Abstract
The cervical system of extant penguins (Aves: Sphenisciformes) is organised into morphological modules, each with its biomechanical function. Indeed, for these marine pelagic birds to acquire hydrodynamic morphology, the folding of the neck is essential. Despite a common general structure, the cervical vertebrae exhibit morphological differences depending on their positioning. These characteristics are identified as apparent cases of complete natural homeotic transformations—therefore, the composition of some modules varies. Two types of complete cervical homeoses are identified between species, but the second type can also occur within some species when the post hatching development is considered. The fossil material analysed here makes it apparent that the two modular configurations characterising the anterior part of the neck—a consequence of the first homeosis—existed 36 My and 25 My ago, for one, and circa 10 My ago, for the other. These comparisons also reveal a clear differentiation in vertebral features between the fossil species of the Oligocene–Miocene ages and the more recent and extant penguins. Ultimately, these observations make the proposal of a hypothesis in relation to the ontogenetic influence of Hox genes, and their regulators, based on the changes observed in the cervical segment of Sphenisciformes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Penguins are peculiar birds in that they easily arouse interest and scientific curiosity, raising questions related to various aspects of their ecology. Despite all the attention paid to them, some characteristics of their anatomy have remained unknown and poorly identified. For example, the restructuring of the forelimbs of penguins as flippers has largely focused on inquisitiveness, in connection with the pelagic aquatic activity, but the cervical system—involved in locomotion—has not been given the same honours. Mechanical folding of the neck is important in penguins, as it allows the head to connect with the thorax during swimming (“underwater flight”), and on land, this folding is used to maintain the balance of erect posture. This lack of consideration was corrected in a previous study (Guinard et al. 2010) devoted to the cervical system of four species of extant penguins: Aptenodytes patagonicus, Pygoscelis papua, Eudyptes chrysolophus, and Spheniscus humboldti.
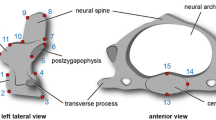
The concept of modularity has been applied to the cervical vertebrae. The neck has been divided into six different morphological zones, noted as Mo 1 to Mo 6 (Fig. 1), each associated with a function in the neck folding. It was found that: (1) the first three modules, especially Mo 3 (curvature 1), form a short and massive structure used to pull the head back, with solid areas of muscle attachments, plus the atlas and the axis (Mo 1 and Mo 2) playing a role both in connection to the skull and rotation; (2) a vertebra of dual morphology (Mo 4) makes the transition from Mo 3 and Mo 5; (3) there is a median zone (Mo 5), where the mechanic folding of the neck occurs; (4) a structure (Mo 6) with the dual role to keep the base of the neck backwards and to form curvature 2.
An example of the modular repartition of the cervical system in two extant species of penguins, based on the morphological structural features. a Aptenodytes patagonicus (MNHN 1229); b Pygoscelis papua (MNHN 1922-224). The left lateral views shows the complete cervical sequence for each species, along with cranial views of cervical C X and C XI for A. patagonicus, as well as C IX and C X for P. papua, in order to see the morphological differences clearly. Module 4 is represented by the transitional vertebra (anterior half characterised by the morphology of the preceding module and the posterior half is shaped as the following modular type). The drawn lines of connection between sequences a and b indicate morphological correspondences. A shift is observed with the position of the transitional vertebra (Mo 4) between the two species, leading to a continuation of the morphological plan—for one vertebra—of module 3 in A. patagonicus (C V) and of module 6 for P. papua (C XIII). Note that the ventral apophysis (processus ventralis corporis) is broken on C XII and C XIII of the A. patagonicus species example. Scale bars = 1 cm
Beyond these mechanical considerations, the morphological criteria and anatomical features used for the modular separation revealed differences in modularity between adult species (Fig. 1). The first of these variations concerns the position of the transitional vertebra (Mo 4), either the fifth or the sixth along the cervical sequence. Configuration Tr V or Tr VI characterise the membership to one or another of these two models. As for the second variation, it concerns the location of the vertebra marking the beginning of the sixth module: the ninth, tenth, or eleventh position, depending on the species. Moreover, this difference of positioning sometimes occurs in the same species during the post-hatching growth. Thus, as a juvenile a penguin of a given species may have the modular arrangement of an adult penguin of another species, either the whole or part of the cervical system. Table 1 shows an inventory of key modular configurations in penguins, drawn from observations on specimens belonging to the comparative anatomy collections of the Muséum National d’Histoire Naturelle de Paris (MNHN).
These results concern extant species, but in the evolving context of the entire group of the Sphenisciformes, it is imperative to focus on the cervical modular distribution in fossil representatives. This informative supplement will be done through enriched, actualised, and new descriptions of specimens listed in the literature. Subsequent to this contribution to the cervical modularity of penguins, it will be necessary to address the nature of the differences and their developmental origins.
The Contribution of Fossil Material
The oldest known penguins date from the early Paleocene period (New Zealand), circa—60 My (Slack et al. 2006). The fossil representatives, more than 40 species (Jadwisczak 2006), are usually identified from tarsometatarsus and limb bones (the most common remains). However, vertebrae are sometimes fossilised, allowing comparisons with extant species.
Three fossil representatives have been chosen with regard to the number of cervical vertebrae: Icadyptes salasi—late Eocene, −36 My (Clarke et al. 2007; Ksepka et al. 2008); Paraptenodytes antarcticus—early Miocene, −25 My (Simpson 1946; Bertelli et al. 2006); and Madrynornis mirandus (Acosta Hospitaleche et al. 2007)—early Late Miocene, circa −10 My. The extant species compared are those previously studied by Guinard et al. (2010): Aptenodytes patagonicus, Pygoscelis papua, Eudyptes chrysolophus, and Spheniscus humboldti. It is important to keep in mind the difference between the two transitional modular configurations. Thus, comparing a vertebra VI of an extant or extinct species of penguin classified Tr V implies a correlation with the cervical VII of an individual classified as Tr VI. Similarly, a comparison of the C XIII of a penguin exhibiting a configuration Tr V is only possible with an individual of similar configuration. The anatomical terms used in the descriptions are given following the common and Latin denominations (Baumel et al. 1993).
Paraptenodytes antarcticus
Simpson (1946) described the remains of this fossil penguin from South America. Of the nine vertebrae preserved, six were attributed to the neck. Simpson estimated that the fossil had probably 13 cervical vertebrae, since this number is invariant among living species of penguins. Bertelli et al. (2006) re-described the remains of P. antarcticus studied by Simpson, focusing mainly on the skull. Although the authors do not consider the cervical system for their description of postcranial material, they provide photographs of the preserved vertebrae (which Simpson did not).
First Cervical Vertebra, the Atlas: Mo 1 (Fig. 4a)
The depth of the incisura fossae is well defined, as in Pygoscelis papua and Eudyptes chrysolophus. This feature appears to be connected with the general shape of the vertebra. Indeed, the more it is stretched vertically, the more the depth is clear. Although well developed, the ventral surface presents no significant side peaks, unlike P. papua. As a matter of interest, the cranial extremity of the second vertebra, the dens axis, is preserved in connection with the atlas.
Determination of the Positioning of the Transitional Vertebra: Mo 4
Simpson (1946) compiled a table of measurements from two variables: the width between the zygapophysis cranialis and the length of the centrum (Table 2). Considering the measurements, and compared with those made in the previous study (Guinard et al. 2010), the first variable, “width between prezygapohyses”—or zygapophysis cranialis—is our front width 1 (Fw1), and the second variable “Length of centrum” is our Length 4 (L4) (Fig. 2). Simpson only used two extant species: Aptenodytes forsteri and Pygoscelis adeliae. Unfortunately, he did not specify the number of individuals measured, and it is unclear whether the values given are means or not. As a reminder, a direct observation on samples of the MNHN assigns a configuration Tr VI to A. forsteri and a configuration Tr V to P. adeliae.
Some parameters of biometrical measurements (in mm) used in the study of cervical vertebrae in extant penguins (Guinard et al. 2010). a Cervical VIII (top view); b cervical VII (ventral view). a–b Front width 1 (Fw1); c–d length 4 (L4). N.B The species represented here is Aptenodytes patagonicus. Scale bars = 1 cm
For Paraptenodytes antarcticus, Fw1 is relatively constant in the succession of vertebrae identified by Simpson as C VI, C VIII, C XI, and C XIII (Table 2). A constant Fw1 parameter indicates erected zygapophysis cranialis, characteristic of the vertebrae of module 5 and the beginning of module 6. Thus, it is easy to deduce from Table 2 that the transitional vertebra of P. antarcticus is C V, as for Pygoscelis, and Megadyptes, and surely, Eudyptes.
In order to define the similarity of organisations graphically, an index I gp (gap index) is determined from the variable Fw1, using personal data together with Simpson’s data from his study (application to vertebrae VI, VIII, XI and XIII). I gp = (Fw1 max − Fw1 min)/Fw1 max, where Fw1 max and Fw1 min are the maximum and minimum widths in the group of vertebrae considered. The following species, which belong to configuration Tr V, are added to the comparison: Eudyptes chrysolophus, Pygoscelis papua, Pygoscelis antarctica and Megadyptes antipodes (from the MNHN collections). Individuals of the species Aptenodytes forsteri and Pygsocelis adeliae from the same collections are also added to complete Simpson’s data.
Note a clear link between Paraptenodytes antarcticus and extant species such as Pygoscelis papua and Eudyptes chrysolophus (Fig. 3). We can therefore conclude that in P. antarcticus, the transitional vertebra (Mo 4) is the fifth cervical. However, the unusual nature of Simpson’s specimen of Pygoscelis adeliae is important to point out. The individual for this species is characterised by a value of I gp superior to that of other penguins classified in the configuration of Tr V model. Despite erected zygapophysis cranialis on C VI (first vertebra of module 5) in P. adeliae (MNHN 1914-161), the width is still important. Some variation in the values between individuals of the same species is suspected to be linked with the age of the bird (and then the use of the cervical system over time). For example, Eudyptes chrysolophus MNHN 1922-224 seems older than MNHN 1993-90.
The graphic model of the Paraptenodytes antarcticus situation regarding the gap index (I gp) between zygapophysis cranialis. Numbers indicate the individuals from the MNHN collections and arrows show those from Simpson (1946). The localisation of P. antarcticus confirms the descriptive data about fossil species belonging to the configuration Tr V model. Note the particular position of P. adeliae (especially the individual measured by Simpson) despite its Tr V characterisation
Sixth Cervical: Mo 5 (Fig. 4b)
Simpson estimated it was the sixth cervical rather than the seventh, and Bertelli et al. (2006) described it as “presumed VI”. It is observed that the arcus vertebra shows a longitudinal ridge. The latter, towards the centre of the vertebra, forms a low and blunted processus spinosus. In addition, there is a pair of strong processus caroticus, which for Simpson, is found on C VII or C VIII of the extant representatives. From the previous study of penguins (Guinard et al. 2010), it is considered that the trueFootnote 1 processus caroticus appear for Aptenodytes patagonicus and Spheniscus humboldti on C VII and for Pygoscelis papua and Eudyptes chrysolophus on C VI, thus just after the transitional vertebra. In this context, the cervical defined as C VI by Simpson is effectively the sixth. Moreover, the general shape of the vertebra is undeniably characteristic of the first vertebra of module 5. Among extant species, only the processus spinosus for Spheniscus humbodlti is noteworthy after the transitional vertebra. But the shape is not the same: for S. humboldti, it is a spine, and for Paraptenodytes antacticus, a long blade. Tori dorsalis are well developed, as for Eudyptes chrysolophus, but are of different shape. The degree of projection of the processus transversus forwards is low (as for S. humboldti and A. patagonicus, logically referring to vertebra VII).
Cervical vertebrae of Paraptenodytes antarcticus (from Bertelli et al. 2006). a Atlas (cranial view); b cervical VI (left lateral view); c cervical VIII (left lateral view); d cervical XI (cranial view); e cervical XIII (right lateral view). an: ansa costotransversaria; av: arcus vertebrae; d: dens axis; fc: fossa condyloidea; fcr: facies articularis cranialis; fcd: facies articularis caudalis; focr: fovea cranioventralis; ftr: foramen transversarium; if: incisura fossae; pc: processus caroticus; pco: processus costalis; ps: processus spinosus; pt: processus transversus; pvc: processus ventralis corporis; pvt: processus ventrolateralis; td: torus dorsalis (pl. tori dorsalis); zcd: zygapophysis caudalis; zcr: zygapophysis cranialis. The remarkable features characterising this fossil species are the presence of a noticeable processus spinosus on the first vertebra of module 5 (b), a massive appearance of vertebrae VIII (vertically elongated) and XI (horizontal elongation), and an atypical ventral morphology of cervical XIII. Scale bars = 1 cm
Eighth Cervical: Mo 5 (Fig. 4c)
On what Simpson defined as the eighth cervical, but may be the ninth, the neural arch still shows a tip, although the processus spinosus is no more distinctive. The processus transversus are similar to those of C VI but the processus caroticus are closer, longer, and well developed, as for Spheniscus humboldti.
The tori dorsalis are similar to those found in this latter species but slightly more pronounced. Any doubt about the position can be resolved through the orientation of the processus transversus (lateral view), more typical of an eighth cervical than a ninth in the context of a Tr V cervical system. Indeed, the orientation of the processus costalis is near to horizontal, although the bony part is not fully preserved. If it were a C IX, the process would have been directed obliquely downward. Other features, such as facies articularis caudalis not yet covered with the vertebral arch (visible in top view), and the position of tori dorsalis and zygapophysis cranialis (shape and contact with the vertebral body) reinforce the determination of C VIII. For the morphologically corresponding vertebra, the nature of zygapophysis cranialis is similar to that of S. humboldti. However, a lateral view reveals quite a different situation. The zygapophysis cranialis are more erect than in extant species, therefore the vertical lengthening of the vertebra follows this tendency. This moves them away from ansa costotransversaria, since their position remains identical to that of extant species—just above the foramen transversarium. This thickened morphology is similar to that found for vertebra X of living Pygoscelis papua and Eudyptes chrysolophus. Other differences with the extant representatives, such as the fossil’s zygapophysis caudalis, are more oriented laterally. Finally, there is a knob at the base of the facies articularis caudalis on the ventral side, which is completely absent in extant species.
Eleventh Cervical and the Supposed Positioning of Processus Ventralis Corporis Marking the Beginning of the Last Module: Mo 6 (Fig. 4d)
C XI also exhibits a crested arcus vertebrae with a small thorn, as on C XII of A.patagonicus. As a matter of interest, considering this feature, Simpson noticed this correlated shift involving one vertebra for this trait. This difference makes sense in regards to the classification of P. antarcticus in the configuration Tr V, whereas A. patagonicus belongs to the configuration of the Tr VI model. However, note that the processus costalis is more developed than for A. patagonicus. This fossil vertebra resembles the eleventh cervical of E. chrysolophus and P. papua, with its compressed processus ventralis corporis, preceded by a large fovea cranioventralis. The ventral apophysis can be linked with another feature of the C VIII and its processus caroticus near to one another. As a processus ventralis corporis is present on C XI, the feature is, with certainty, present on C X, as with P. papua and S. humboldti or even may be from C IX as in E. chrysolophus. Indeed, the large size of processus caroticus that are getting close to one another is a sign of the upcoming appearance of the processus ventralis corporis. Unlike living species, the processus transversus is more massive, hence the absence in the fossil of the lower and upper apophyses to the level of the ansa costotransversaria. However, the most striking feature is the lateral extension of the entire vertebra (cranial view), which leads to a thinning and elongation of the facies articularis cranialis. In the extant representatives, an aspect of this feature is found in P. papua.
Thirteenth Cervical: Mo 6 (Fig. 4e)
The ansa costotranversaria, unlike P. papua and E. chrysolophus, are horizontal and broad. The zygapophysis caudalis are more rounded and more massive than the living species of configuration Tr V. But the most striking feature is the unusual ventral morphology of the last vertebra of the neck. While the extant representatives have a processus ventralis corporis, although small, P. antarcticus shows an excavation of the ventral surface. In addition, lateral areas of the facies articularis cranialis each seem to have a well-defined processus ventrolateralis.
Paraptenodytes antarcticus is mainly characterised by the configuration Tr V, shared by the majority of living species. Unfortunately, the absence of vertebrae IX and X does not allow a statute on the position of the vertebra marking the beginning of the last cervical module. From a morphological standpoint, the fossil P. antarcticus differs from extant penguins through a more massive appearance of certain vertebrae (C VIII and C IX). In addition, the ventral morphology is completely original to this fossil on the last cervical vertebra.
Icadyptes salasi
Icadyptes salasi belongs to the giant fossil penguins. The remains of this specimen from South America are characterised by a narrow and elongate skull, a complete forelimb, and an especially robust cervical system represented by nine cervical vertebrae.
Axis: Mo 2
The processus ventralis corporis is more backward oriented than it is for A. patagonicus. The processus spinosus is moderately backward oriented, just as in A. patagonicus, but its basis is anteroposteriorly longer—closer to the anterior facies articularis. The upper part of the dens axis is horizontal, but among extant species, this surface is obliquely upward oriented. The degree of development of the tori dorsalis is similar to that is observed in A. patagonicus.
Ksepka et al. (2008) identified the following vertebrae, sometimes connected together, ranging from IV to XI. This determination is confirmed here, through the position of tori dorsalis. Indeed, they are found just before the zygapophysis caudalis on C VI, C VII, and C VIII, then on the zygapophyisis caudalis on C IX, C X and CXI.
This arrangement is identical to that observed in extant species, Pygoscelis papua and Eudyptes chrysolophus. This suggests that in Icadyptes salasi, the transitional vertebra or Mo 4 would be the fifth, as in the two living representatives mentioned above.
Fourth Cervical: Mo 3 (Fig. 5a)
Only the left zygapophysis caudalis is preserved as being connected to the corresponding zygapophysis cranialis of the next vertebra. Its orientation is similar to that found in A. patagonicus: it is the same about the shape. In comparison with existing species, tori dorsalis of module 3—at least the second of its vertebra—are less developed than in Icadyptes salasi.
Some cervical vertebrae of Icadyptes salasi (from Ksepka et al. 2008). a Cervical IV and cervical V (top view); b cervical VI (left lateral view); c cervical VII (left latertal view); d: cervical VIII (top view); e: cervical X (top view). fcr: facies articularis cranialis; pco: processus costalis; ps: processus spinosus; td: torus dorsalis (pl. tori dorsalis); zcd: zygapophysis caudalis; zcr: zygapophysis cranialis. Although the vertebrae have suffered from the conditions of fossilisation, it can be underlined that the fifth vertebra (a) is of transitional nature; I. salasi thus belongs to the configuration Tr V model. Also note the presence of a processus spinosus on the first vertebra of module 5, the sixth, that is still identifiable on C VII. Scale bars = 1 cm
Fifth Cervical: Mo 4 (Fig. 5a)
The morphology confirms the transitional nature. The zygapophysis cranialis are still directed forward (lateral view), and the zygapophysis caudalis are characteristic of the next module, the fifth. The transitional modular configuration of Icadyptes salasi is therefore similar to that of Pygoscelis and Eudyptes genera. Note that the tori dorsalis are poorly developed, unlike in the living species, and there is no preeminent processus spinosus on this vertebra. Its total absence would be surprising, since it is mostly present on the transitional vertebra for the last time before reappearing on module 6. Maybe it was not preserved. Anyway, while taking into account the compression factor in the fossilisation, this transitional vertebra is more elongated than in extant species of configuration Tr V. Moreover, it is surprising that the vertebral arch covers the posterior centrum, as it is clearly visible in living penguins. The crushing of fossilisation may also be to blame.
Sixth Cervical: Mo 5 (Fig. 5b)
When compared to extant species, the processus costalis are more obliquely and downwards oriented. This may be connected with a facies articularis caudalis of C V covered by the vertebral arch. Nevertheless, this suggests an important cranial projection of the facies articularis cranialis. Finally, as for fossil P. antarticus, the processus spinosus is still present on the first vertebra of module 5, here as a straight blade (flattened at the top). Among extant species, Spheniscus humboldti exhibits a processus spinosus but its form is a slight peak. This may be a primitive feature.
Seventh Cervical: Mo 5 (Fig. 5c)
There is still a processus spinosus but it is a very low spike. The upright and size of the zygapophysis cranialis are more defined. Considering that stretch upwards, A. patagonicus presents this characteristic on the morphological corresponding vertebra, the eighth, but it is slightly less pronounced. Bring to mind that this type of morphology is observed on C VIII of the fossil representative P. antarcticus, but stronger. The cranial projection of the facies articularis cranialis is still important.
Eighth Cervical: Mo 5 (Fig. 5d)
The processus spinosus is no more apparent but a clear sagittal crest runs on the back of the vertebra. The inward orientation of zygapophysis cranialis is more important than in the previous vertebra. In fact, this feature increases gradually from the beginning of the module. However, the shape is not lengthened upwards as much; the zygapophysis cranialis and caudalis are on the same alignment, unlike the previous vertebra (lateral view).
Ninth Cervical: Mo?
Even if fossilisation has not preserved the lower and ventral part, it is clear that the cranial projection of the facies articularis cranialis is important. The shape and location of the tori dorsalis are similar to that found in A. patagonicus on the morphological corresponding vertebra, namely C X.
Tenth Cervical: Mo 6 (?) (Fig. 5e)
Morphology with more forward-projected zygapophysis cranialis is a sign of involvement in the second curve of the neck (of convex type). The degree of projection of the facies articularis cranialis seems less important than in the living species of configuration Tr V. This is unusual because C IX and C XI exhibit a marked development of this feature. Compressions and constraints of fossilisation may be to blame. The lateral orientation of zygapophysis caudalis is also lower than in the extant penguins.
Eleventh Cervical: Mo 6
Although the ventral part is not preserved—making the identification of a processus ventralis corporis impossible—it is likely that the eleventh cervical belongs to the last module of the cervical system. Indeed, this characteristic is proven in the living representatives, regardless of their transitional configuration. The tori dorsalis are similar in shape and development to those of P. papua.
Icadyptes salasi is also characterised by the configuration Tr V, but because of the poor preservation of the ventral area of the vertebrae C IX and C X, it is not possible to determine which vertebra marks the beginning of module 6. I. salasi predates Paraptenodytes antarcticus, and one can note some similarities between these two fossil representatives in the persistence of a noticeable processus spinosus on the first vertebra of module 5 (C VI) and in vertical elongation of the zygapohysis cranialis (C VII) compared to living species. But Icadyptes salasi differs from P. antarcticus by other characteristics. The condyle, the connection between the skull and first cervical vertebra, is large. This suggests a massive atlas, the first cervical vertebra. Ksepka et al. (2008) noted that the vertebrae of the middle section of the neck are almost as wide as the skull. It is clear that this factor is due to the elongation of the cranium and its median shortening (top view). The vertebrae of the middle zone of the neck are clearly longer, but the shortening is marked between the eighth and ninth cervical (Ksepka et al. 2008). The absence of extant species of large sizes with a configuration Tr V also strengthens the singularity of Icadyptes salasi; the genus Aptenodytes is the tallest among living representatives, being of configuration Tr VI.
Madrynornis mirandus
An outstanding particularity of this other South American fossil penguin, more recent than the two species studied above, is the preservation of all of thirteen cervical vertebrae (Fig. 6). Therefore, the study of the full modular distribution of the neck is possible. The description of vertebrae is given here for the first time.
Cervical vertebrae of Madrynornis mirandus. a Cervical I, atlas—Mo 1 (cranial view); b cervical II, axis—Mo 2 (top view); c cervical III—Mo 3 (left side view); d cervical IV—Mo 3 (top view); e cervical V—Mo 3 (top view); f cervical VI—Mo 4 (top view); g cervical VII—Mo 5 (left lateral view); h cervical VIII—Mo 5 (left lateral view); i cervical IX—Mo 5 (top view); j cervical X—Mo 6 (left lateral view); k cervical XI—Mo 6 (left lateral view); l cervical XII—Mo 6 (top view); m cervical XIII—Mo 6 (top view). The conservation of the entire cervical vertebrae allows one to define the exact modularity of this axial skeleton segment of M. mirandus. The transitional vertebra is the sixth and cervical X marks the beginning of module 6. The very particular characteristic of this fossil species is a more important development of the ansa costotransversaria on C XIII in comparison to the extant species classified in the configuration Tr VI model (the proportion is similar to that of the extant species characterised by configuration Tr V). Scale bars = 1 cm
First Cervical, Atlas: Mo 1 (Fig. 6a)
The depth of the incisura fossae is equivalent to that of S. humboldti, P. papua and E. chrysolophus. The tori dorsalis are more backward oriented than in living species, as are the edges of the associated parts of arcus atlantis. The general morphology is close to that of P. papua, although the ventral surface is similar to that of S. humboldti.
Second Cervical, Axis: Mo 2 (Fig. 6b)
The axis is mostly similar to that of S. humboldti. However, the processus spinosus is closer to the vertical orientation and larger. Conversely, the processus ventralis corporis is more backward oriented, approaching the alignment of A. patagonicus, although the shape is thinner and longer, and its tip is similar to that of E. chrysolophus. The extension and the projection of tori dorsalis are equivalent for A. patagonicus.
Third Cervical: Mo 3 (Fig. 6c)
The tori dorsalis are shaped like a collarette, as in E. chrysolophus and S. humboldti, but more developed, and their orientation is similar to that of these two species. The processus transversus are straight, as in P. papua, and their basis is similar to that of E. chrysolophus. The processus spinosus has a caudal projection similar to that of S. humboldti but its tip is oriented upwards. As for the processus ventralis corporis, it is larger and finer than in extant species.
Fourth Cervical: Mo 3 (Fig. 6d)
The processus spinosus is backwards oriented in its entirety, as in S. humboldti, and shaped as a blade of constant proportion throughout its length (as in E. chrysolophus). However, its size is more important than in living penguins and the tori dorsalis are broader, with their extremities pointing in the caudal direction (as in E. chyrsolophus and P. papua). The processus ventralis corporis, logically reduced compared to the previous vertebra, is rather well developed. In spite of the fracture at the root of this apophysis it is clear that its size is still noteworthy; at least to a degree similar to that found in S. humboldti (the size is smaller in the other three living species).
Fifth Cervical: Mo 3 (Fig. 6e)
The processus spinosus is oriented vertically, as in A. patagonicus, but of a larger size, preserving the morphology of a blade rounded at its apex. The forward orientation of zygapohysis cranialis is less pronounced than that in A. patagonicus and is therefore close to S. humboldti. The tori dorsalis are oriented laterally, not forward as in both extant species listed for this comparison, and they are proportionately larger. Another novelty of M. mirandus is the line shape of the vertebral body between the zygapophysis caudalis (top view): it is straight, whereas in the compared living representatives, it is curved. The processus ventralis corporis is very reduced, as in extant A. patagonicus and S. humboldti, but identifiable by a protuberance prior to the facies articularis caudalis. However, in M. mirandus, the spot is positioned a little before.
Sixth Cervical: Mo 4 (Fig. 6f)
The orientation of the zygapophysis cranialis, similar to that of A. patagonicus—still tilted forward but starting to be erect compared to the previous vertebrae of module 3—defines the transitional position. Madrynornis mirandus is of configuration Tr VI; a group also including S. humboldti. The morphology of the posterior half of the vertebra confirms this determination (zygapophysis caudalis backward oriented). The processus spinosus is a vertical spike, thickened at its apex, and the tori dorsalis are similar to those of S. humdoldti. However, M. mirandus differs from extant species because the processus transversus are more developed and bend backwards, with the ansa costotransversaria following this movement as well.
Seventh Cervical: Mo 5 (Fig. 6g)
The fully erected zygapophysis cranialis are characteristic of the first vertebra of module 5, with the facies articularis cranialis projected forward. There is the presence of a slight protuberance as a remainder of the processus spinosus, as for S. humboldti. Tori dorsalis also have a similar morphology to that of the latter species. The processus transversus are similar in size to the living representatives. The line separating the zygapophysis caudalis is less curved than in the preceding vertebra (top view).
Eighth Cervical: Mo 5 (Fig. 6h)
The vertebral arch is slightly curved as in the corresponding morphological vertebra of P. papua (the seventh), but there are slight crested lines that reach the midline of the back vertebrae (as in S. humboldti, but clearer). Tori dorsalis are developed in a proportion similar to that found in E. chrysolophus (C VII). There are massive processus transversus, as for S. humboldti, but the ventral line of the vertebral body is straighter. As for ansa costotraversaria, they are developed but the extremities are not directed backwards (as for E. chrysolophus). The shape of zygapophysis cranialis is close to that of E. chrysolophus (C VII), and the line separating the zygapophysis caudalis is less curved than in comparable extant species.
Ninth Cervical: Mo 5 (Fig. 6i)
The tori dorsalis are visible and developed as for A. patagonicus and E. chrysolophus (C VIII) but the crested lines joining the central axis of the vertebral arch are more erased than in the previous vertebra. The size of ansa costotransversaria is slightly reduced compared to C VIII, with a proportion similar to extant species A. patagonicus, E. chrysolophus (C VIII), and P. papua (C VIII), without sharing the characteristic of being oriented slightly backwards at the extremity. The line between zygapophysis caudalis is still less curved than in comparable living species.
Tenth Cervical: Mo 6 (Fig. 6j)
The appearance of a processus ventralis corporis, succeeding the double processus caroticus of the previous module, marks the beginning of the sixth. Its position on C X is identical to that of S. humboldti and juvenile A. patagonicus. It is a rounded blade directed forwards as in S. humboldti. However, an extra blade is just in front of the facies articularis caudalis, a feature also present on C X in E. chrysolophus. However, it must be remembered that M. mirandus and E. chrysolophus are respectively classified as configuration Tr VI and configuration Tr V; therefore, the corresponding cervical morphology of E. chrysolophus is C IX. This similarity is interesting to underline. The tori dorsalis are equivalent to that of A. patagonicus. Generally, we note that the morphology of the vertebra is similar to that of S. humboldti, but that the top of the vertebra is more rounded.
Eleventh Cervical: Mo 6 (Fig. 6k)
The morphology and orientation of the processus ventralis corporis are similar to those of S. humboldti. However, unlike this extant species, and P. papua for the morphological corresponding vertebra (C X), there is no small processus spinosus. Instead, the vertebral arch is very flat. The tori dorsalis are developed in identical proportion to those of A. patagonicus and E. chrysolophus (C X). While zygapophysis cranialis are slightly forward oriented, orientations of processus caroticus and processus transversus are similar to those of A. patagonicus but the features are larger. In addition, M. mirandus, unlike the extant species, shows a greater development of ansa costotransversaria.
Twelfth Cervical: Mo 6 (Fig. 6l)
The orientation of the processus ventralis corporis is close to vertical, as for S. humboldti, but is larger. In addition, the apophysis conserves its rounded blade morphology. The subtle reappearance of the processus spinosus as a tiny spike converges with the observation of A. patagoncius, as well as of P. papua (C XI) and E. chrysolophus (C XI). The tori dorsalis are still easily identifiable. An increase in the size of ansa costotranversaria continues and exceeds that of A. patagonicus and S. humboldti. The curve of the line separating the zygapophysis caudalis is still less apparent than in extant species.
Thirteenth Cervical: Mo 6 (Fig. 6m)
There are small asperities on the posterior end of the vertebral arch, on each side of the axis of the processus spinosus—a low blade running on the top of the vertebra (as for A. patagonicus). The processus transversus are slightly more developed than in extant species. The axis of elongation of the ansa costotranversaria corresponds to that of S. humboldti (perpendicular), but the size of these processes is more important in M. mirandus (substantially close to that in P. papua and E. chrysolophus for C XII). The morphology of the posterior part of the vertebral arch is similar to that of P. papua and E. chrysolophus. As for the processus ventralis corporis, its orientation and proportion are similar to E. chrysolophus (C XII). In species with a similar transitional configuration, it is either more (A. patagonicus) or less (S. humboldti) developed.
Madrynornis mirandus is the first known fossil species to be characterised by the configuration Tr VI. With the tenth cervical marking the beginning of module 6, the cervical system of this extinct penguin has a modular arrangement similar to that of Spheniscus humboldti, but is also comparable to that of a juvenile Aptenodytes patagonicus. Unlike the older fossil penguins, such as P. antarcticus and I. salasi, M. mirandus shares general morphological similarities with extant species and those that are common with S. humboldti are not negligible. However, M. mirandus also possesses its particularities. The most surprising finding is found in module 6, in the posterior part of the neck. When compared with extant species of configuration Tr VI, M. mirandus shows a large development of the ansa costotransversaria. This feature follows an extension degree similar to that of extant species of configuration Tr V, a surprising association. Similarly, the morphology of the module 5—with the posterior portion of the vertebral arches—is specific to this fossil species.
Complete Natural Homeoses and Variations of the Cervical Modularity
Identification of Two Types of Homeotic Transformations
During the development of an organism the activity of the Hox genes is crucial to mediate the antero-posterior identity of the individual (Morgan 1997), and more genes are involved in controlling the structure of morphogenesis and in particular the positioning and identity of the vertebrae. Mutations in homeotic genes lead to homeotic transformation or homeosis. This is the formation of a normal organ structure in place of another at an abnormal anatomical site.
In the context of the vertebral column, homeoses describe a class of phenotypes in which one vertebra is characterised by the features of its immediate neighbour, anterior or posterior, while the number of vertebrae is constant—such states are qualified as anterior or posterior homeotic transformations. Natural cases have been identified in the human skeleton (Oostra et al. 2005), for example, with the thoracalisation of the first lumbar vertebra, carrying “lumbar ribs”. The identity transformation usually affects certain specific vertebral structures and not the entire vertebra. In the vertebral skeleton, one can also find numerical anomalies (too many or too few vertebrae). In this latter case, for example, there may be the presence of an additional vertebra in the thoracic segment leading, in particular, to the formation of a transitional thoracolumbar vertebra with articulating rib rudiment (Oostra et al. 2005). Numerical anomalies and segmental homeotic transformations are different conditions but both result from an altered homeotic pattern (Oostra et al. 2005). It is interesting to note that this type of occurrence is at the limit of two different identity segments within the vertebral skeleton (e.g., thoracic/lumbar limit).
In the light of these definitions, it is clear that homeotic transformations apply to the differences highlighted between the extant species of penguins (Fig. 1). The first shift observed in the morphological correspondences shows that the penguin species with a transitional vertebra (Mo 4) in position six have an extra vertebra in the previous module. This is compensated by the reduction of one vertebra at the end of the cervical sequence in comparison with species where the transitional vertebra (Mo 4) is in the fifth position. The morphological plan of a given module is extended in both transitional configurations, where the total number of cervical vertebrae is still thirteen. Thus, cervical five compared in two species of penguins with different transitional configurations does not have the same identity. This characteristic represents the first type of homeosis observed in Sphenisciformes. The position of the transitional vertebra (Mo 4) is a feature unique to each species and does not change during post-hatching growth (Fig. 7). Consequently, this first type of homeotic transformation is called a complete fixed homeosis—complete identity transformation from one vertebra to another (and, therefore, implying a different function).
Modular repartition of the cervical system in four extant species of penguins (adult and juvenile stages) based on morphological features. juv. Apt. p.: juvenile Aptenodytes patagonicus; ad. Apt. p.: adult Aptenodytes patagonicus; Sph. h.: Spheniscus humboldti; Eud. c.: Eudyptes chrysolophus; juv. Pyg. p.: juvenile Pygoscelis papua; ad. Pyg. p.: adult Pygoscelis papua; Tr: Transitional vertebra (Mo 4 Module 4)
The second shift observed in the cervical sequence concerns the beginning of module 6. Surprisingly, it can be identified between adults of different species (Fig. 1) but sometimes within the same species during the growth of the chick (Fig. 7). In this second state of occurrence, one vertebra acquires the ventral identity characteristic of the preceding vertebra, thus shifting the distribution within modules 5 and 6—transformation from a processus ventralis corporis to two processus caroticus (Fig. 8). This variation of identity during the growth of the chick has been identified in Aptenodytes patagonicus and Pygoscelis papua from ontogenetic follow-up of several individuals at different stages of growth (Guinard et al. 2010).
Modification of the ventral morphology of cervical IX during growth in Pygoscelis papua with the transition of a processus ventralis corporis (pvc) to two processus caroticus (pc)—cranial views. a Sub-adult; b adult. Note that parts of the processus transversus are missing in a, this is because they are not completely fused with the rest of the vertebra in juveniles. An intermediate phase of transformation between the two morphologies can also be observed in A. patagonicus, where C X undergoes the same change (see Fig. 12 in Guinard et al. 2010). Scale bar = 1 cm
Eudyptes chrysolophus and Spheniscus humboldti do not appear to exhibit this trait in their various stages after hatching (but the possibility that it takes place during the development in the egg cannot be excluded). Qualifying this homeosis is more subtle because of its dual identification—between adults of different species and within the same species during the post-hatching growth. At first glance, the variation of identity may be less drastic than with complete fixed homeosis. Indeed, only a part of the identity of the vertebra is transformed (an apophysis). However, this is the only difference that defines belonging to two different modules: the fifth and sixth. The processus ventralis corporis is a powerful muscle attachment (Fig. 1) that implies a different mechanical function compared to module 5. Obviously, the processus ventralis corporis is small in a growing penguin, like all the other processes of the vertebrae, but the analysis does not consider this state. The fact is that the identity variation of this character is complete; the two new processus caroticus morphologies are not rudimentary but entire bony features. In conclusion, this second homeotic transformation is complete in its occurrence during growth, as well as between adults of different species. Anyway, the dual nature of this homeotic transformation leads to qualify it as a complete inter/intraspecific homeosis.
Influence of the Homeotic Genes
In vertebrates, the number of units (somites) that contribute to the plan of each part of the anterior-posterior axis is variable—“transposition” is used to describe this state. Experiments conducted on Hox genes in mice have shown that they are partly responsible for the specialisation of a particular segmental identity along the anterior-posterior axis. Thus, a Hox code determining the morphology of each vertebra was proposed (Kessel et al. 1990). Burke et al. (1995) conducted a comparative study of the expression of the Hox genes during development in two animals having the same regulatory genes but very different morphologies: the mouse and the chicken. It is therefore interesting to try to detect which somites might be involved and which Hox genes would control the areas recognised in penguins.
The vertebrae come from the evolution of an internal part of the somites called sclerotome, which differentiates into vertebrae following their position along the anterior-posterior axis. The different anatomical zones (cervical, thoracic, lumbar, sacral, and caudal) illustrate this. The morphological differentiation is also possible within these major segments: the modules identified in the cervical part. It is commonly accepted that each plan of a vertebra come from halves of two consecutive sclerotomes, as shown in Table 3 with the example of the chicken (fourteen cervical vertebrae). Thus, somites 1–5 constitute the neurocranium and the first half of the atlas (C I); the next somites build the rest of the vertebral skeleton.
Nevertheless, it must be underlined that the fifth somite participates in the development of the anterior extremity of the second cervical vertebra, the axis, namely the part that fits into the atlas (dens axis), as demonstrated by work on the quail (Huang et al. 2000). The difference between the somitic origin of the anterior extremity of the axis and the remaining area of the anterior cranial part of this vertebra is confirmed by the existence of a joint visible only during the juveniles stages of the king penguin, for example (Fig. 9)—apparently not described before.
New formed somites already possess the anteroposterior information necessary for generating their final vertebral identity. Indeed, when the places of somites are experimentally changed, they generate a characteristic structure of their origin and not of their new position (Kant and Goldstein 1999; Nowicki and Burke 2000; Alexander et al. 2009). The determination of the overall pattern of somites is therefore conducted prior to formation, and it seems that the pre-somitic mesoderm plays an important role in structuring (Carapuço et al. 2005). Thus, the observed changes in penguins are the consequence of processes occurring early in the development of the bird (Fig. 10).
By extension from the chicken and quail, it is likely that in penguins, somites 1–5 are also involved in the formation of the neurocranium and in the anterior part of the atlas (plus the anterior extremity of the axis—dens axis). In continuity, somites 6–18 constitute the remaining cervical vertebrae (thirteen in number). Burke et al. (1995) established a table summarising the correspondence of the anterior limits of expression of somitic Hox for the chicken and the mouse. For the cervical region in chicken, Hoxb-4 and Hoxd-4 express from somites 7–8, Hoxa-4 and Hoxc-4 from somites 10–11, and Hoxc-5 from somites 17–18. Even though in the mouse and the chicken, the number of cervical vertebrae is not the same (7 vs. 14) the limits of expression between the main axial units (cervical and thoracic) are identical. In both cases, the identical gene Hoxc-6 marks the boundary between the cervical and thoracic segments. It is the same with the goose, with 17 cervical vertebrae. These boundary correspondences, despite the number of vertebrae, show a fundamental link with limb position and spinal nerves (Burke et al. 1995). This also suggests that the development of homologous axial structures conserves the Hox code (Gaunt 1994).
According to Burke et al. (1995), Hoxb-4 and Hoxd-4 in the chicken are associated with the anterior part of the neck and stop activity at the level of somites 9 and 10 (cervical V), where Hoxa-4 and Hoxc-4 begin full expression, with somites 10 and 11 (posterior s10 + anterior s11 = C VI). The results are observed in embryos at 4½ and 5 days old (stages 25–26 of development). It must be underlined that, according to these authors, the precise counting of somites is difficult at the embryological stages studied. As the anterior somites cannot be accurately counted, the limbs were used as points of reference to determine the level of somites. Nevertheless, they consider that the level of somites shown in their study is accurate to plus or minus one somite.
Besides, in a similar work, Gaunt (2000) obtained a different result, since the anterior limit of expression of Hoxa-4 is placed at somites 9 and 10 (posterior s9 + anterior s10 = cervical V) and 5½ days of growth. Moreover, Gaunt (2000) states that the anterior limit of expression of Hoxa-4 in the chick is different when observed at 1½ and 5½ days of growth. Finally, Horan et al. (1994) also showed that in the mouse, Hoxa-4 plays a role in the positioning information of the identity of certain vertebrae along the anterior-posterior axis. Observations on skeletons of ckickens (Gallus gallus and Gallus domesticus, collections of the MNHN) show that in these birds, the transitional vertebra (Mo 4) is the cervical V. The basis of the reasoning which follows, applied to penguins, will therefore be based on the results of Gaunt (2000), assuming that the beginning of the expression of at least Hoxa-4 and Hoxc-4 implies the formation of the transitional vertebra in the cervical avian system. Thus one might wonder if a variation of the expression of at least genes Hoxa-4 and Hoxc-4 in the first days of embryonic development could be responsible for the shift of the transitional vertebra between the different species of penguins (Fig. 5).
The coupling activity of Hoxa-4 and Hoxc-4 is more important to take into account than the hypothetical individual mutations of each of these genes (Horan et al. 1995). Indeed, the Hox genes 1–13 of vertebrates are placed in four groups—A, B, C and D—called clusters. Those of paralogous clusters play complementary role(s) in relation to each other (Suemori and Noguchi 2000). Vertebral morphology is the result of the combination of features provided by the Hox paralogs. In the mouse, vertebrae C III to C V have a similar morphology, with a unique combination of Hox expression; for C VI and C VII, each is different and they have their unique combination (Kessel and Gruss 1991; Wellik 2007). This suggests that each vertebral module would have its own combination of Hox expression. In addition, at least two paralogous groups contribute to the pattern of each vertebra: the axial skeleton finding a normal pattern after and prior to homeotic transformations (Wellik 2007).
Influence of Molecules Intervening in the Regulation of Hox Genes
The developmental regulation of Hox genes is achieved through different types of signal molecules. As an example, the retinoids (Marshall et al. 1996), whose signal defect can cause a misregulation of the Hox expression leading to shifts in the rostral expression domain of Hox genes containing functional RAREs—retinoid acid responses elements (Colon 1995; Alexander et al. 2009). Other interveners and mechanisms in the Hox regulation process can affect the homeotic transformation (Abu-Abed et al. 2000; Ikeya and Takada 2001; Cordes et al. 2004). The complexity of interactions during the spatial and temporal expression of Hox genes through development has been reviewed in the literature (Deschamps et al. 1999; Deschamps and Van Nes 2005; Young et al. 2009).
The Case of the Inter/Intraspecific Homeosis and the Limit of Mo 5/Mo 6
One aspect of the inter/intraspecific complete homeosis is its occurrence during the growth of some chick penguins. As the development of the vertebrae is already established in very early stages of development, the evolution of this transformation is already planned in the concerned species (A. patagonicus and P. papua). In this case, the involvement of Hox genes is more difficult to understand. Their role after birth remains poorly characterised, but their expression exists (Aubin and Jeanotte 2001). Specifically, a variety of mechanisms could be employed to ensure the spread of early patterns of Hox expression in paraxial mesoderm in late development and adulthood (Alexander et al. 2009).
Conclusion
The variations in the organisation of the cervical system in extant penguins illustrate outstanding cases of complete natural homeotic transformations: modification of the identity of a vertebra into another, in the direct neighbourhood, without modification of the total number of vertebral elements (thirteen). Incomplete or transitional homeotic transformations naturally observed, or induced by experiences, are individual variations of a dominant morphological plan—considered as abnormalities—and may be associated with other anatomical peculiarities. However, in penguins, the homeoses are complete and do not fall within the above category: they define a new morphological vertebral plan. Complete homeotic transformations in penguins also show a real evolutionary ability and the production of natural new phenotypes, through interactions and mechanisms investigated by others in laboratory experiments.
-
1)
The first type is a complete fixed homeosis, with a transitional vertebra (Mo 4) as C V or C VI. Identity of future vertebrae are established very early in development, thus this variation is probably connected with modifications involving Hox genes, whose influence on the vertebral homeoses is not lacking in the experimental literature. This might be, at least, the coupled action of Hoxa-4 and Hoxc-4, starting their activity in the future cervical V in the chicken (which is also the transitional vertebra), which could play a role in this transformation. Since the limits of expression of Hoxa-4 and Hoxc-4 are not fixed in the early stages of embryonic development in the chicken, a spatial shift of expression in some species of penguins would not be surprising. The involvement of other Hox paralogs is possible, as well as the influence of some molecules implicated in the activity of the Hox code.
-
2)
The second type of homeotic transformation affects the first vertebra of the module 6—sometimes even during growth of some species—with an identity shift characteristic of the preceding vertebra and last of module 5. A. patagonicus and P. papua are, at this time, the only penguins identified for the post-hatching occurrence of this homeotic transformation. But there is no evidence that this change does not occur within the egg for other species. The vertebral identity is determined very early in development; the Hox genes involved in the formation of the vertebrae in the early embryonic stages probably establish this complete inter/intraspecific homeosis. However, those Hox genes do not necessarily govern the further transformation, so their role in growth and adulthood implies significant additional investigations. Yet, the fact that this second homeotic transformation can also occur during post-hatching growth, and is independent of the position of the transitional vertebra (A. patagonicus and P. papua differ on this feature but they are concerned by the complete inter/intra homeosis during growth), suggests a certain complexity of genetic mixing in the group of Sphenisciformes. It is also interesting to consider that this complete inter/intraspecific homeosis, still in the case of expression during growth, occurs on the fourth vertebra after the transitional vertebra in the concerned species, despite the difference in position of that transitional vertebra.
Moreover, it is useful to remember that these transformations of identities do not affect the mechanical folding of the cervical system. As a consequence, the presence of an extra vertebra characteristic of modules 3 or 6 may “help” the first or second morphological curvature of the neck. The system may be balanced in this way between the species affected by these changes. Differences in a more or less used folding—in both intensity and duration—concern, for example, features, such as the cranial projection of the facies articularis cranialis (Guinard et al. 2010).
Nevertheless, in theory, embryological studies on penguins would test hypotheses of the involvement of Hox genes, plus their regulators and later vectors, about these two types of homeotic transformations. However, this biological model is difficult to consider for such investigations. The road remains open to further research in molecular genetics and in the genetics of development on avian representatives available for use in a laboratory.
A comparison with some fossil representatives has allowed one to clarify that the general structure of penguins’ cervical systems does not appear to have fundamentally changed during the evolution of the group. The modular configuration characterising the positioning of the transitional vertebra (module 4) at the level of cervical V seems to have existed during the late Eocene (circa −36 My) with Icadyptes salasi, along with Paraptenodytes antarcticus (Miocene, −25 My). The other configuration—transitional vertebra in the sixth position—exists with certainty at the end of the Miocene (circa −10 My) with Madrynornis mirandus.
The question is whether these two configurations have emerged following that order, or if they had cohabited since a period prior to Icadyptes salasi. Although the sufficiently comprehensive fossil material to the cervical region is low, the dominance of the configuration Tr V in extant species would suggest that this is the basic model and that the configuration Tr VI is derived from it via a posterior shift of the expression of the Hox genes involved in its formation. Then, the two complete natural homeotic transformations identified in penguins would be anteriorisations (since in both cases it would be the acquirement of the morphology of the adjacent vertebra from the previous module). This hypothesis will be preferred for now. The clear distinction in structure between the ancestral species I. salasi and P. antartcticus, with vertebral features clearly absent in extant species (and even in M. mirandus) suggests that these two genera are from a different stem than the recent species and later fossil representatives come from.
Notes
Adult A. patagonicus possess tiny ventral apophyses on their transitional vertebra but these cannot be regarded as the strong processus caroticus characteristic of module 5 in all species. This is why the word “true” is used.
References
Abu-Abed, S., Dolle, P., Metzger, D., Beckett, B., Chambon, P., & Petkovich, M. (2000). The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes and Development, 15, 226–240.
Acosta Hospitaleche, C., Tambussi, C., Donato, M., & Cozzuol, M. (2007). A new Miocene penguin from Patagonia and its phylogenetic relationships. Acta Palaeontologica Polonica, 52(2), 299–314.
Alexander, T., Nolte, C., & Krumlauf, R. (2009). Hox Genes and segmentation of the hindbrain and axial skeleton. Annual Review of Cell and Developmental Biology, 25, 431–456.
Aubin, J., & Jeanotte, L. (2001). Implication des gènes Hox dans les processus d’organogenèse chez les mammifères. Medicine and Science, 17, 54–62.
Baumel, J. J., King, A. S., Breazile, J. E., Evans, H. E., & Vanden Berge, J. C. (1993). Handbook of avian anatomy: Nomina anatomica avium (2nd ed.). Cambridge, MA: Publications of the Nuttall Ornithological Club.
Bertelli, S., Giannini, N. P., & Ksepka, D. T. (2006). Redescription and phylogenetic position of the early Miocene penguin Paraptenodytes antarcticus from Patagonia. American Museum Novitates, 3525, 1–36.
Burke, A. C., Nelson, C. E., Morgan, B. A., & Tabin, C. (1995). Hox genes and the evolution of vertebrate axial morphology. Development, 121, 333–346.
Carapuço, M., Nóvoa, A., Bobola, N., & Mallo, M. (2005). Hox genes specify vertebral types in the presomitic mesoderm. Genes and Development, 19, 2116–2121.
Clarke, J. A., Ksepka, D. T., Stucchi, M., Urbina, M., Giannini, N., Bertelli, D., et al. (2007). Paleogene equatorial penguins challenge the proposed relationship between biogeography, diversity, and Cenozoic climate change. Proceedings of the National Academy of Science, 104(28), 11545–11550.
Colon, R. A. (1995). Retinoic acid and pattern formation in vertebrates. Trends in Genetics, 11(8), 314–319.
Cordes, R., Schuster-Gossler, K., Serth, K., & Gossler, A. (2004). Specification of vertebral identity is coupled to Notch signaling and the segmentation clock. Development, 131, 1221–1233.
Deschamps, J., Van Den Akker, E., Forlani, S., De Graaff, W., Oosterveen, T., Roelen, B., et al. (1999). Initiation, establishment and maintenance of Hox gene expression patterns in the mouse. International Journal of Developmental Biology, 43, 635–650.
Deschamps, J., & Van Nes, J. (2005). Developmental regulation of the Hox genes during axial morphogenesis in the mouse. Development, 132(13), 2931–2942.
Gaunt, S. J. (1994). Conservation in the Hox code during morphological evolution. International Journal of Developmental Biology, 38(3), 549–552.
Gaunt, S. J. (2000). Evolutionary shifts of vertebrate structures and Hox expression up and down the axial series of segments: a consideration of possible mechanisms. International Journal of Developmental Biology, 44, 109–117.
Guinard, G., Marchand, D., Courant, F., Gauthier-Clerc, M., & Le Bohec, C. (2010). Morphology, ontogenesis and mechanics of cervical vertebrae in four species of Penguins (Aves: Spheniscidae). Polar Biology, 33, 807–822.
Horan, G. S., Ramirez-Solis, R., Featherstone, M. S., Wolgemuth, D. J., Bradley, A., & Behringer, R. R. (1995). Compound mutants for the paralogous Hoxa-4, Hoxb-4, and Hoxd-4 genes show more complete homeotic transformations and a dose-dependent increase in the number of vertebrae transformed. Genes and Development, 9, 1667–1677.
Horan, G. S. B., Wu, K., Woldgemuth, D. J., & Behringer, R. R. (1994). Homeotic transformation of cervical vertebrae in Hoxa-4 mutant mice. Proceedings of the National Academy of Science USA, 91, 12644–12648.
Huang, R., Zhi, Q., Patel, K., Wilting, J., & Christ, B. (2000). Contribution of single somites to the skeleton and muscles of the occipital and cervical regions in avian embryos. Anatomy and Embryology, 202(5), 375–383.
Ikeya, M., & Takada, S. (2001). Wnt-3a is required for somite specification along the anteropsoterior axis of the mouse embryo and for regulation of Cdx-1 expression. Mechanical Development, 103, 27–33.
Jadwisczak, P. (2006). Eocene penguins of Seymour Islands, Antarctica: Taxonomy. Polish Polar Research, 21(1), 3–62.
Kant, R., & Goldstein, R. S. (1999). Plasticity of axial identity among somites: Cranial somites can generate vertebrae without expressing Hox Genes appropriate to the trunk. Developmental Biology, 216, 507–520.
Kessel, M., Bailling, R., & Gruss, P. (1990). Variations of cervical vertebrae after expression of a Hox-1.1 transgene in mice. Cell, 61(2), 301–308.
Kessel, M., & Gruss, P. (1991). Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell, 67, 89–104.
Ksepka, D. T., Clarke, J. A., DeVries, T. J., & Urbina, M. (2008). Osteology of Icadyptes salasi a giant penguin from the Eocene of peru. Journal of Anatomy, 213, 131–147.
Marshall, H., Morrison, M. H., Studer, M., Pöpperl, H., & Krumlauf, R. (1996). Retinoids and Hox genes. The Federation of American Societies for Experimental Biology Journal, 10, 969–978.
Morgan, B. A. (1997). Hox genes and embryonic development. Poultry Science, 76, 96–104.
Nowicki, J. L., & Burke, A. C. (2000). Hox genes and morphological identity: Axial versus lateral patterning in the vertebrate mesoderm. Development, 127, 4265–4275.
Oostra, R. J., Hennekam, R. C. M., De Rooij, L., & Moorman, A. F. M. (2005). Malformations of the axial skeleton in museum Vrolik I: homeotic transformations and numerical anomalies. American Journal of Medical Genetics, 134A, 268–281.
Simpson, G. G. (1946). Fossil penguins. Bulletin of the American Museum of Natural History, 87.
Slack, K. E., Jones, C. M., Ando, T., Harrison, G. L. (Abby), Fordyce, R. E., Arnason, U., & Penny, D. (2006). Early penguin fossils, plus mitochondrial genomes, calibrate avian evolution. Molecular Biology and Evolution, 23(6), 1144–1155.
Suemori, H., & Noguchi, S. (2000). Hox C cluster genes are dispensable for overall body plan of mouse embryonic development. Developmental Biology, 220, 333–342.
Wellik, D. M. (2007). Hox patterning of the vertebrate axial skeleton. Developmental Dynamics, 236, 2454–2463.
Young, T., Rowland, J. E., Van de Ven, C., Bialecka, M., Novoa, A., Carapuco, M., et al. (2009). Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Developmental Cell, 17, 516–526.
Acknowledgments
Carolina Acosta Hospitaleche and Claudia Tambussi (Museo de La Plata), as well as Eduardo Ruigómez (Museo Egidio Feruglio Paleontólogico), for providing photographs of the cervical vertebrae of Madrynornis mirandus, thus allowing the study of this fossil species. Daniel Ksepka (North Carolina State University) for the transmission of additional photographs of the cervical V of Icadyptes salasi, in order to confirm the first impressions on this important vertebra. Rémi Laffont (Université de Bourgogne) for his help in providing some references, René Guinard for checking the manuscript and the department of comparative anatomy of the Muséum National d’Histoire Naturelle de Paris for access to the osteological collections. Benedikt Hallgrimsson—the editor in chief—and two anonymous reviewers, whose very pertinent comments have helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guinard, G., Marchand, D. Modularity and Complete Natural Homeoses in Cervical Vertebrae of Extant and Extinct Penguins (Aves: Sphenisciformes). Evol Biol 37, 210–226 (2010). https://doi.org/10.1007/s11692-010-9097-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-010-9097-0