Abstract
It is well known that there is a strong relationship among the environment, selection, and extinction, but the underlying role of genetics and genetic constraints in contributing to extinction is less appreciated. Integration of characters may enhance survivability for species, providing that selective pressure is parallel with the patterns of morphological integration. However, we hypothesize that, if the direction of selection shifts, integration may also prevent populations from responding quickly enough to the new directions of selection. This would lead to the inability to find a successful adaptive solution, causing downward pressure on the population, and ultimately, extinction. We test this model with a computer simulation, using an adaptive landscape model. We generate populations of varying levels of multivariate integration and generate selection pressures to test the ability of the populations to respond to selection both parallel and orthogonal to the axis of maximum variation. In these simulations, more highly integrated populations survived longer when selection was in the direction of maximum variation. However, when selection was closer to orthogonal to the axis of maximum variation, extinction was more rapid in highly integrated populations. These results suggest that integration may play a strong role in both survivability and extinction. Tightly integrated populations are highly persistent when selection pressure is close to the axis of maximum variation, which is expected to frequently be the case since integration is likely often a product of selection. However, these highly integrated taxa are more susceptible to extinction when the direction of selection shifts, and is closer to orthogonal to the axis of maximum variation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
That environmental change plays a role in evolutionary transitions has been long known (Simpson 1953; Wright 1949). However, the complex interplay of environment, developmental mechanisms, and phenotypic expression, all of which play a role in extinction, is less well-understood (Berger and Lynch 1995; Lande and Shannon 1996; Gomulkiewicz and Holt 1995; Chevin et al. 2010). One of the key elements in biology on which evolutionary change rests is population variation (Darwin 1859; Maynard Smith 1976; Gomulkiewicz and Houle 2009). Selection requires sufficient variation in a population so that some part of the gene pool can survive to reproduce. This is especially the case when strong selection is acting on a species and only particular anatomical solutions may exist to the environmental circumstances. If the variation in a population is too limited, there may not be a sufficient number of individuals with phenotypes that successfully respond to the environment, and the species may face extinction (Maynard Smith 1976; Kellermann et al. 2009).
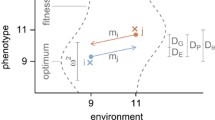
Morphological integration, which constrains elements of population variation by warping the overall pattern so that it falls along particular axes, also serves to facilitate evolutionary change. Selection requires variation, but in some circumstances the constraint of integration can be seen as enhancing a species’ ability to respond to selection pressure (Cheverud 1984; Berger 1986; Chevin et al. 2010). If individual characters are under independent genetic controls, any complex morphological change would require multiple independent genetic mutations, and as the number of independent genes required for a morphological change increases, the probability of any coordinated morphological change, even under selection pressure, decreases (Riedl 1978; Cheverud 1984). Therefore, pleiotropy and morphological integration facilitate the evolution of complex traits (Cheverud 1984, 1996; Wagner 1988; Hansen 2003). Perhaps more importantly, pleiotropy can increase the ability of a species to rapidly evolve in a particular direction to meet the demands of selection. Using the language of the adaptive landscape (Wright 1932), a shift to a particular pattern of integration would be advantageous if adaptive peaks fall along a line in the landscape (Wagner 1988). As environmental circumstances change, integration of traits would allow the ability to shift rapidly along a specific adaptive ridge (Hansen 2003), as there would be maximal variation on which selection could operate (Fig. 1a).
a, b (following Klingenberg 2004)—The morphological integration of characters X and Y facilitates their evolution along the red vector, which describes the maximum axis of variation. As long as the direction of selection is along the vector of maximum variation, species A can rapidly shift toward the adaptive peak. There is relatively little variation perpendicular to the red vector, so if there is strong selection pressure in that direction (a grade shift), there is little variation on which selection can act (Fig. 3). If the selection pressure along any direction orthogonal to the main axis is strong enough, the species will suffer extinction (Color figure online)
There appear to be multiple causes for morphological integration (Hallgrimson et al. 2009). Phenotypic integration may appear even if the traits are under independent genetic controls, if coordinated patterns of selection are present (Klingenberg 2004). Due to the adaptive advantages listed above, integration may also be the product of selection (Cheverud 1984; Wagner 1988). In this vein, Lande (1979) suggested that the patterns of past selection could be inferred from these structures of population covariances (see Pachut 1992 for a paleontological example). Finally, integration is often expected to be the product of highly conserved regulatory architecture (Schluter 1996; Shubin and Wake 1996). For example, fore to hind limb patterns of integration may be closely linked, as serial homologs under the influence of deeply primitive regulatory genes (Schmidt and Fischer 2009; Young and Hallgrimsson 2005).
Schluter (1996) argued that evolution often follows ‘genetic lines of least resistance’. In a multivariate sense, these lines of least resistance are the axes of maximum trait variation along which evolution may easily drive population means, likely a product of directional and stabilizing selection (Wagner 1996). The first eigenvector of a population is this line of maximum variation. Schluter (1996) found the evolution of sticklebacks, sparrows, finches, flycatchers, and mice to follow this line of least resistance, and Marroig and Cheverud (2005, 2010) found that craniofacial variables in New World monkeys followed similar patterns.
The origins of these patterns of integration are likely to influence the ability of a population to respond to different directions of selection (Lande 1979). Traits that show patterns of integration, yet are under independent genetic control, are likely to be highly evolvable, since the traits are not genetically coupled. But traits that are under highly conserved pleiotropic regulation are less likely to be able to vary independently, even under stronger selection pressure (see Berger 1986 for a review of early debates over evolvability and constraint). The paradoxical relationship between variation and integration is highlighted when examining integration in the historical context, as patterns of adaptive integration, which facilitated evolution at one point, may later constrain evolution when selective patterns shift.
Fundamental to understanding the limits of adaptive potential are the concepts of evolvability and constraint. There are multiple concepts applied to the term ‘evolvability’ (Klingenberg 2004; Pigliucci 2008). Whereas it has been used to refer to the mutational potential of genetic architecture (Wagner and Alternberg 1996), it is also used in a very specific quantitative sense to indicate the ability of a population (and its gene pool) to respond to random selection vectors (Hansen and Houle 2008). A constraint is simply anything that limits the ability of a population to respond to selection, and while this typically refers to genetic limitations (e.g. Gomulkiewicz and Houle 2009), it also may refer to broader physical limitations, such as biomechanical constraints due to body size (Berger 1986; Maynard Smith et al. 1985; Wroe and Milne 2007).
The complete absence of variation in phenotypic space is an obvious constraint on evolutionary potential (the “absolute constraint” of Mezey and Houle 2005), but any limitation on a population’s ability to respond to selection, even if modest, can equally be considered a degree of constraint (Gomulkiewicz and Houle 2009). In many circumstances, modest constraint (“quantitative constraint”, Mezey and Houle 2005) will have relatively little effect if selection in the direction of the constraint is also modest, and over a long period of time (Gomulkiewicz and Houle 2009).
If pleiotropy is often the result of selection (Cheverud 1984), the direction of selection will often fall along the axis of greatest variation (Jones et al. 2007). However, if selection pressure on a highly integrated population shifts, and moving along the first eigenvector is no longer selected, the patterns of integration would no longer fall in line with the direction of selection. Under these circumstances, in highly integrated populations there would be relatively little variation available for selection (Fig. 1b). Gomulkiewicz and Houle (2009) have proposed a general model for the role of constraints in evolution that incorporates such factors as population demography, strength of selection, time, and the patterns of genetic covariance. One expectation of their model is that, under identical levels of selection pressure, selection along these minor axes is more likely to result in population decrease or extinction as a result of the limitations on variation.
The structure of multivariate variation is such that the greater the first eigenvalue, the smaller the others will necessarily be. A logical implication of this mathematical relationship is that more integrated populations, with more variation in the first eigenvector, will also have less variation along other axes to respond to selection (Lande 1979; Berger 1986; Schluter 1996; Gomulkiewicz and Houle 2009). This, then, means that highly integrated populations are at higher risk for extinction when the direction of selection deviates from the first eigenvector.
Here we propose to test, using a computer simulation approach, the hypothesis that integration of morphological traits has an effect on rates of extinction. Our expectation is that, if the adaptive peak falls along the main axis of variation, the more integrated population will be able to persist under strong selection pressure; however if the adaptive peak does not fall along the main axis but closer to perpendicular to it, more highly integrated populations will go extinct more rapidly.
Methods
We tested this hypothesis using computer simulations of population growth and multivariate selection in multiple directions. We generated ‘populations’ with four quantitative characters. The data for the four characters were drawn from (Gaussian) normally-distributed data with standardized parameters (x = 0, σ = 1). Covariance matrices were calculated and were subjected to eigen analysis. Eigenvectors and eigenvalues were derived and variances for the eigenvalues (VE) of each population were calculated.
Selection along the axis of maximum variance was compared with other orthogonal directions of selection. The axis of maximal variance is derived using eigen analysis, and is represented by the first principal component. Other orthogonal axes are represented by the other PCs. We expect successful response to selection along the first PC to correlate with the overall level of integration, as quantified by the variance of eigenvalues (Pavlicev 2009), but successful response to selection along other PCs to inversely correlate with the variance of eigenvalues. The metric for the response to selection is the number of generations before extinction, with more successful populations lasting longer before extinction.
Selection was simulated, following on the principles of Jones et al. (2004) by ‘culling’ individuals in the population based on a probability (P) determined by the individual’s (z) distance from the adaptive peak (θ):
with c as a constant representing the strength of overall selection and n as a random number between 0 and 1. Each generation, every individual in the population is subject to selection following this algorithm, and if an individual is selected against (“culled”), it is no longer used to calculate population parameters, for population size, mean population value, or for reproduction. This algorithm is designed to produce an increasing level of selective pressure towards the adaptive peak each succeeding generation, with pressure greatest further from the peak. The population increases its population by reproduction, which is fixed at a 0.5 % growth rate, per generation. New individuals are drawn from a normal distribution centered on the current population mean, using the standard deviation of the current population (remaining after selection). The strength of selection (c) was set to be slightly stronger than the reproduction rate so that, even though the mean does shift towards the adaptive peak, all populations eventually went extinct in this simulation (Fig. 2) before reaching the peak. To examine the effects of other parameters on extinction, population size and distance from adaptive peak were varied in the model (in this model, distance from the adaptive peak factors into the overall strength of selection). It is important to emphasize that these model populations had a fixed covariance structure, so no mutations that could relax or increase integration were available to them.
Growth curve for populations under this simulation model. The red line represents population growth unaffected by selection, whereas the blue population is the population under selection, as modeled here. Population growth is unconstrained until the first (a) arrow, at which point selection has started. The second (b) arrow indicates extinction (Color figure online)
We calculated several parameters for these populations, including the VE, variance along all PCs, and the number of generations before extinction for all selection simulations. We expect that populations with greater VE (more integrated species) will persist longer when selection is along PC1 than populations with lower variances of eigenvalues. Conversely, when the vector of selection is orthogonal to the axis of greatest variation, or along a random vector, we expect that populations with higher VE will go extinct sooner than those with lower variances of eigenvalues.
Results
There appears is a strong relationship between the variance of eigenvalues and survivability when selection acts along the axis of greatest variation (Fig. 3). Populations with higher variances of eigenvalues survived for more generations than those that were less integrated (Table 1; Fig. 3a). However, when selection was orthogonal to the axis of greatest variation, populations that were less integrated persisted longer, while more integrated populations became extinct sooner (Table 1; Figs. 4, 5).
a–d Correlation between the variance of eigenvalues and the number of generations before extinction under the selection model here, for each of the axes of variation. For the axis of maximal variation, PC1 (Fig. 4a), there is a strong positive correlation between the variance of eigenvalues and the number of generations until extinction, but for the other axes (Fig. 4b–d), there are strong negative correlations. This suggests that highly integrated populations respond well to selection along the axis of maximum variation, but poorly to selection perpendicular to that axis
Test of the effects of varying population size (PC1 in blue, PC2 in red). Starting population size affected the number of generations for each population before extinction (left), but had no effect on the relationship between integration (as measured by the VE) and the evolvability of the populations (here for clarity we show the correlation between the VE and the 1st and 2nd PC scores, but the patterns for PC3 and PC4 were the same seen in PC2) (Color figure online)
The effect of varying the distance from the adaptive peak (PC1 in blue, PC2 in red). The distance to the peak affected the strength of selection, so this factor affected survivability for populations under any axis of selection (PC1: r = −0.9907, p = 0.000; PC2: r = −0.8721, p = 0.00000001). When examining the effect of distance on the correlations between the VE and the number of generations until extinction, the results are highly significant for both eigenvectors (PC1: r = −0.5729, p = 0.0014; PC2: r = 0.9059, p = 0.000). However, when overall survivability (the sum of populations under selection pressure along and orthogonal to the 1st PC) was controlled using residual analysis, these effects disappeared (PC1: r = −0.1425, p = 0.4696; PC2: r = 0.0777, p = 0.7121). As in Fig. 4 the correlation shown are between the VE and the 1st and 2nd PC scores, but PC3 and PC4 have similar results to PC2) (Color figure online)
The effect of population size behaved in a predictable ways and appeared not to have any impact on the relationship between integration and extinction. When sample size was tested (populations ranging from 50 to 1,000 in increments of 50) there was similarly no effect on the relationship between integration and evolvability, although larger populations had more outliers and survived longer (Fig. 4).
However, when the effect of distance of the population from the adaptive peak was examined, the results were more complex, although ultimately resulting in a similar conclusion (Fig. 5). The further the population was from the adaptive peak when selection started, the stronger the relationship between the VE and the survival of the population when selection was along eigenvector 2, but the effect was reversed for eigenvector 1 (Fig. 5b). Further from the adaptive peak, the populations were less likely to show the strong positive effect of persistence under selection pressure along the first eigenvector but a greater effect along eigenvector 2. However, the relationships between the VE and population persistence (whether positive or negative) were always highly significant. So, in effect, even though overall survival was hampered by the strong selection pressure created by the greater distance (Fig. 5a), the correlation between the VE and population persistence was strongly maintained. When the effect of the overall rate of survival was controlled by regressing the correlations for each distance (as in Fig. 5b) against the overall rate of survival at that distance (the total survival rate of populations under selection with and orthogonal to the first principal component—the sum of values on the vertical axis at each distance increment in Fig. 5a), the effect disappeared, and the residuals showed no correlation between the distance and the residuals (Fig. 5c).
Discussion and Conclusion
The results indicate that there is a clear relationship between integration and the survivability of a species, and that the critical factor in highly integrated populations is the direction of selection. Even under extremely strong selection pressure, when selection was in the direction of the axis of maximum variation, the population was able to persist for many generations before going extinct. However, when selection pressure was orthogonal to the pattern of maximum variation, extinction was rapid, and more rapid in more highly integrated populations. Consistent with what we might expect based on Gomulkiewicz and Houle (2009), this effect was much stronger in more integrated populations.
These results may appear to contradict, to some extent, the results of Hansen (2003), who found that moderate pleiotropy increased overall variation, as variation of the third pleiotropic gene ‘added’ the variation of that gene. In a two-trait study, he found that if each gene controlling a single trait possessed 42 % of the total variation, and the pleiotropic gene had 16 % of the variation, evolvability was maximized. He did not examine the patterns of evolvability for more than two traits, but he did find that, as the correlation due to pleiotropy increased above this level, evolvability approached zero. So high levels of integration do act as significant constraints. However, with regard to the present study, the results cannot be directly compared to Hansen (2003), as the genetic patterns for each population were treated as fixed; pleiotropic effects were constant and no variation was ‘added’ as the effect of a pleiotropic gene.
The survivability of a species is determined by a variety of external factors, but there are a host of intrinsic factors that influence the likelihood of a species going extinct when faced with new external pressures, such as reproduction rate (Gomulkiewicz and Holt 1995; Chevin et al. 2010) and adaptive specialization (Cody 1966; Huey and Hertz 1984). The relationship between evolvability and species survival is generally not as well understood, but key among the intrinsic factors is the ability to adapt from one fitness peak to another (Futuyma 2010; Wagner and Alternberg 1996). If species cannot evolve rapidly enough when selection factors change, they face the danger of extinction (Orr and Unkless 2008; Gomulkiewicz and Houle 2009). Therefore, constraints on variation can enhance survivability by speeding evolutionary changes along particular adaptive trajectories (Huey and Hertz 1984; Rice 1998). If there is, for example, selection pressure for longer limbs, the integration of these traits ensures that the organism can respond. However, if a local fitness maximum cannot be found along the adaptive trajectory, then the integration may contribute to population reduction or extinction. More variable (and less integrated) species are less likely to go extinct than less variable ones when external selection forces dramatically change direction, because there is more of a likelihood that somewhere in the population is a morph that can survive and reproduce in the new circumstances (Pavlicev et al. 2010). Interestingly, there may be some relationship between adaptive specialization and constrained variation; niche specialization may act to limit ranges, contributing to the limiting of gene flow (Simpson 1953; Watkins 2001), or stabilizing selection may be strong enough to restrict the genetic variation to one or a very few alleles.
If evolutionary pressures select for morphological integration Cheverud (1984, 1996), then integration is one of the key elements affecting the survivability of a species. It is clear that integration is essential to maintaining functional equivalence in organisms of varying size and shape. Canids and felids, for example, need to have a (relatively) fixed ratio of fore to hindlimb length, so locomotor efficiency is retained irrespective of the size of the individual in a population (Schmidt and Fischer 2009). This integration ensures that changes along particular axes can occur rapidly (Hansen 2003). In the adaptive landscape, this axis would form a ridge with peaks along the ridge at specific adaptive maxima. However, some mammalian orders appear to be more evolvable with regard to limb proportion; primates, for example, have differing levels of integration of the limbs and dramatically different proportion of limb element length, depending on the locomotor pattern (Rolian, 2009; Villmoare et al. 2011; Young et al. 2010). This means that the adaptive peaks do not fall along any particular ridge, and that a taxon must be more evolvable to find those peaks.
Integration, therefore, only enhances survivability when the adaptive peaks fall along the axis of morphological integration. Since integration is likely often (but not always) to be the result of selection on a particularly successful combination of trait expression (Cheverud 1984, 1996), we expect that this is often the case. And the more highly integrated the traits, the more quickly it can respond to selection along that axis. However, if selection shifts and is no longer along this axis of integration, it appears that integration works to limit the variation on which selection can act. In these circumstances, integration contributes to extinction. It appears that integration may have been a significant constraint on the evolvability and survivability of populations throughout evolutionary history.
References
Berger, R. (1986). Constraints for the evolution of functionally coupled characters: A nonlinear analysis of a phenotypic model. Evolution, 40, 182–193.
Berger, R., & Lynch, M. (1995). Evolution and extinction in a changing environment: A quantitative-genetic analysis. Evolution, 49, 151–163.
Cheverud, J. (1984). Quantitative genetics and developmental constraints on evolution by selection. Journal of Theoretical Biology, 110, 155–171.
Cheverud, J. (1996). Developmental integration and the evolution of pleiotropy. American Zoologist, 36, 44–50.
Chevin, L., Lande, R., & Mace, G. (2010). Adaptation, plasticity, and extinction in a changing environment: Towards a predictive theory. PLoS Biology, 8, e1000357.
Cody, M. (1966). A general theory of clutch size. Evolution, 20, 174–184.
Darwin, C. (1859). On the origin of species. London: John Murray.
Futuyma, D. (2010). Evolutionary constraint and ecological consequences. Evolution, 64(7), 1865–1884.
Gomulkiewicz, R., & Holt, R. (1995). When does evolution by natural selection prevent extinction? Evolution, 49, 201–207.
Gomulkiewicz, R., & Houle, D. (2009). Demographic and genetic constraints on evolution. The American Naturalist, 174, E218–E229.
Hallgrimson, B., Jamniczky, H., Young, N., Rolian, C., Parsons, T., Boughner, J., et al. (2009). Deciphering the palimpsest: Studying the relationship between morphological integration and phenotypic covariation. Evolutionary Biology, 36, 355–376.
Hansen, T. (2003). Is modularity necessary for evolvability? Remarks on the relationship between pleiotropy and evolvability. Biosystems, 69, 83–94.
Hansen, T., & Houle, D. (2008). Measuring and comparing evolvability and constraint in multivariate characters. Evolutionary Biology, 21, 1201–1219.
Huey, R., & Hertz, P. (1984). Is a jack-of-all-temperatures a master of none? Evolution, 38, 441–444.
Jones, A., Arnold, S., & Bürger, R. (2004). Evolution and stability of the G-Matrix on a landscape with a moving optimum. Evolution, 58, 1639–1654.
Jones, A., Arnold, S., & Bürger, R. (2007). The mutation matrix and the evolution of evolvability. Evolution, 61, 727–745.
Kellermann, V., van Heerwaarden, B., Sgrò, C., & Hoffmann, A. (2009). Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science, 325, 1244–1246.
Klingenberg, C. (2004). Integration, modules, and development: Molecules to morphology to evolution. In M. Pigliucci & K. Preston (Eds.), Phenotypic integration: Studying the ecology and evolution of complex phenotypes (pp. 213–230). New York: Oxford University Press.
Lande, R. (1979). Quantitative genetic analysis of multivariate evolution, applied to brain: Body-size allometry. Evolution, 33, 402–416.
Lande, R., & Shannon, S. (1996). The role of genetic variation in adaptation and population persistence in a changing environment. Evolution, 50, 434–437.
Marroig, G., & Cheverud, J. (2005). Size as a line of least evolutionary resistance: Diet and adaptive morphological variation in New World monkeys. Evolution, 59, 1128–1142.
Marroig, G., & Cheverud, J. (2010). Size as a line of least resistance II: Direct selection on size or correlated response due to constraints? Evolution, 64, 1470–1488.
Maynard Smith, J. (1976). What determines the rate of evolution? The American Naturalist, 110, 331–338.
Maynard Smith, J., Burian, R., Kauffman, S., Alberch, P., Campbell, J., Goodwin, B., et al. (1985). Developmental constraints and evolution: A perspective from the Mountain Lake conference on development and evolution. The Quarterly Review of Biology, 60, 265–287.
Mezey, J. G., & Houle, D. (2005). The dimensionality of genetic variation for wing shape in Drosophila melanogaster. Evolution, 59, 1027–1038.
Orr, H., & Unkless, R. (2008). Population extinction and the genetics of adaptation. The American Naturalist, 172, 160–169.
Pachut, J. (1992). Morphological integration and covariance during astogeny of an ordovician trepostome bryozoan from communities of different diversities. Journal of Paleontology, 66, 750–757.
Pavlicev, M. (2009). Measuring morphological integration using eigenvalue variance. Evolutionary Biology, 36, 157–170.
Pavlicev, M., Cheverud, J., & Wagner, G. (2010). Evolution of adaptive phenotypic variation patterns by direct selection for evolvability. Proceedings of the Royal Society B. doi:10.1098/rspb.2010.2113.
Pigliucci, M. (2008). Is evolvability evolvable? Nature Reviews Genetics, 9, 75–82.
Rice, S. (1998). The evolution of canalization and the breaking of von Baer’s law: Modeling the evolution of development with epistasis. Evolution, 52, 647–656.
Riedl, R. (1978). Order in living organisms: A systems analysis of evolution. New York: Wiley.
Rolian, C. (2009). Integration and evolveability in primate hands and feet. Evolutionary Biology, 36, 100–117.
Schluter, D. (1996). Adaptive radiation along genetic lines of least resistance. Evolution, 50, 1766–1774.
Schmidt, M., & Fischer, M. (2009). Morphological integration in mammalian limb proportions: Dissociation between function and development. Evolution, 63(3), 749–766.
Shubin, N., & Wake, D. (1996). Phylogeny, variation, and morphological integration. American Zoologist, 36, 51–60.
Simpson, G. (1953). The meaning of evolution. New Haven: Yale University.
Villmoare, B., Fish, J., & Jungers, W. (2011). Selection, integration, and strepsirrhine locomotor adaptations. Evolutionary Biology, 38, 88–99.
Wagner, G. (1988). The influence of variation and of developmental constraints on the rate of multivariate phenotypic evolution. Journal of Evolutionary Biology, 1, 45–66.
Wagner, G. (1996). Homologues, natural kinds and the evolution of modularity. American Zoologist, 36, 36–43.
Wagner, G., & Alternberg, L. (1996). Complex adaptations and the evolution of evolvability. Evolution, 50, 967–976.
Watkins, T. (2001). A quantitative genetic test of adaptive decoupling across metamorphosis for locomotor and life-history traits in the pacific tree frog, Hyla regilla. Evolution, 55, 1668–1677.
Wright, S. (1932). The roles of mutation, inbreeding, crossbreeding, and selection in evolution. In Proceedings of the sixth international congress on genetics (pp. 355–366). Reprinted in William B. Provine (1986), Sewall wright: evolution: selected papers (pp. 161–177). Chicago: University of Chicago Press.
Wright, S. (1949). Population structure in evolution. Proceedings of the American Philosophical Society, 93, 471–478.
Wroe, S., & Milne, N. (2007). Convergence and remarkably consistent constraint in the evolution of carnivore skull shape. Evolution, 65, 1251–1260.
Young, N., & Hallgrimsson, B. (2005). Serial homology and the evolution of mammalian limb covariation structure. Evolution, 59(12), 269.
Young, N., Wagner, G., & Hallgrimson, B. (2010). Development and the evolvability of human limbs. Proceedings of the National Academy of Sciences of the United States of America, 107, 3400–3405.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Villmoare, B. Morphological Integration, Evolutionary Constraints, and Extinction: A Computer Simulation-Based Study. Evol Biol 40, 76–83 (2013). https://doi.org/10.1007/s11692-012-9186-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-012-9186-3