Abstract
Genetic theories of sexual selection predict that most ornamental secondary sexual traits provide reliable indication of the genetic quality of their bearers. Accordingly, also the offspring of mates with elaborate mating display should perform better than those of less conspicuous counterparts. In this study, we used Arctic charr (Salvelinus alpinus) as a model species to investigate whether the variation in a carotenoid-based red breeding coloration (a sexually dichromatic trait) in different sexes would reflect differences in individual genetic variability, one measure of individual quality, and/or indirectly, be manifested in variation in the offspring’s early viability and growth. We created maternal half-sibling families by artificially fertilizing the eggs with milt from bright- and pale-coloured males and then held the resulting progenies under identical hatchery conditions. The expression of red coloration among parental fish was not associated with their genetic diversity estimates in either sex nor did offspring sired by bright males consistently differ in terms of embryo survival or endogenous growth efficiency from offspring sired by pale males. By contrast, maternal effects were notably strong and, additionally, the degree of female coloration was negatively linked to their reproductive potential. The more intensely coloured females had a smaller relative fecundity and they also produced offspring of lower viability, implying a significant trade-off in resource allocation between ornamentation and offspring. Our results indicate that the red breeding ornamentation of Arctic charr is likely to be informative rather among females than males when the reproductive quality is predicted on grounds of the number of offspring produced. Nevertheless, this study does not support the direct selection hypothesis in explaining the evolution of female ornamentation, but rather suggests that the less intense coloration of female charr compared to males may reflect inter-sexual differences in the trade-off between natural and sexual selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development and maintenance of most sexually selected ornamental traits are believed to involve significant costs (Zahavi 1975; Andersson 1994; Tomkins et al. 2004), and thus they may be otherwise opposed by natural selection (e.g., Stuart-Fox and Ord 2004; Hamon and Foote 2005). As a consequence, only the most vigorous individuals should be capable of allocating a significant proportion of their resources into elaborated ornaments without suffering a reduction in viability. This prediction is, in particular, a key premise for the genetic theories of inter-sexual selection. Costly secondary sexual traits provide a reliable indication of an individual’s heritable quality and are therefore used by the opposite sex (mostly females) to assess potential mates (Cotton et al. 2004; Neff and Pitcher 2005).
In a variety of animal species, both males and females are ornamented (Kraaijeveld et al. 2007), but most studies of sexual selection have, however, focused on ornamental traits in only one sex (males). As a consequence, examples of selection acting on such traits in different sexes remain rare. Furthermore, the expression of ornamental traits may not necessarily reflect specific superior alleles (i.e., traditional ‘good genes’; Kokko et al. 2003; Hunt et al. 2004), but it can as well be correlated with the general allelic diversity and/or inbreeding level (i.e., a ‘good-genes-as-heterozygosity’ hypothesis by Brown 1997; see also Aparicio et al. 2001; van Oosterhout et al. 2003; Reid et al. 2005), suggesting a possible means by which a directional mating preference could also be targeted at relatively heterozygous or outbred individuals (Kempenaers 2007; Fromhage et al. 2009).
Carotenoid-based yellow–red colours are among the most conspicuous visual signals used by animals to attract mates, and they are often considered costly condition-dependent indicators of an individual quality (Møller et al. 2000). Carotenoids also have important nutritional, antioxidant and immunostimulant properties in the body (Olson and Owens 1998; Blount et al. 2003; McGraw 2005), and hence, by using these pigments for ornamental purposes, an individual may reveal its superior health status (e.g., McGraw and Ardia 2003; Mougeot et al. 2007; Baeta et al. 2008; see also Pike et al. 2007). In the three-spined stickleback (Gasterosteus aculeatus L.), for example, females prefer to mate with colourful males (Milinski and Bakker 1990; Bakker and Mundwiler 1994), presumably because the red coloration signals their good physical condition (Milinski and Bakker 1990; Barber et al. 2000), high heritable resistance against parasitic infections (Folstad et al. 1994; Barber et al. 2001) and high antioxidant defence (Pike et al. 2007). The studies on two other fish species, the European minnow (Phoxinus phoxinus L.) (Müller and Ward 1995) and guppy (Poecilia reticulata Peters) (van Oosterhout et al. 2003), on the other hand, have shown that male carotenoid-based breeding display can also provide information about differences between individuals in their genetic diversity (or inbreeding levels). Thus, it is possible that the bright carotenoid coloration would signal individual heritable quality, either directly (e.g., additive genetic breeding value for fitness; Hunt et al. 2004) or indirectly through associations between heterozygosity and other fitness parameters such as disease resistance (e.g., Reid et al. 2005; Rosen and Tarvin 2006).
In females, by contrast, the informative content and evolution of carotenoid-based ornaments may be even more problematic as they must not only allocate carotenoids to their own somatic maintenance, and possibly sexual signals, but often also to their offspring (through the eggs) (Fitzpatrick et al. 1995; Blount et al. 2000; Blount 2004). This is because the developing embryos are particularly dependent on maternally derived antioxidants due to their rapid metabolism incurring high rates of free radical production. In sockeye salmon (Oncorhynchus nerka Walbaum), for example, up to 85% of the total body carotenoids may be present in ripening eggs (Crozier 1970). Egg carotenoids are known to reduce the susceptibility of embryonic tissues to oxidative stress (Blount et al. 2000), and their positive effects on fertilization rates, early survival and early growth rates have been documented in fishes (Torrissen 1984; Salze et al. 2005; Ahmadi et al. 2006; Sawanboonchun et al. 2008; Tyndale et al. 2008) as well as in a wide range of other taxa (e.g., George et al. 2001; McGraw et al. 2005a). Consequently, there might be strong selection pressure on females to invest large amounts of these valuable resources to eggs, instead of ‘wasting’ them on ornaments and the question has been asked whether female colour may have evolved solely as a non-adaptive, correlated trait resulting from female selection for male colour in species expressing sexual coloration in both sexes (i.e., the genetic correlation hypothesis; Lande 1980; Amundsen 2000). Carotenoid-based ornaments could thus be regarded, at least in some systems, as examples of traits that are subject to sexual conflict, i.e., are favoured in males but selected against in females (Wedell et al. 2006; but see MacDougall and Montgomerie 2003; Gladbach et al. 2010).
The Arctic charr (Salvelinus alpinus L.) is a fish species exhibiting a carotenoid-based abdominal ornamentation that is correlated with parasite intensities and immune activity (Skarstein and Folstad 1996; Skarstein et al. 2005). The breeding coloration is clearly a sexually dichromatic trait, males being redder than females (Skarstein and Folstad 1996). The expression of redness can, however, considerably vary among reproductively active fish within both sexes (Skarstein et al. 2005; Nordeide et al. 2008), though the role of these colours in signalling and mating has yet remained unclear. Nonetheless, because the mating system of Arctic charr is clearly non-resource-based, i.e., neither sex provides parental care after spawning (Sigurjónsdóttir and Gunnarson 1989), mate choice is likely to be based, in addition to potential direct benefits from gamete quality and quantity (see Måsvær et al. 2004; Janhunen et al. 2009), on indirect genetic benefits. In their recent laboratory experiment, Eilertsen et al. (2009) found a positive relationship between male redness and offspring’s endogenous growth rate, though the effect appeared only among the smaller-sized sires. However, this finding suggests that at least the paternal red coloration may indeed be linked to some beneficial genetic effects also in Arctic charr.
In the present study, we used a Finnish population of Arctic charr as a model to investigate whether the breeding coloration in either sex would reflect the degree of individual genetic diversity. In addition, we established maternal half-sib families by artificially crossing (in vitro) females with two males that had developed a breeding coloration of different degree in the same rearing environment. By experimentally controlling for the confounding maternal and environmental effects (Sheldon 2000), it was possible to test whether differently coloured males differ in their genetic quality, determined as their offspring’s early survival and growth performance. On the other hand, because maternal effects are known to be pronounced in early developmental phases of fish (see Kamler 2008 and references therein), and because charr females might be constrained to trade-off carotenoids between investment in ornamentation and investment in eggs (Nordeide et al. 2006, 2008), we also examined whether there are any correlative evidence that the variation in sexual coloration among females (dams) would be related to their reproductive potential.
Materials and Methods
Sampling of Parental Fish
We started the experiment in November 2002 at the Saimaa Fisheries Research and Aquaculture station, in eastern Finland. Reproductively active Arctic charr (n ♀ = 30 and n ♂ = 60) from a cultivated brood stock of Lake Saimaa (Kuolimo region; 61°14′N, 27°36′E) were used as parental fish for the study (year-classes 1996–1999; second hatchery generation). The fish were anaesthetized with buffered MS-222 (tricaine methane sulphonate), and measured for their total length (L T to the nearest mm), body mass (to the nearest g), and abdominal coloration (Table 1). In addition, we took a fin sample from each individual for later microsatellite DNA analyses. These samples were stored in 99.5% pure ethanol until extraction.
The males were sampled on the basis of their redness (see the procedure below), that is, thirty bright-coloured and thirty pale-coloured individuals were selected. After the measurements, we stripped the fish of gonadal products with light hand pressure along their abdomens. Special care was taken to keep the gametes uncontaminated by water and urea. Assuming the amount of residual eggs in the body cavity to be small and approximately equal for each female, we also weighed the stripped egg batches without ovarian fluid in order to determine the female-specific fecundities, or gonadosomatic indices [GSI% = (gonad weight/total body weight) × 100]. Eggs were stored with ovarian fluid in open plastic containers and milts were stored in oxygenated plastic bags on ice at <5°C awaiting further handling.
Quantifying Breeding Coloration
The bright and pale males were initially ranked so that the difference in their red spawning coloration was evident to bare eye. Since ocular inspection gives, however, a very subjective estimate of the degree of ornamentation, we also quantified the skin colour of each fish with a hand-held Minolta CR-10 colorimeter (Konica Minolta Sensing Americas Inc. NJ, USA) in the CIE 1976 L*a*b* colour system mode (Commission Internationale de l’Eclairage, CIE 1986). The three continuous parameters describe lightness (L*), redness (+a*) and yellowness (+b*). Colour readings were performed five times on the left flank at a standard position: the abdominal region between pectoral and pelvic fins. The five consecutive measurements were then averaged to provide an individual colour assessment for each fish. A calculatory definition of colour chroma (saturation) was given as: C* = (a*2 + b*2)0.5. Chroma was highly correlated with both a* and b* (Spearman’s rank correlation, both r s > 0.97, P < 0.001, n = 90), and thus its value can be expected to represent well the amount of carotenoid-based pigmentation (astaxanthin) in Arctic charr skin (Hatlen et al. 1998; see also Scalia et al. 1989). The males assigned to the bright and pale groups differed highly significantly in their C*-values (t = 15.66, P < 0.001, n = 60; Table 1), but not in their total lengths or mass (t = −0.060, P = 0.953 and t = 0.231, P = 0.818, respectively; n = 60). There was neither a significant relationship between the C*-value and total length or mass among the sampled females (Pearson’s correlation: r = 0.162, P = 0.391 and r = 0.158, P = 0.404, respectively; n = 30).
Fertilizations and Incubation Procedure
All fertilizations were done within 24 h after the gametes were collected. Before the fertilizations, the functionality (i.e., full motility) of sperm was verified for each male using a microscope. We divided the egg batches of each female into two approximately equally sized portions. One portion was then fertilized with milt of a bright male of a father pair and the other half was fertilized with milt from a pale male, resulting in total of 60 families (or 30 maternal half-sibships). Excess quantities of milt were used in order to ensure maximal fertilization rates. Different males were used for each female. Crossing pairs were randomly formed, though with following preconditions: both males fertilizing the eggs of a given female were from the same age-class and rearing tank, and they were approximately size-matched. In order to avoid matings between siblings, the females were always chosen from different cohort than their mates.
After fertilisation, the swollen eggs from each full-sib family were divided into four batches of 100 eggs. Each batch was put into individual floating cylindrical containers (depth 20 cm, ø 10 cm) with plastic grid bottom (mesh size c. 2 mm). The containers were introduced into four round hatchery tanks (ø 1.5 m) with a flow-through water supply with one container of each family being placed in each of the four tanks, resulting in 60 containers per tank. Rearing temperature followed that of the ambient waterway throughout the study period. The number of non-fertilized eggs (i.e., those turning partly or totally opaque) was estimated for each incubation unit 1 day after the fertilizations (grand mean across all families = 1.4 ± 0.3 (SE) %). Thereafter, dead eggs were regularly counted and removed from the incubators to minimize the risk of fungal and bacterial infections.
Progeny Parameters
Embryonic survival was determined in late March 2003, when all eggs in each container had developed visible eyespots. The eggs were given a mechanical treatment by pouring them out from incubators into a plastic cup, after which the eggs that contained aborted embryos turned partially or completely white and could be discarded. Family-specific survival rates were then expressed as a mean percentage of live, remaining embryos from the initial number of eggs. It is perhaps noteworthy that only a minor proportion of the total variation in this viability measure appears to be attributable to variation in fertilization success.
Shortly before hatching in spring, the replicated egg batches within 30 families were pooled and moved to separate family-specific flow-through rearing tanks (ø 50 cm). The number of paired half-broods was reduced to fifteen due to the high mortality experienced by several families during the incubation period. High mortality at the early phase of development is typical for this Arctic charr population in hatchery conditions (pers. obs.). Thirty days after the transfer (c. 220 degree days post-fertilization), a sub-sample of fifteen endogenously feeding larvae from each family was randomly selected and photographed from the lateral view on a Petri dish. The standard length (L S, excluding caudal fin) as well as the length (L) and height (H) of the yolk sacs were measured to 0.1 mm accuracy from the digital images using the graphic software Image-Pro PLUS 3.0 for Windows (Media Cybernetics Inc. Silver Spring, MD, USA). The yolk sac volume was calculated according to the formula (Kamler 2008):
Estimation of Individual Genetic Diversity
The level of individual genetic variability of the parental fish was estimated using 11 microsatellite markers previously shown to be polymorphic in Finnish Arctic charr populations as outlined in Kekäläinen et al. (2009). ‘Internal relatedness’ (IR; Amos et al. 2001) which estimates the similarity of parental half-genotypes within an individual was used as a measure of genetic diversity. IR was highly correlated with the two other genetic diversity estimates measured, the mean observed heterozygosity (H OBS) and heterozygosity weighted by locus (HL; Aparicio et al. 2006) (Pearson’s correlation, |r| = 0.98, n = 90, P < 0.001) and thus only the results for IR are reported.
Statistical Analyses
Statistical analyses were performed using SPSS 13.0 for Windows (SPSS Inc. Chicago, IL, USA). Proportional data (GSI, embryo survival) were arcsine square-root-transformed and C*-values were log-transformed prior to statistical analyses to render them normally distributed. The difference between sire coloration groups (bright vs. pale) was tested in respect of IR using student’s t-test. Further, association between IR and abdominal redness (C*-value) was studied for both sexes separately using Spearman’s rank correlation (males; a non-normal distribution of chroma) and Pearson’s product moment correlation (females).
To test for the presence of maternal effects in embryo survivorship and larval size measurements, Pearson’s correlation analysis was used on data from paired bright- and pale-sired maternal half-siblings. A paired-samples t-test was used to examine if there were consistent differences in the mean survival rates and growths of bright- and pale-sired maternal half-siblings. The GSI and each of the progeny traits (female-specific grand means) were separately tested against the abdominal coloration of females using a multiple linear regression model, in which female C* and total length were entered as predictor variables. Then, plots of residuals against predicted values were investigated to verify the assumption of linearity. All statistical significances were evaluated as two tailed and at a critical value α = 0.05.
Results
Relationship Between Coloration and Genetic Diversity
Polymorphism was observed in seven of the 11 microsatellites assessed, and the results reported are based on data for these seven loci. The average number of alleles observed per locus was 3.0 and the average observed heterozygosity was 0.51. Internal relatedness (IR) did not differ significantly between the males ranked as either bright- or pale-coloured individuals (t = −1.21, P = 0.230, n = 60). There was neither dependence between IR and abdominal C*-values among males (Spearman’s rank correlation, r s = −0.098, P = 0.454, n = 60) nor among females (Pearson’s correlation, r = −0.127, P = 0.503, n = 30).
Offspring Viability and Growth
Embryo survival rates from fertilization to eyed stage varied substantially among families, the grand means being 33.4 ± 5.4 (SE) % (range 3–92%) for bright-sired half-clutches and 33.0 ± 5.1 (SE) % (range 3–93%) for pale-sired half-clutches. A high positive correlation was found between the proportions of bright- and pale-sired half-siblings surviving to eyed stage (Pearson’s correlation, r = 0.946, P < 0.001, n = 30), indicating a notable maternal effect on incubation success. Amongst paired half-sibships, however, survival was not consistently associated with sire brightness (paired-samples t-test, P = 0.996, n = 30; Fig. 1).
There was a strong maternal effect also in larval endogenous growth, as indicated by the strong positive correlations between the post-hatching standard lengths and yolk sac volumes of bright- and pale-sired half-siblings (Pearson’s correlation, r = 0.770, P = 0.001 and r = 0.704, P = 0.003, respectively, n = 15). Nevertheless, neither of these size variables differed between offspring sired by bright and pale males (paired-samples t-test, both P > 0.420, n = 15; Fig. 2).
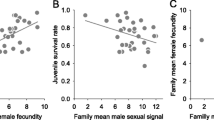
Female redness (C*) was negatively associated with both GSI (Fig. 3a) and embryo survival (Fig. 3b), but did not significantly predict larval standard length or larval yolk sac volume when a potentially confounding effect of female total length was controlled for by using multiple linear regressions (see Table 2). None of the measured attributes were mediated through female size.
The relationship between female abdominal coloration and a residual gonadosomatic index (GSI), and b residual embryo survival (female-specific grand means) in Arctic charr. Standardized residual values on the y-axes are from the linear regressions, in which the corresponding variables were regressed on female total length
Discussion
Condition-dependent ornaments are often assumed to result from directional sexual selection as they may provide honest information about the mate’s heritable quality to the prospective partners (Andersson 1994; Møller and Alatalo 1999; Cotton et al. 2004). In the present experiment, our objective was to evaluate whether an ornamental sexual trait in Arctic charr, carotenoid-based breeding coloration, predicts variation in individuals’ own genetic diversity and/or their reproductive success, as determined by the offspring’s early performance. We did not find evidence for the hypothesis presented by Brown (1997) that the degree of ornamental expression in either sex would signal information about individual genetic diversity. Furthermore, when controlling for the maternal effects by means of artificial half-sib fertilizations, we neither observed that the offspring sired by the bright-coloured males would consistently differ in terms of embryonic survival or endogenous growth efficiency from offspring sired by pale-coloured males. Hence, male redness may not be associated with the beneficial genetic effects underlying these few, albeit essential aspects of progeny quality. By contrast, we did find that the degree of colour expression in females was negatively linked to their reproductive potential. Besides having a smaller relative fecundity (gonadosomatic index), more intensely coloured females also produced offspring of lower viability. These results suggest that charr males should not express directional preference for red females owing to their lower fertility.
Several recent studies have shown that some sexually selected traits can be indicative of individual genetic diversity (Aparicio et al. 2001; Foerster et al. 2003; Marshall et al. 2003; Reid et al. 2005; Rosen and Tarvin, 2006), and this link presumably plays an important role in populations that exhibit inbreeding avoidance as a part of their mating strategies (Fromhage et al. 2009). Then, mate choice on more ornamented individuals (males) could be expected to evolve simply due to the advantages of producing genetically more diverse and consequently higher quality offspring (Lehmann et al. 2006; Kempenaers 2007; see also Primmer et al. 2003). Multi-locus heterozygosity has been strongly linked, for example, to increased metabolic efficiency, developmental stability (e.g., Mitton 1993) and resistance to disease (Reid et al. 2005), i.e., factors that might allow the heterozygous individuals to better afford the costs of developing carotenoid-based ornamentation, in relation to their homozygous (or inbred) counterparts. Nevertheless, we did not observe any association between the measure of individual genetic diversity (IR) and abdominal brightness, suggesting that the expression of this ornamental trait may not reflect an overall genetic quality (e.g., a general effect caused by variance in inbreeding) (see however Müller and Ward 1995; van Oosterhout et al. 2003). On the other hand, the extent to which the genetic diversity estimates across a relatively small number of microsatellite markers actually predict genome-wide variability is not clear (see Slate et al. 2004).
It is perhaps not so surprising that we also failed to detect a genetic connection between male coloration and progeny traits. Although the fitness consequences arising from directional sexual selection occur widely across animal taxa, they have mostly proven to be minor (Møller and Alatalo 1999). According to a meta-analysis conducted on the available data on ‘good-genes’ effects, the male traits chosen by females would reveal, on average, only 1.5% of the genetic component of variance in offspring viability (Møller and Alatalo 1999). In a recent half-sib experiment on brown trout (Salmo trutta L.), however, the number of surviving embryos was found to negatively correlate with the sires’ red-coloured spots, whereas the positive genetic effects were rather linked to the dark melanin-based pigmentation (Wedekind et al. 2008a). It is important to note that genetic benefits do not entirely result from the additive effects of genetically superior individuals, but they also encompass interactions between the genes that offspring inherit from both parents (i.e., dominance genetic effects; Mays and Hill 2004; Puurtinen et al. 2009; see also Wedekind et al. 2001; Rudolfsen et al. 2005; Wedekind et al. 2008b). Such genetic compatibility effects between males and females may cause large variation in fitness and thus complicate or even weaken directional sexual selection on males (Neff and Pitcher 2005). For example, in a crossing experiment on Chinook salmon (Oncorhynchus tshawytscha Walbaum) Pitcher and Neff (2007) found no relationship between male sexually selected traits (a few morphological measures) and offspring survival or growth, but instead, they concluded that an optimal mate selection could still increase these offspring attributes by between 3 and 19% during the endogenous feeding period alone. Further, and perhaps most importantly, the early viability and developmental variation in fishes are largely attributable to the female parent (e.g., Nagler et al. 2000; Perry et al. 2004; Janhunen et al. 2010), and this feature may undermine any independent effects of paternal genes. Yet, one can expect a gradual shift towards sire-mediated effects, and depending on the magnitude of the maternal influence through egg deposit, the genetic fitness benefits associated with paternal sexual coloration might be manifested only later in life (see however Eilertsen et al. 2009).
In conformity with the findings of other half-sib experiments on fish (e.g., Barber et al. 2001; Wedekind et al. 2001; Kortet et al. 2004), our results indicate strong maternal effects on both the embryo viability and larval size traits. Such effects may arise from genetic causes as well as from differences in egg size and quality (e.g., altering amounts of nutrients and hormones deposited in the yolk; reviewed by Kamler 2008). Non-genetic maternal effects were mediated partly through dam coloration in the present study. The redness of females was negatively associated with their reproductive output (measured as both the relative amount of eggs produced and the number of surviving embryos), suggesting a significant trade-off in resource allocation between ornamentation and the offspring. This finding conforms with the hypothesis proposed by Fitzpatrick et al. (1995) that males choosing excessively ornamented females would receive fewer and/or poorer eggs than they could have obtained from a similar quality female which allocated less to ornamentation and more to reproduction. A possible physiological basis for our finding about the negative relationship between female redness and the early viability of her offspring could be that the development of carotenoid-based breeding coloration in females might reduce the availability of these valuable pigments (antioxidants) to developing embryos, thus leading to lower incubation success per brood (see e.g., Ahmadi et al. 2006; Tyndale et al. 2008). In support of this idea, Nordeide et al. (2006, 2008) found that the intensity of red ornamental coloration in both the stickleback and Arctic charr females may, indeed, be negatively correlated with the amount of carotenoids in the eggs, though the relationship was only marginally significant in the latter case.
The direct (mutual) selection hypothesis proposes that ornamental elaboration of both males and females is positively linked to some aspect of individual quality, and hence, these ornaments are a consequence of directional mate choices (Kraaijeveld et al. 2007). Because a preference for more colourful females would seem to disadvantage Arctic charr males, however, the direct selection hypothesis seems to be a somewhat illogical explanation for the existence of female ornamentation in this species (see also Nordeide et al. 2008). Further, it seems unlikely that mutual breeding ornamentation has resulted from reproductive competition separately in both sexes (see LeBas 2006; Watson and Simmons 2010) because only male charr have been shown to compete over spawning territories and mates (Sigurjónsdóttir and Gunnarson 1989). In contrast, based on the present results, it seems more likely that female ornamental coloration in Arctic charr has evolved as a non-adaptive, genetically correlated trait resulting from sexual selection acting on males (Lande 1980).
Carotenoid-based coloration can have a strong genetic basis (Elvingson and Nilsson 1994; Craig et al. 2005; Hughes et al. 2005), but it may also involve an environmental component, reflecting differences in individual nutritional state (McGraw et al. 2005b; Hadfield and Owens 2006). Consequently, using cultivated fish instead of wild fish for this sort of experiment may result in the causes of variation in coloration being different from those in the environment where the signalling system evolved. In hatchery-reared Arctic charr, within-population variability in carotenoid pigmentation is known to result partly from the differential feed uptake and growth among individuals (Hatlen et al. 1997). However, there was no relationship between individual colour intensity and body size in our study. Additionally, the other potentially confounding factors—age and rearing conditions—were the same within each male pair. Thus, the observed variation in colour expression is likely, to a considerable degree, due to genetic causes (e.g., intrinsic ability to sequester carotenoids from food and mobilize/allocate them further for different purposes).
To conclude, our data do not provide evidence for the conjecture that the degree of carotenoid-based sexual ornamentation in Arctic charr would reflect individual genetic quality in the form of genome-wide variability. Nor does our study lend support to the ‘good genes’ theories of sexual selection, that is to say, charr females may not receive indirect genetic benefits through their offspring’s early survival and growth by preferentially mating with males with intense red coloration. However, we cannot exclude the possibility that the bright red males would still be fitter in a heritable sense and possess a better breeding value, on average, for the total fitness. In females, instead, the investment into carotenoid ornamentation appears to involve significant reproductive costs, i.e., it occurs at the expense of fecundity and offspring viability. This finding does not support the direct selection hypothesis when explaining the evolution of female ornamentation. On the contrary, it conforms to the view that the less intense coloration of females in comparison with males reflects inter-sexual differences in the intensity of sexual selection, or, in particular, in the trade-off between natural and sexual selection. Either way, the carotenoid-based breeding coloration of Arctic charr is presumably far more informative among females than among males in predicting their quality by the number of offspring produced.
References
Ahmadi, M. R., Bazyar, A. A., Safi, S., Ytrestoyl, T., & Bjerkeng, B. (2006). Effects of dietary astaxanthin supplementation on reproductive characteristics of rainbow trout (Oncorhynchus mykiss). Journal of Applied Ichthyology, 22(5), 388–394.
Amos, W., Worthington-Wilmer, J., Fullard, K., Burg, T. M., Croxall, J. P., Bloch, D., et al. (2001). The influence of paternal relatedness on reproductive success. Proceedings of the Royal Society of London Series B, 268(1480), 2021–2027.
Amundsen, T. (2000). Why are female birds ornamented? Trends in Ecology & Evolution, 15(7), 149–155.
Andersson, M. (1994). Sexual selection. Princeton: Princeton University Press.
Aparicio, J. M., Cordero, P. J., & Veiga, J. P. (2001). A test of the hypothesis of mate choice based on heterozygosity in the spotless starling. Animal Behaviour, 62(5), 1001–1006.
Aparicio, J. M., Ortego, J., & Cordero, P. J. (2006). What should we weigh to estimate heterozygosity, alleles or loci? Molecular Ecology, 15(14), 4659–4665.
Baeta, R., Faivre, B., Motreuil, S., Gaillard, M., & Moreau, J. (2008). Carotenoid trade-off between parasitic resistance and sexual display: An experimental study in the blackbird (Turdus merula). Proceedings of the Royal Society of London Series B, 275(1633), 427–434.
Bakker, T. C. M., & Mundwiler, B. (1994). Female mate choice and male red coloration in a natural 3-spined stickleback (Gasterosteus aculeatus) population. Behavioral Ecology, 5(1), 74–80.
Barber, I., Arnott, S. A., Braithwaite, V., Andrew, J., & Huntingford, F. A. (2001). Indirect fitness consequences of mate choice in sticklebacks: Offspring of brighter males grow slowly but resist parasitic infections. Proceedings of the Royal Society of London Series B, 268(1462), 71–76.
Barber, I., Arnott, S. A., Braithwaite, V., Andrew, J., Mullen, W., & Huntingford, F. A. (2000). Carotenoid-based sexual coloration and body condition in nestling male sticklebacks. Journal of Fish Biology, 57(3), 777–790.
Blount, J. D. (2004). Carotenoids and life-history evolution in animals. Archives of Biochemistry and Biophysics, 430(1), 10–15.
Blount, J. D., Houston, D. C., & Møller, A. P. (2000). Why egg yolk is yellow? Trends in Ecology & Evolution, 15(2), 47–49.
Blount, J. D., Metcalfe, N. B., Arnold, K. E., Surai, P. F., Devevey, G. L., & Monaghan, P. (2003). Neonatal nutrition, adult antioxidant defences and sexual attractiveness in the zebra finch. Proceedings of the Royal Society of London Series B, 270(1525), 1691–1696.
Brown, J. L. (1997). A theory of mate choice based on heterozygosity. Behavioral Ecology, 8(1), 60–65.
CIE (International Commission on Illumination). (1986). Colorimetry. Vienna: CIE Publication No. 15.2.
Cotton, S., Fowler, K., & Pomiankowski, A. (2004). Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proceedings of the Royal Society of London Series B, 271(1541), 771–783.
Craig, J. K., Foote, C. J., & Wood, C. C. (2005). Countergradient variation in carotenoid use between sympatric morphs of sockeye salmon (Oncorhynchus nerka) exposes nonanadromous hybrids in the wild by their mismatched spawning colour. Biological Journal of the Linnean Society, 84(2), 287–305.
Crozier, G. F. (1970). Tissue carotenoids in prespawning and spawning sockeye salmon (Oncorhynchus nerka). Journal of the Fisheries Research Board of Canada, 27, 973–975.
Eilertsen, E. M., Bårdsen, B.-J., Liljedal, S., Rudolfsen, G., & Folstad, I. (2009). Experimental evidence for paternal effects on offspring growth rate in Arctic charr (Salvelinus alpinus). Proceedings of the Royal Society of London Series B, 276(1654), 129–136.
Elvingson, P., & Nilsson, J. (1994). Phenotypic and genetic parameters of body and compositional traits in Arctic charr, Salvelinus alpinus (L.). Aquaculture Research, 25(7), 677–685.
Fitzpatrick, S., Berglund, A., & Rosenqvist, G. (1995). Ornaments or offspring: Costs to reproductive success restrict sexual selection processes. Biological Journal of the Linnean Society, 55(3), 251–260.
Foerster, K., Delhey, K., Johnsen, A., Lifjeld, J. T., & Kempenaers, B. (2003). Females increase offspring heterozygosity and fitness through extra-pair matings. Nature, 425(6959), 714–717.
Folstad, I., Hope, A. M., Karter, A., & Skorping, A. (1994). Sexually selected color in male sticklebacks: A signal of both parasite exposure and parasite resistance? Oikos, 69(3), 511–515.
Fromhage, L., Kokko, H., & Reid, J. M. (2009). Evolution of mate choice for genome-wide heterozygosity. Evolution, 63(3), 684–694.
George, S. B., Lawrence, J. M., Lawrence, A. L., Smiley, J., & Plank, L. (2001). Carotenoids in the adult diet enhance egg and juvenile production in the sea urchin Lytechinus variegatus. Aquaculture, 199(3–4), 353–369.
Gladbach, A., Gladbach, D. J., Kempenaers, B., & Quillfeldt, P. (2010). Female-specific colouration, carotenoids and reproductive investment in a dichromatic species, the upland goose Chloephaga picta leucoptera. Behavioral Ecology and Sociobiology, 64(11), 1779–1789.
Hadfield, J. D., & Owens, I. P. F. (2006). Strong environmental determination of a carotenoid-based plumage trait is not mediated by carotenoid availability. Journal of Evolutionary Biology, 19(4), 1104–1114.
Hamon, T. R., & Foote, C. J. (2005). Concurrent natural and sexual selection in wild male sockeye salmon, Oncorhynchus nerka. Evolution, 59(5), 1104–1118.
Hatlen, B., Arnesen, A. M., Jobling, M., Siikavuopio, S., & Bjerkeng, B. (1997). Carotenoid pigmentation in relation to feed intake, growth and social interactions in Arctic charr, Salvelinus alpinus (L.), from two anadromous strains. Aquaculture Nutrition, 3(3), 189–199.
Hatlen, B., Jobling, M., & Bjerkeng, B. (1998). Relationships between carotenoid concentration and colour of fillets of Arctic chair, Salvelinus alpinus (L.), fed astaxanthin. Aquaculture Research, 29(3), 191–202.
Hughes, K. A., Rodd, F. H., & Reznick, D. N. (2005). Genetic and environmental effects on secondary sex traits in guppies (Poecilia reticulata). Journal of Evolutionary Biology, 18(1), 35–45.
Hunt, J., Bussière, L. F., Jennions, M. D., & Brooks, R. (2004). What is genetic quality? Trends in Ecology & Evolution, 19(6), 329–333.
Janhunen, M., Piironen, J., & Peuhkuri, N. (2010). Parental effects on embryonic viability and growth in Arctic charr Salvelinus alpinus at two incubation temperatures. Journal of Fish Biology, 76(10), 2558–2570.
Janhunen, M., Rudolfsen, G., Kekäläinen, J., Figenschou, L., Peuhkuri, N., & Kortet, R. (2009). Spawning coloration and sperm quality in a large lake population of Arctic charr (Salmonidae: Salvelinus alpinus L.). Biological Journal of the Linnean Society, 98(4), 794–802.
Kamler, E. (2008). Resource allocation in yolk-feeding fish. Reviews in Fish Biology and Fisheries, 18(2), 143–200.
Kekäläinen, J., Vallunen, J. A., Primmer, C. R., Rättyä, J., & Taskinen, J. (2009). Signals of major histocompatibility complex overdominance in a wild salmonid population. Proceedings of the Royal Society of London Series B, 276(1670), 3133–3140.
Kempenaers, B. (2007). Mate choice and genetic quality: A review of the heterozygosity theory. Advances in the Study of Behaviour, 37, 189–278.
Kokko, H., Brooks, R., Jennions, M. D., & Morley, J. (2003). The evolution of mate choice and mating biases. Proceedings of the Royal Society of London Series B, 270(1515), 653–664.
Kortet, R., Vainikka, A., Rantala, M. J., Myntti, J., & Taskinen, J. (2004). In vitro embryo survival and early viability of larvae in relation to male sexual ornaments and parasite resistance in roach, Rutilus rutilus L. Journal of Evolutionary Biology, 17(6), 1337–1344.
Kraaijeveld, K., Kraaijeveld-Smit, F. J. L., & Komdeur, J. (2007). The evolution of mutual ornamentation. Animal Behaviour, 74(4), 657–677.
Lande, R. (1980). Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution, 34(2), 292–305.
LeBas, N. R. (2006). Female finery is not for males. Trends in Ecology & Evolution, 21(4), 170–173.
Lehmann, L., Keller, L. F., & Kokko, H. (2006). Mate choice evolution, dominance effects, and the maintenance of genetic variation. Journal of Theoretical Biology, 244(2), 282–295.
MacDougall, A. K., & Montgomerie, R. (2003). Assortative mating by carotenoid-based plumage colour: A quality indicator in American goldfinches, Garduelis tristis. Die Naturwissenschaften, 90(10), 464–467.
Marshall, R. C., Buchanan, K. L., & Catchpole, C. K. (2003). Sexual selection and individual genetic diversity in a songbird. Proceedings of the Royal Society of London Series B, 270(Suppl 2), S248–S250.
Måsvær, M., Liljedal, S., & Folstad, I. (2004). Are secondary sexual traits, parasites and immunity related to variation in primary sex traits in the Arctic charr? Proceedings of the Royal Society of London Series B, 271(Suppl), S40–S42.
Mays, H. L., & Hill, G. E. (2004). Choosing mates: Good genes versus genes that are a good fit. Trends in Ecology & Evolution, 19(10), 554–559.
McGraw, K. J. (2005). The antioxidant function of many animal pigments: Are there consistent health benefits of sexually selected colourants? Animal Behaviour, 69(4), 757–764.
McGraw, K. J., Adkins-Regan, E., & Parker, R. S. (2005a). Maternally derived carotenoid pigments affect offspring survival, sex ratio, and sexual attractiveness in a colourful songbird. Die Naturwissenschaften, 92(8), 375–380.
McGraw, K. J., & Ardia, D. R. (2003). Carotenoids, immunocompetence, and the information content of sexual colors: An experimental test. The American Naturalist, 162(6), 704–712.
McGraw, K. J., Hill, G. E., & Parker, R. S. (2005b). The physiological costs of being colourful: Nutritional control of carotenoid utilization in the American goldfinch, Carduelis tristis. Animal Behaviour, 69(3), 653–660.
Milinski, M., & Bakker, T. C. M. (1990). Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature, 344(6264), 330–333.
Mitton, J. B. (1993). Enzyme heterozygosity, metabolism and developmental stability. Genetica, 89(1–3), 47–65.
Møller, A. P., & Alatalo, R. (1999). Good-genes effects in sexual selection. Proceedings of the Royal Society of London Series B, 266(1414), 85–91.
Møller, A. P., Biard, C., Blount, J. D., Houston, D. C., Ninni, P., Saino, N., et al. (2000). Carotenoid-dependent signals: Indicators of foraging efficiency, immunocompetence or detoxification ability? Avian and Poultry Biological Reviews, 11, 137–159.
Mougeot, F., Pérez-Rodríguez, L., Martínez-Padilla, J., Leckie, F., & Redpath, S. M. (2007). Parasites, testosterone and honest carotenoid-based signalling of health. Functional Ecology, 21(5), 886–898.
Müller, G., & Ward, P. I. (1995). Parasitism and heterozygosity influence the secondary sexual characters of the European minnow, Phoxinus phoxinus (L.) (Cyprinidae). Ethology, 100(4), 309–319.
Nagler, J. J., Parsons, J. E., & Cloud, J. G. (2000). Single pair mating indicates maternal effects on embryo survival in rainbow trout, Oncorhynchus mykiss. Aquaculture, 184(1), 177–183.
Neff, B. D., & Pitcher, T. E. (2005). Genetic quality and sexual selection: An integrated framework for good genes and compatible genes. Molecular Ecology, 14(1), 19–38.
Nordeide, J. T., Mohus, Å., Nicolaisen, O., Volden, R., & Egeland, E. S. (2008). Offspring or ornaments? Is carotenoid-based ornamentation in female Arctic charr, Salvelinus alpinus (L.), condition-dependent and traded off against offspring? Ecology of Freshwater Fish, 17(2), 328–339.
Nordeide, J. T., Rudolfsen, G., & Egeland, E. S. (2006). Ornaments or offspring? Female sticklebacks (Gasterosteus aculeatus L.) trade off carotenoids between spines and eggs. Journal of Evolutionary Biology, 19(2), 431–439.
Olson, V. A., & Owens, I. P. F. (1998). Costly sexual signals: Are carotenoids rare, risky or required? Trends in Ecology & Evolution, 13(12), 510–514.
Perry, G. M. L., Audet, C., Laplatte, B., & Bernantchez, L. (2004). Shifting patterns in genetic control at the embryo-alevin boundary in brook charr. Evolution, 58(9), 2002–2012.
Pike, T. W., Blount, J. D., Lindström, J., & Metcalfe, N. B. (2007). Availability of non-carotenoid antioxidants affects the expression of a carotenoid-based sexual ornament. Biology Letters, 3(4), 353–356.
Pitcher, T. E., & Neff, B. D. (2007). Genetic quality and offspring performance in Chinook salmon: Implications for supportive breeding. Conservation Genetics, 8(3), 607–616.
Primmer, C. R., Landry, P.-A., Ranta, E., Merilä, J., Piironen, J., Tiira, K., et al. (2003). Prediction of offspring fitness based on parental genetic diversity in endangered salmonid populations. Journal of Fish Biology, 63(4), 909–927.
Puurtinen, M., Ketola, T., & Kotiaho, J. S. (2009). The good-genes and compatible-genes benefits of mate choice. The American Naturalist, 174(5), 741–751.
Reid, J. M., Arcese, P., Cassidy, A. L. E. V., Marr, A. B., Smith, J. N. M., & Keller, L. F. (2005). Hamilton and Zuk meet heterozygosity? Song repertoire size signals inbreeding and immunity in song sparrows (Melospiza melodia). Proceedings of the Royal Society of London Series B, 272(1562), 481–487.
Rosen, R. F., & Tarvin, K. A. (2006). Sexual signals of the male American Goldfinch. Ethology, 112(10), 1008–1019.
Rudolfsen, G., Figenschou, L., Folstad, I., Nordeide, J. T., & Søreng, E. (2005). Potential fitness benefits from mate selection in the Atlantic cod (Gadus morhua). Journal of Evolutionary Biology, 18(1), 172–179.
Salze, G., Tocher, D. R., Roy, W. J., & Robertson, D. A. (2005). Egg quality determinants in cod (Gadus morhua L.): Egg performance and lipids in eggs from farmed and wild broodstock. Aquaculture Research, 36(15), 1488–1499.
Sawanboonchun, J., Roy, W. J., Robertson, D. A., & Bell, J. G. (2008). The impact of dietary supplementation with astaxanthin on egg quality in Atlantic cod broodstock (Gadus morhua L.). Aquaculture, 283, 97–101.
Scalia, S., Isaksen, M., & Francis, G. W. (1989). Carotenoids of the Arctic charr, Salvelinus alpinus (L.). Journal of Fish Biology, 34(6), 969–970.
Sheldon, B. C. (2000). Differential allocation: Tests, mechanisms and implications. Trends in Ecology & Evolution, 15(10), 397–402.
Sigurjónsdóttir, H., & Gunnarson, K. (1989). Alternative mating tactics of Arctic charr. Salvelinus alpinus, in Thingvallavatn, Iceland. Environmental Biology of Fishes, 26(3), 159–176.
Skarstein, F., & Folstad, I. (1996). Sexual dichromatism and the immunocompetence handicap: An observational approach using Arctic charr. Oikos, 76(2), 359–367.
Skarstein, F., Folstad, I., & Rønning, H. P. (2005). Spawning colouration, parasites and habitat selection in Salvelinus alpinus: Initiating speciation by sexual selection? Journal of Fish Biology, 67(4), 969–980.
Slate, J., David, P., Dodds, K. G., Veenvliet, B. A., Glass, B. C., Broad, T. E., et al. (2004). Understanding the relationship between the inbreeding coefficient and multilocus heterozygosity: Theoretical implications and empirical data. Heredity, 93(3), 255–265.
Stuart-Fox, D. M., & Ord, T. J. (2004). Sexual selection, natural selection and the evolution of dimorphic coloration and ornamentation in agamid lizards. Proceedings of the Royal Society of London Series B, 271(1554), 2249–2255.
Tomkins, J. L., Radwan, J., Kotiaho, J. S., & Tregenza, T. (2004). Genic capture and resolving the lek paradox. Trends in Ecology & Evolution, 19(6), 323–328.
Torrissen, O. J. (1984). Pigmentation of salmonids—Effects of carotenoids in eggs and startfeeding diet on survival and growth rate. Aquaculture, 43(1–3), 185–193.
Tyndale, S. T., Letcher, R. J., Heath, J. W., & Heath, D. D. (2008). Why are salmon eggs red? Egg carotenoids and early life survival of Chinook salmon (Oncorhynchus tshawytscha). Evolutionary Ecology Research, 10(8), 1187–1199.
van Oosterhout, C., Trigg, R. E., Carvalho, G. R., Magurran, A. E., Hauser, L., & Shaw, P. W. (2003). Inbreeding depression and genetic load of sexually selected traits: How guppy lost its spots. Journal of Evolutionary Biology, 16(2), 273–281.
Watson, N. L., & Simmons, L. W. (2010). Reproductive competition promotes the evolution of female weaponry. Proceedings of the Royal Society of London Series B, 277(1690), 2035–2040.
Wedekind, C., Evanno, G., Urbach, D., Jacob, A., & Müller, R. (2008a). ‘Good genes’ and ‘compatible genes’ effects in an Alpine whitefish and the information content of breeding tubercles over the course of the spawning season. Genetica, 134(1), 21–30.
Wedekind, C., Jacob, A., Evanno, G., Nusslé, S., & Müller, R. (2008b). Viability of brown trout embryos positively linked to melanin-based but negatively to carotenoid-based colours of their fathers. Proceedings of the Royal Society of London Series B, 275(1644), 1737–1744.
Wedekind, C., Müller, R., & Spicher, H. (2001). Potential genetic benefits of mate selection in whitefish. Journal of Evolutionary Biology, 14(6), 980–986.
Wedell, N., Kvarnemo, C., Lessells, C. M., & Tregenza, T. (2006). Sexual conflict and life histories. Animal Behaviour, 71(5), 999–1011.
Zahavi, A. (1975). Mate selection: A selection for a handicap. Journal of Theoretical Biology, 53(1), 205–214.
Acknowledgments
This study was funded by the Ministry of Agriculture and Forestry (310613), Jenny and Antti Wihuri Foundation, and by Finnish Game and Fisheries Research Institute. We wish to thank Saimaa Fisheries Research and Aquaculture (FGFRI) for providing the experimental fish and facilities and especially Tapani Heikkinen, Maija Hyttinen and Jorma Sorjonen for their assistance in the study. Two anonymous reviewers are thanked for their constructive suggestions on a previous draft of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janhunen, M., Peuhkuri, N., Primmer, C.R. et al. Does Breeding Ornamentation Signal Genetic Quality in Arctic charr, Salvelinus alpinus?. Evol Biol 38, 68–78 (2011). https://doi.org/10.1007/s11692-010-9100-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-010-9100-9