads and parasitoid age at the species (GLM: F 6, 161 = 15.5, P < 0.001), but also at the genus level (REML: F 3, 11.9 = 3.99, P = 0.035). There was a tendency for species of Microplitis to mature eggs more slowly and to reach the maximum egg load at an earlier age. As a result, Microplitis species have smaller maximum egg loads than species of Cotesia (Fig. 6). Maximum egg load in the Japanese Microplitis sp. was only about 15–20 % of that observed in C. rubecula. Note also that wasps with the lowest egg loads also tended to live the longest.
Discussion
This study reports that the different species of parasitoids in the genera Cotesia and Microplitis exhibited some trait convergence, whereas other traits were either genus- or species-specific. Host growth manipulation showed a strong degree of convergence in all associations, with the growth and development of hosts being greatly reduced compared with healthy (=unparasitized) hosts. However, at the same time each parasitoid species regulated host growth in accordance with the nutritional requirements of their own progeny. The exception was the C. vestalis–P. xylostella association; unlike all of the host species studied here, P. xylostella is in the micro-Lepidoptera and thus is only marginally larger than the adult parasitoid. All other host species are macro-Lepidoptera whose healthy larvae grow many times larger than their respective parasitoids. Importantly, the maximum mass of the different host-parasitoid complexes reflected parasitoid adult mass more than the growth potential of the host species parasitized. For instance, although fully-grown healthy larvae of P. rapae are considerably smaller than larvae of the noctuids that served as hosts for C. marginiventris and all four Microplitis sp., they attained a relatively larger terminal mass when parasitized by the largest parasitoid studied here, C. rubecula.
Host regulation and parasitoid resource utilization strategies have become ‘finely-tuned’ over evolutionary time in order to optimize fitness-related traits such as development time, body mass and survival (Godfray 1994; Harvey and Strand 2002; Brodeur and Boivin 2004; Pennacchio and Strand 2006). Moreover, ‘option sets’ for parasitoid decision-making processes during larval development are probably rather limited. Therefore, deviation for a narrow range of developmental responses would invariably have strong fitness-related costs, particularly on survival. Hosts must not be allowed to grow too large or too small. Consequently, most solitary endoparasitoids of macro-Lepidoptera have probably been selected to regulate host growth in accordance with their own nutritional requirements, irrespective of their phylogeny (Harvey 2005). Given that many of these parasitoids are of approximately the same size at eclosion, this has probably led to broad convergence in regulatory strategies amongst many endoparasitoid species with different degrees of relatedness (Vinson and Iwantsch 1980). However, for C. vestalis that attacks tiny micro-lepidopteran hosts, like Pl. xylostella, this pattern is clearly absent, for the simple reason that caterpillars must grow to nearly their full size to satisfy the minimal nutritional requirements of the parasitoid.
In contrast with host growth manipulation, traits associated with the adult parasitoid diverged amongst the species studied here. When developing in hosts of the same instar, sexual size dimorphism, in which emerging female wasps were larger than males, was pronounced in all 3 Cotesia species but completely absent in Microplitis. An exception is M. mediator, in which we found that the larger wasps were males (see also Harvey and Strand 2003). In most parasitoids thus far studied, sexual-size dimorphism, if any, generally favors females over males, although in some ichneumonid clades, the reverse is found (Hurlbutt 1987; King 1989; Mackauer 1996). The benefits of large size are generally assumed to be translated in reproductive characters of parasitoids and other insects, such as longevity and fecundity (Charnov 1982). In many animals, including parasitoids, it has often been assumed that eggs are more costly to produce than sperm and consequently that fitness returns for large females in terms of egg production are correspondingly much higher than in large males in terms of sperm production (Charnov 1982: Jervis et al. 2008). However, such claims have recently been challenged by Boivin (2012), who found that male sperm production is highly limited in some parasitoid species. Furthermore, adult size may affect other demographic traits with clear fitness related implications for males, such as dispersal capability and in competitive interactions for mating opportunities. Indeed, studies with a variety of vertebrate and invertebrate taxa have found that, where competition for access to females is strong, an increase in male size is favored (Ghiselin 1974). Unfortunately, no studies thus far have examined this area in the parasitic Hymenoptera, and, therefore, the factors responsible for the production of larger males in some species are not well understood.
Development times varied amongst the parasitoid species, but were generally shorter in C. rubecula and C. vestalis, that are both attack specialist-feeding herbivores, than in the other parasitoids. In koinobiont endoparasitoids, there is often a very strong physiological integration between the host and the developing parasitoid larvae (Lawrence 1990), such that parasitoid growth is strongly tailored to host species- and stage-specific changes in the host’s endocrine milieu (Pennacchio and Strand 2006). Specialist herbivores are better adapted to plant-related traits such as secondary chemistry, and may even require plant allelochemicals to be present as cues for oviposition or as feeding stimulants (Schoonhoven et al. 2005). Moreover, specialist herbivores have evolved a range of finely-tuned mechanisms to deal with even high concentrations of plant secondary metabolites, adaptations which are often lacking in generalists (Awmack and Leather 2002; Schoonhoven et al. 2005). A consequence is that generalist herbivores—and perhaps their parasitoids—are less able to deal with high levels of plant secondary metabolites and must trade-off the ecological benefits of a broad diet against the physiological and developmental costs of dealing with plant toxins. As a result, the development of their endoparasitoids will also be closely linked by the physiological constraints in their hosts.
All of the parasitoid species studied here are synovigenic, meaning that females continue to mature eggs after adult eclosion (Flanders 1950; Jervis and Kidd 1986). In all species studied here, the egg loads increased after eclosion, although more in some species than in others. Egg production dynamics and maximum egg loads of the parasitoids differed to some extent between Cotesia and Microplitis species, as well as between the two more specialized Cotesia species and the others. In particular, initial and maximum egg loads were much lower in M. mediator and Microplitis sp., with adult females storing less than half of the ripe eggs in their oviducts than all of the other parasitoid species. On the other hand, maximum egg loads in C. rubecula were largest amongst the species tested. A suite of ecophysiological factors are considered to play an important role in the dynamics of egg production by parasitoids (Flanders 1950; Ellers et al. 2000; Jervis et al. 2001, 2008; Riddick 2006; Richard and Casas 2009, 2012). Host abundance is an important factor, with parasitoids attacking numerous, early stages of hosts (e.g. eggs, young larvae) expected to invest in the production of larger numbers of smaller eggs than parasitoids attacking more scarce later stages (e.g. older larvae and pupae [Price 1972; Pexton and Mayhew 2004; Jervis et al. 2008]).
Adult parasitoid longevity also varied significantly across the different species, with a clear tendency of an inverse relationship between this parameter and egg production. Female wasps in two of the four Microplitis species lived significantly longer than males, whereas in all three Cotesia species the difference in longevity was not significant. Our data suggests that there are possible trade-offs between investment in somatic and maintenance tissue, even amongst closely related koinobiont endoparasitoids, and further supports the argument that host ecology can drive micro-evolutionary changes in important life-history traits, even amongst very closely related species. The inability of Blackburn (1991a, b) and Mayhew and Blackburn (1999) to find many life-history correlations in parasitoids such as between size and longevity and reproduction at a large phylogenetic scale is, therefore, hardly surprising. Parasitoids are an immensely diverse and complex group of organisms, and as we have shown, many of these correlations do not even exist amongst species, such as those in the higher Microgastrinae, that share a common recent phylogenetic history.
The factors influencing the evolution of resource allocation to reproduction and maintenance in parasitoids has attracted considerable attention over the years (Rosenheim 1996; Ellers and van Alphen 1997; Sevenster et al. 1998; Rivero and Casas 1999; Ellers et al. 2000; Ellers and Jervis 2003; Desouhant et al. 2005; Pelosse et al. 2007, 2011; Le Lann et al. 2012; reviewed by Jervis et al. 2001, 2008, 2012). One of the most important factors that influence the evolution of trade-offs between maintenance and reproduction in parasitoids is host availability, particularly if this parameter is predictable in certain environments (Price 1972; Ellers et al. 2000; Jervis et al. 2008). We do not know how abundant or aggregated the various hosts are in nature, but this factor should be investigated to determine if this may play an important role in selecting for differences in maintenance and egg production in the different parasitoids studied here. Clearly our results show that these traits are not tightly conserved at the phylogenetic level, and that species- and habitat-related constraints have led to divergence in some of these traits even among species in the same genus. In this study we have identified important variation in life-history traits amongst closely related species in two genera of the higher Microgastrinae. One area that we have not explored, but would be profoundly interesting, is to measure genetic variation in these traits amongst different populations of each species in different habitats across their ranges. It has been well established for many years that organisms (plants and animals) exhibit genetic variation in the expression of various traits that enable them to become locally adapted to their environment (Kawecki and Ebert 2004). Given that the abundance of hosts may vary spatially and temporally, it is certainly likely that reproductive traits in parasitoids could vary intra-specifically in response to long-term selection, provided conditions remain predictable over time. A recent study by Vuarin et al. (2012) found that local populations of the koinobiont parasitoid Leptopilina heterotoma exposed to similar conditions exhibited differences in reproductive and somatic traits. They argued that these differences in traits in the parasitoid may be responses to local selective pressures, such as microclimate, microhabitats, or intensity of competition. Interestingly, they did not find significant differences in these traits from populations in contrasting environments. It is clear that much more research needs to be done in this area in order to determine the extent of intra-specific trait-mediated variation in response to divergent selection pressures in parasitoids.
The late pioneering parasitoid biologist George Salt once famously wrote, that, “…far from being a purely passive victim, obliterated without a trace, the host is often able to impress it’s mark, and a very clear mark, upon the insect parasitoid that destroys it” (Bateson 2003). In comparing various life history, development and reproductive traits amongst closely related parasitoid species in two ‘sister’ genera, we can verify Salt’s observation. Our findings suggest that phylogeny plays an important role in the conservation of certain traits in parasitoids, but that evolutionary processes generated from the local environment ‘fine-tune’ these traits even further down to the species level. Therefore, although broad phylogenetic comparisons are of some utility in establishing and better understanding some evolutionary relationships at the broader taxonomic level, more studies should aim to explore life-histories amongst closely related species, or even within species. Intra- and interspecific competition can drive speciation in parasitoids (Hood et al. 2012). Therefore, adaptive syndromes may emerge at much smaller scales and in more ecologically refined settings than was previously thought.
References
Askew, R. R., & Shaw, M. R. (1986). Parasitoid communities: their size, structure and development. In J. Waage & D. Greathead (Eds.), Insect parasitoids (pp. 225–264). London, UK: Academic Press.
Awmack, C. S., & Leather, S. R. (2002). Host plant quality and fecundity in herbivorous insects. Annual Review of Entomology, 47, 817–844.
Bateson, P. (2003). George Salt. 12(December), pp. 1903–17, February 2003. Biological Memoirs of Fellows of the Royal Society, 49, 447–459.
Benrey, B., & Denno, R. F. (1997). The slow growth-high mortality hypothesis: A test using the Cabbage Butterfly. Ecology, 78, 987–999.
Blackburn, T. M. (1991a). A comparative examination of life-span and fecundity in parasitoid Hymenoptera. Journal of Animal Ecology, 60, 151–164.
Blackburn, T. M. (1991b). Evidence for a ‘fast-slow’ continuum of life-history traits among parasitoid Hymenoptera. Functional Ecology, 5, 65–74.
Boivin, G. (2012). Sperm as a limiting factor in mating success in Hymenoptera parasitoids. Entomologia Experimentalis et Applicata, 146, 149–155.
Brodeur, J., & Boivin, G. (2004). Functional ecology of immature parasitoids. Annual Review of Entomology, 49, 27–49.
Charnov, E. L. (1982). The theory of sex allocation. Princeton, NJ: Princeton University Press.
Desouhant, E., Driessen, G., Amat, I., & Bernstein, C. (2005). Host and food searching in a parasitic wasp Venturia canescens: a trade-off between current and future reproduction. Animal Behaviour, 70, 145–152.
Dinnage, R., Cadotte, M. W., Haddad, N. M., Crutsinger, G. M., & Tilamn, D. (2012). Diversity of plant evolutionary lineages promotes arthropod diversity. Ecology Letters, 15, 1308–1317.
Ehrlich, P. R., & Raven, P. H. (1964). Butterflies and plants—a study in coevolution. Evolution, 18, 586–608.
Ellers, J., & Jervis, M. A. (2003). Body size and the timing of egg production in parasitoid wasps. Oikos, 102, 164–172.
Ellers, J., Sevenster, J. A., & Driessen, G. (2000). Egg load evolution in parasitoids. American Naturalist, 156, 650–665.
Ellers, J., & van Alphen, J. J. M. (1997). Life history evolution in Asobara tabida: plasticity in allocation of fat reserves to survival and reproduction. Journal of Evolutionary Biology, 10, 771–785.
Flanders, S. E. (1950). Regulation of the ovulation and egg disposal in the parasitic Hymenoptera. Canadian Entomologist, 82, 134–140.
Ghiselin, M. T. (1974). The economy of nature and the evolution of sex. Berkeley: University of California Press.
Godfray, H. C. J. (1994). Parasitoids : Behavioral and evolutionary ecology. Princeton: Princeton University Press.
Harvey, J. A. (2005). Factors affecting the evolution of development strategies in parasitoid wasps: The importance of functional constraints and incorporating complexity. Entomologia Experimentalis et Applicata, 117, 1–13.
Harvey, J. A., Harvey, I. F., & Thompson, D. J. (1994). Flexible larval growth allows use of a range of host sizes by a parasitoid wasp. Ecology, 75, 1420–1428.
Harvey, J. A., & Strand, M. R. (2002). The developmental strategies of endoparasitoid wasps vary with host feeding ecology. Ecology, 83, 2439–2451.
Harvey, J. A., & Strand, M. R. (2003). Sexual size and development time dimorphism in a parasitoid wasp: An exception to the rule? European Journal of Entomology, 100, 485–492.
Hood, G. R., Egan, S. P., & Feder, J. L. (2012). Interspecific competition and speciation in parasitoids. Evolutionary Biology, 39, 219–230.
Hurlbutt, B. (1987). Sexual size dimorphism in parasitoid wasps. Biological Journal of the Linnean Society, 30, 63–89.
Janz, N. (2011). Ehrlich and Raven revisited: Mechanisms underlying codiversification of plants and enemies. Annual Review of Ecology Evolution and Systematics, 42, 71–89.
Jervis, M. A., Ellers, J., & Harvey, J. A. (2008). Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annual Review of Entomology, 53, 361–385.
Jervis, M. A., Heimpel, G. E., Ferns, P. N., Harvey, J. A., & Kidd, N. A. C. (2001). Life-history strategies in parasitoid wasps: A comparative analysis of “ovigeny”. Journal of Animal Ecology, 70, 442–458.
Jervis, M. A., & Kidd, N. A. C. (1986). Host-feeding strategies in hymenopteran parasitoids. Biological Reviews, 61, 395–434.
Jervis, M. A., More, A., & Heimpel, G. E. (2012). The evolution of parasitoid fecundity: A paradigm under scrutiny. Ecology Letters, 15, 357–364.
Kawecki, T. J., & Ebert, D. (2004). Conceptual issues in local adaptation. Ecology Letters, 7, 1225–1241.
Kellermann, V., Overgaard, J., Hoffmann, A. A., Fløjgaard, C., Svenning, J. C., & Loeschcke, V. (2012). Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proceedings of the National Academy of Sciences, 109, 16228–16233.
King, B. H. (1989). Host-size dependent sex ratios among parasitoid wasps: Does host size matter? Oecologia, 78, 420–426.
Lawrence, P. O. (1990). The biochemical and physiological effects of insect hosts on the development and ecology of their parasites: an overview. Archives of Insect Biochemistry and Physiology, 13, 217–228.
Le Lann, C., Visser, B., van Baaren, J., van Alphen, J. J. M., & Ellers, J. (2012). Comparing resource exploitation and allocation of two closely related aphid parasitoids sharing the same host. Evolutionary Ecology, 26, 79–94.
Mackauer, M. (1996). Sexual-size dimorphism in parasitoid wasps: Influence of host quality. Oikos, 76, 265–272.
Mackauer, M., & Sequeira, R. (1993). Patterns of development in insect parasites. In N. E. Beckage, S. N. Thompson, & B. A. Federici (Eds.), Parasites and pathogens of insects (Vol. 1, pp. 1–23). New York: Academic Press.
Mayhew, P. J., & Blackburn, T. N. (1999). Does development mode organize life history traits in the parasitoid Hymenoptera? Journal of Animal Ecology, 68, 906–916.
McKitrick, M. C. (1993). Phylogenetic constraint in evolutionary theory: has it any explanatory power? Annual Review of Entomology, 24, 307–330.
Murphy, N., Banks, J. C., Whitfield, J. B., & Austin, A. D. (2008). Phylogeny of the parasitic microgastroid subfamilies (Hymenoptera : Braconidae) based on sequence data from seven genes, with an improved time estimate of the origin of the lineage. Molecular Phylogenetics and Evolution, 47, 378–395.
Pelosse, P., Bernstein, C., & Desouhant, E. (2007). Differential energy allocation as an adaptation to different habitats in the parasitic wasp Venturia canescens. Evolutionary Ecology, 21, 669–685.
Pelosse, P., Jervis, M. A., Bernstein, C., & Desouhant, E. (2011). Does synovigeny confer reproductive plasticity upon a parasitoid wasp that is faced with variability in habitat richness? Biological Journal of the Linnean Society, 104, 621–632.
Pennacchio, F., & Strand, M. R. (2006). Evolution of developmental strategies in parasitic Hymenoptera. Annual Review of Entomology, 51, 233–258.
Pexton, J. J., & Mayhew, P. J. (2004). Competitive interactions between parasitoid larvae and the evolution of gregarious development. Oecologia, 141, 179–190.
Price, P. W. (1972). Parasitoids utilizing the same host: adaptive nature of differences in size and form. Ecology, 53, 190–195.
Price, P. W. (1973). Reproductive strategies in parasitoid wasps. American Naturalist, 107, 684–693.
Quicke, D. L. J., Shaw, M. R., Takahashi, M., & Yanechin, B. (2004). Cocoon silk chemistry of non-cyclostome Braconidae, with remarks on phylogenetic relationships within the Microgastrinae (Hymenoptera : Braconidae). Journal of Natural History, 38, 2167–2181.
Richard, R., & Casas, J. (2009). Stochasticity and controllability of nutrient sources in foraging: Host-feeding and egg resorption in parasitoids. Ecological Monographs, 79, 465–483.
Richard, R., & Casas, J. (2012). A quantitative framework for ovarian dynamics. Functional Ecology, 26, 1399–1408.
Riddick, E. W. (2006). Egg load and body size in lab-cultured Cotesia marginiventris. BioControl, 51, 603–610.
Rivero, A., & Casas, J. (1999). Rate of nutrient allocation to egg production in a parasitic wasp. Proceedings of the Royal Society of London B, 266, 1169–1174.
Rosenheim, J. A. (1996). An evolutionary argument for egg limitation. Evolution, 50, 2089–2094.
Schoonhoven, L. M., van Loon, J. J. A., & Dicke, M. (2005). Insect-plant biology (2nd ed.). United Kingdom: Oxford University Press.
Sequeira, R., & Mackauer, M. (1992). Covariance of adult size and development time in the parasitoid wasps Aphidius ervi in relation to its host, Acyrthosiphum pisum. Evolutionary Ecology, 6, 34–44.
Sevenster, J. G., Ellers, J., & Driessen, G. (1998). An evolutionary argument for time limitation. Evolution, 52, 1241–1244.
Shaw, M. R. & Huddleston, T. (1991). Classification and biology of braconid wasps. Royal Entomological Society of London.
Shorey, H. H., & Hale, R. L. (1965). Mass-rearing of larvae of nine noctuid species on a simple artificial medium. Journal of Economic Entomology, 58, 522–524.
Slansky, F, Jr. (1986). Nutritional ecology of endoparasitic insects and their hosts: An overview. Journal of Insect Physiology, 32, 255–261.
Stearns, S. C. (1992). The evolution of life-histories. Oxford, UK: Oxford University Press.
Vinson, S. B., & Iwantsch, G. F. (1980). Host regulation by insect parasitoids. Quarterly Review of Biology, 55, 143–165.
Vuarin, P., Allemand, R., Moiroux, J., van Baaren, J., & Gibert, P. (2012). Geographic variations of life history traits and potential trade-offs in different populations of the parasitoid Leptopilina heterotoma. Natuurwissenschaften, 99, 903–912.
Whitfield, J. B. (1997). Molecular and morphological data suggest a common origin for the polydnaviruses among braconid wasps. Naturwissenschaften, 84, 502–507.
Acknowledgments
The authors wish to thank the lab of Ted Turlings at Neuchâtel University, Switzerland, for kindly supplying S. littoralis, C. marginiventris and Microplitis mediator; Toshiharu Tanaka at Nagoya University kindly supplied Microplitis sp and its host M. separata. Workspace was provided by Gregor Disveld and insect cultures were maintained by Roel Wagenaar. Hans Smid, Nina Fatouros and Tibor Bukovinszky at ‘Bugs-in-the-Picture’ kindly supplied photographs of C. rubecula, C. vestalis and M. mediator and M. rufiventris; Mike Strand and Jena Johnson at University of Georgia kindly supplied the photograph of M. demolitor, and Koppert Bv. Ltd. (Rotterdam) kindly supplied the photograph of C. marginiventris.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harvey, J.A., Visser, B., Le Lann, C. et al. Convergence and Divergence in Direct and Indirect Life-History Traits of Closely Related Parasitoids (Braconidae: Microgastrinae). Evol Biol 41, 134–144 (2014). https://doi.org/10.1007/s11692-013-9253-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-013-9253-4