Abstract
Mosaic evolution describes different rates of evolutionary change in different body units. Morphologically these units are described by more relationships within a unit than between different units which relates mosaic evolution with morphological integration and modularity. Recent evidence suggests mosaic evolution at the human basicranium due to different evolutionary rates of midline and lateral cranial base morphology but this hypothesis has not yet been addressed explicitly. We this hypothesis and explore how mosaic evolution relates to modular development. Evolutionary data sets on midline (N = 186) and lateral (N = 86) basicranial morphology are compared with 3D data on pre- and postnatal basicranial ontogeny (N = 71). Our results support the hypothesis of mosaic evolution and suggest a modular nature of basicranial development. Different embryological basicranial units likely became differently modified during evolution, with relatively stable midline elements and more variable lateral elements. In addition, developmental data suggests that modularity patterns change throughout ontogeny. During prenatal ontogeny lateral basicranial elements (greater sphenoid wings and petrosal pyramids) change together compared with the midline base. Close to birth the greater sphenoid wings keep a spatially stable position, while the petrosal pyramids become dissociated and shifted posteriorly. After birth the greater sphenoid wings and petrosal pyramids change again jointly and with respect to midline cranial base elements. This sequential pattern of integration and modularization and re-integration describes human basicranial ontogeny in a way that is potentially important for the understanding of evolutionary change. Phylogenetic modifications of this pattern during morphogenesis, growth, and development may underlie the mosaic evolution of the hominin basicranium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mosaic Evolution and its Relation to Modularity and Integration
Mosaic evolution describes different rates of evolutionary change in different units (body structures, or functions) of an organism/species and is informative about the evolution of complex morphological structures (Raff 1996; Wagner 1996; Wagner and Altenberg 1996; Klingenberg et al. 2003; Rosas and Bastir 2004).
Mosaic evolution (or mosaicism) of different morphological units can be closely related with morphological integration and modularity (Olson and Miller 1958; Chernoff and Magwene 1999; Bastir 2008). Morphological units of evolutionary change are characterised by more relationships within a unit than between different units, a definition that has been applied to modularity (Wagner 1996; von Dassow and Munro 1999; Klingenberg et al. 2003; Klingenberg 2005). In addition, more relationships within a module than between modules is equivalent to increased levels of morphological integration within a module versus weak integration levels between different modules (Olson and Miller 1958; Chernoff and Magwene 1999).
Modularity has been suggested to allow for evolvability (Riedl 1975; Wagner 1996; Wagner and Altenberg 1996) because low levels of morphological integration among different units permit evolutionary change in some units but not in others. Consequently, also the rate of evolutionary change can be faster in one module and slower in others leading then to mosaic evolution (Bastir 2008).
Since the work of Cheverud (1982, 1995, 1996) morphological integration and modularity have been addressed frequently in research on the evolution of primate and human skull (e.g. Ross and Ravosa 1993; Ackermann and Cheverud 2000; Marroig et al. 2004; Lieberman et al. 2000; Bookstein et al. 2003; Bastir and Rosas 2005; Bastir et al. 2005; Mitteroecker and Bookstein 2007, 2008). One interesting field of research in this context is the relationship of integration and modularity to mosaicism in human skull evolution. In particular, there is evidence (Ross and Henneberg 1995; Lieberman et al. 2000; Bastir et al. 2008) that the evolution of the basicranium might offer an interesting example for the study of mosaicism and modularity and its “evo–devo” relationships.
This paper analyses whether there is empirical support for mosaic evolution in human basicranial evolution. We further address the question whether and how mosaicism might relate to modular development. To what degree are evolutionary basicranial units comparable with developmental ones? Possible answers to this question require a combined analysis of evolutionary and developmental data.
Mosaic Evolution in the Hominin Basicranium
The extreme flexion of the human cranial base has attracted the attention of paleoanthropologists since the early days of anthropology and has classically been related to brain evolution and encephalisation (Virchow 1857; Dabelow 1931; Ford 1958; Biegert 1963; Ross and Ravosa 1993; Ross and Henneberg 1995; Lieberman et al. 2000). Anatomically, basicranial flexion is interesting because, due to its key position between the brain and the face, modification of the basicranial angle interacts with the position of the face (Dabelow 1931; Weidenreich 1941; Hofer 1952; Biegert 1957; Gould 1977; Lieberman et al. 2008; Bastir 2008).
Main stream ideas in paleoanthropology assert a strong correlation between encephalisation and cranial base flexion across ontogeny and phylogeny (Biegert 1957; Gould 1977; Ross and Ravosa 1993; Ross and Henneberg 1995; Spoor 1997; Lieberman et al. 2000; Ross et al. 2004), despite the fact that purely mechanistic principles assessed on the basis of prenatal data do not explain the underlying causal principles (Jeffery 2002a; Jeffery and Spoor 2002).
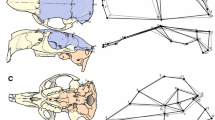
However, paleontological data have shown that basicranial flexion apparently overlaps within hominins of very different cranial capacities (Ross and Henneberg 1995). This can be assumed because within the evolution of modern humans comparative anatomical studies have shown that “modern” midline base morphology, that is, the cranial base angle between the pre-sphenoid plane and the spheno-occipital clivus (Fig. 1a) is achieved early in some fossils of Australopithecus and H. erectus (Fig. 1); (Ross and Henneberg 1995; Lieberman et al. 2000; Baba et al. 2003). In turn, a modern human pattern of lateral basicranial morphology has appeared late with the appearance of H. sapiens (Bastir et al. 2008).
These differences in the evolutionary timing of appearance of modern human basicranial features indicate different rates of morphological evolution of midline and lateral basicranial structures. However, no study has so far addressed empirically such a hypothesis of evolutionary mosaicism. Therefore, the first part of this paper analyses evolutionary patterns of midline and lateral base morphology, while the second part relates these data to basicranial development.
Developmental Modularity in the Basicranium
Embryologically, the basicranium consists of several developmental units. At the midline prechordal precursors of the presphenoid and the mesethmoid can be distinguished from the chordal basisphenoid and parachordal basioccipital (Sperber 1989; Larsen 2001). The midline consists of eight ossification centres, three of the presphenoid, four of the postsphenoid, and one of basioccipital (Sperber 1989). The orbitosphenoid, alisphenoid and otic capsules are parachordal elements and located at the lateral floor of the basicranium. Ontogenetic studies suggest differences in the onset of ossification as well as the time and rate of growth and development of different basicranial elements (O’Rahilly and Gardner 1972; Sperber 1989; Bach-Petersen and Kjaer 1993; Bach-Petersen et al. 1994; Bach-Petersen et al. 1995; Nemzek et al. 2000; Larsen 2001). While the lateral ali- and orbitosphenoids appear around 8 and 9 weeks respectively, of intra-uterine life, midline elements such as the pre- and post sphenoid start ossifying much later in the embryo, approximately around the 16th week (Sperber 1989; Bach-Petersen et al. 1994). These ossification centres lay the basis for the endochondral bone portions of the basicranium and its morphology (Sperber 1989; Nemzek et al. 2000).
Analysis of shape changes during fetal ontogeny has shown flexion followed by retro-flexion of the cranial base between 10 and 29 gestational weeks and coronal petrosal re-orientation associated with an increase of supratentorial brain volume (Jeffery 2002a; Jeffery and Spoor 2002). Lateral ontogenetic shape changes of the developing prenatal basicranium are relatively unknown and documented mainly (although not exclusively) on a descriptive morphological basis (O’Rahilly and Gardner 1972; Bach-Petersen and Kjaer 1993; Bach-Petersen et al. 1994; Bach-Petersen et al. 1995; Nemzek et al. 2000). 3D shape information about human prenatal basicranial growth and development is scarce (Plavcan and German 1995; Richtsmeier et al. 2006; Morimoto et al. 2008) and no study has so far addressed the endocranial shape changes of the prenatal basicranium.
Postnatal ontogeny shows spatio-temporal ontogenetic differences between midline and lateral basicranial elements. Adult values of midline base angle are achieved early in humans (2 years) (Lieberman and McCarthy 1999). Comparisons of non-adult with adults in longitudinal X-ray data show that adult midline base shape can be documented around 8 years of age (Bastir et al. 2006) whereas the shape of the lateral basicranial floor starts being indistinguishable from adults several years later, at about 12–14 years (Sgouros et al. 1999; Goodrich 2005; Bastir et al. 2006). On these findings a spatio-temporal pattern of basicranial ontogenetic modularity has been suggested implying dissociated ontogenies of midline and lateral elements (Bastir et al. 2006).
Basicranial ontogenetic modularity fits well with modularity detected in adult humans in which morphological semi-independence between variation patterns of midline and lateral elements (Bastir et al. 2004; Bastir and Rosas 2005) and between the anterior, middle and posterior cranial fossae have been identified (Bruner and Ripani 2008). However, again, 3D morphology, spatio-temporal patterning and modularity of the developing lateral basicranium during pre- and postnatal human ontogeny have not been addressed so far.
Methodological Considerations
Modularity and integration have been analysed using several methods, for example, factor analysis, principal components analysis, matrix correlations, path analysis, or partial least squares analysis (Zelditch 1987; Wagner 1990; Cheverud 1995; Chernoff and Magwene 1999; Ackermann and Cheverud 2000; Bookstein et al. 2003; Bastir et al. 2005; Mitteroecker and Bookstein 2007, 2008). However, also analysis of the geometry of ontogenetic trajectories can reflect patterns of integration and modularity (O’Higgins 2000; Bulygina et al. 2006; O’Higgins et al. 2006); (Fig. 2). While linear shape trajectories are not necessarily informative about modularity, curved trajectories apparently contain such information. This is because a linear ontogenetic shape trajectory describes ontogeny of either one module that changes shape in a homogenous way or several modules that change shape simultaneously (Fig. 2a). In turn, if an ontogenetic shape trajectory appears curved then modular development can be inferred. This is due to the fact that shape changes occur in a localized fashion, that is, during one ontogenetic period in one part of the analysed structure, and during a different period in a different part of the structure (Fig. 2b). These differences are indicated by the curved trajectory in shape space. Inflection points of the trajectory indicate switching of ontogenetic change in different anatomical regions. This regional change corresponds to the definition of modularity according to Klingenberg (2005) which describes more relations within that localized area (unit) than between it and others. Thus, if shape trajectories are analysed together with their associated changes in landmark configurations then information about modularity as well as their anatomical basis is provided.
Relationships between the geometry of an ontogenetic trajectory and modularity. a A linear ontogenetic shape trajectory may indicate either integrated development of one module or simultaneous development of several modules. b A curved ontogenetic shape trajectory indicates modular (dissociated) development within a complex morphological structure
Recent geometric morphometric studies of craniofacial growth offer some examples how curved ontogenetic trajectories reflect developmental modularity (Bulygina et al. 2006; O’Higgins et al. 2006). These studies analysed shape data of the ontogeny of basicranial and facial structures together. Because the basicranium grows earlier than the face (Enlow 1990), one fraction of the shape trajectory that represents early ontogeny is influenced by basicranial signals and shows one “orientation” in shape space while another fraction of the shape trajectory which represents later ontogeny shows stronger facial signals and is thus orientated in a different way in shape space. The inflection point indicates when the basicranial module stops changing shape while the facial one, starts, or continues to grow. This geometry facilitates therefore the recognition of developmental modules, a basicranial and a facial one, changing their shape at different ontogenetic periods (Enlow 1990; Bastir et al. 2006).
The second part of this paper uses 3D Procrustes geometric morphometrics to analyze morphological changes in pre- and postnatal ontogeny of the ossified cranial base in humans. The data are interpreted in terms of morphological, developmental integration and modularization and compared with morphological aspects of basicranial evolution.
Materials and Methods
First, the hypothesis of mosaic evolution was evaluated comparing separate analyses of midline and lateral basicranial shape data of the evolutionary data set. Midline base morphology of adult chimpanzees (N = 32) and adult modern humans (N = 144) and eight fossil hominins (Sts5, KNM-ER 3733, KNM-ER 3883; Sambungmacan 4, Bodo, Kabwe, Gibraltar 1, Guattari 1) was analyzed by four standard 2D landmarks of midline basicranial morphology (basion, dorsum sellae, anterior and posterior sphenoid) and compared with fossils of several hominin species. The landmarks of recent species were digitized on lateral radiographs of humans from Asia, Africa and Europe (see Kuroe et al. 2004 and Bastir and Rosas 2005 for details of orientations and X-ray methods) and of chimpanzees (Dean and Wood 2003). The landmarks of the fossils were obtained from midsagittal sections of 3D reconstructions of CT scans of the virtual anthropological collections of the MNCN, Madrid, and images published in literature (SM4, Baba et al. 2003). These data were analyzed by standard geometric morphometrics and thin-plate splines (Bookstein 1991; Zelditch et al. 2004) and principal components analysis of shape data (partial warps) (Rohlf et al. 1996; Zelditch et al. 2004) to assess the role of basicranial flexion as principal factor of variation in different recent and fossil species. Then, the findings of the midline base analysis are compared with principal components scatter plots of published 3D data of lateral basicranial anatomy (middle cranial fossa) of chimpanzees, hominins and humans (Bastir et al. 2008). In the case of mosaic basicranial evolution an overlap of fossil hominins and modern humans is expected for the midline base data which would contrast with lateral basicranial data, of which a clear separation has been reported (Bastir et al. 2008).
In the second step, 3D landmark data of modern human midline and lateral basicranial structures were analyzed together in a developmental data set. We studied size and shape changes in the ontogeny of the complete ossified chondrocranium of a total of 71 CT scans of 21 prenatal fetal crania between 12th and 35th intrauterine weeks (14 of 2nd trimester; 7 of 3rd trimester, Shimada collection, University of Kagoshima, Japan), and 50 postnatal crania of 12 perinatal, 10 pre-M1 dental stage, 8 pre-M2 dental stage, 5 pre-M3 dental stage and 15 adult individuals (Bastir and Rosas 2004). These data come from the MNCN-virtual collections, the ORSA-data base, and NESPOS data base (www.nespos.org).
Amira 4 was used for 3D surface reconstructions, on which 27 landmarks (Table 1) of the pre-chordal, and chordal midline base (pre-sphenoid) and floor (mesethmoid) and the parachordal lateral basicranial floor (ali-sphenoid, otic capsule) were digitized. Landmark data were processed and analyzed by standard Procrustes geometric morphometrics (Rohlf and Slice 1990; Bookstein 1991; O’Higgins 2000; Zelditch et al. 2004), using Morpheus et al. (Slice 1998) and morphologika 2 (O’Higgins 2000) software. Principal components analysis in Procrustes Formspace were performed to analyze the size and shape changes of basicranial ontogeny as well as the geometry of the ontogenetic shape trajectory (Bookstein 1991; O’Higgins 2000; Mitteroecker et al. 2004; Zelditch et al. 2004; Bastir et al. 2007). Amira 4.0 was used for producing the 3D surfaces and landmark warps to visualize the shapes associated to the negative and positive extremes of the corresponding principal components loadings.
Results
Figure 3a shows the scatter plot of the first two principal components of the evolutionary data set indicating distribution patterns of midline cranial base shape. Figure 4 shows the shape changes associated to these principal components. Principal component 1 (Fig. 4b) is importantly driven by variation of basicranial flexion (positive loadings) versus retro-flexion (negative loadings). Principal component 2 (Fig. 4c) reflects variation of spheno-occipital clivus length and also angulation. PC1 and PC2 account for approximately 75% of total variance of this sample (Table 2). Two morphological domains can be identified. Chimpanzees plot on the negative scores of PC1 (Fig. 3a) (retro-flexed base, Fig. 4b) and are separate from fossil hominins, which fully overlap with the modern humans, both plotting with the positive PC1 scores (Fig. 3a) and show a higly flexed cranial base (Fig. 4b). The plots in Fig. 3a clearly indicate that the intraspecific range of basicranial flexion in modern humans completely encloses the interspecific range observed in fossil hominins.
a Scatter plot of principal components analysis of midline cranial base. PC1 versus PC2. Note the overlap of humans and hominins. b Scatter plot of principal components of lateral base (data from Bastir et al. 2008). PC1 versus PC2. Note the separation of humans, hominins and chimpanzees
a Midline cranial base landmarks on sagittal section of a modern humans. b Midline base landmarks and TPS splines indicating the shapes associated to the extremes of PC1. c Midline base landmarks and TPS splines indicating the shapes associated to the extremes of PC2. Note that basicranial flexion is a key feature of these principal components (see scatter plot of Fig. 3a)
Figure 3b shows principal components plots of lateral base evolution, where three different domains for PC1 can be identified; one of the chimpanzees, one of the fossil hominins (except fossil H. sapiens) and another one of the modern humans.
Comparison of the scatter plots in Fig. 3a, b indicates thus different evolutionary distributions of midline and lateral basicranial morphologies. Australopithecines, early, later Homo and modern humans overlap in main features of midline base morphology (Fig. 3a), while they are clearly separated in main features of lateral base morphology (Fig. 3b). This empirical evidence supports the hypothesis of mosaic basicranial evolution in Homo.
3D Form Space Analysis of Endocranial Ontogeny
Three principal components were extracted from the developmental data set, which accounted for approximately 95% of total variance (Table 3). Figure 5 suggests a curved (non-linear) ontogenetic trajectory which implies that basicranial morphology changes differently at different developmental stages.
Geometry of the ontogenetic shape trajectories. Scatterplot of a PC1 vs. PC2 (note the positive peaks on PC2 between 2nd and 3rd prenatal trimester and between preM1 and preM2), and the negative peaks during perinatal and adult periods), b PC1 vs. PC3 (note the negative peak on PC3 during the perinatal period) and c PC2 vs. PC3. These peaks mark inflection points along curved ontogenetic trajectories and indicate modular development
Figure 5a shows a curved ontogenetic trajectory projected into PC1-PC2 subspace. PC1 orders chronologically the developmental stages and PC2 shows two positive and two negative peaks. One positive in the middle of prenatal ontogeny (between second and third trimester), one negative around birth, one positive during the transition of preM1 to preM2 developmental stages and a negative towards the adults. Figure 5b illustrates a curved trajectory in PC1–PC3 subspace with one negative peak on PC3 that occurs around birth. Finally, Fig. 5c shows the projection of the ontogenetic trajectory into the PC2–PC3 subspace, and again, a complex curve can be identified. Early prenatal ontogeny starts in the upper left quadrant of the plot. Later prenatal shapes plot in the centre of both lower quadrants until birth, which is characterised by shapes that plot in the lower left quadrant. Postnatal immature shapes are then found in the centre of right quadrants until adult shapes are achieved which plot again within the upper left quadrant.
These peaks mark inflection points along a 3D curved ontogenetic shape trajectory instead of a linear and straight shape trajectory and imply complex modular development. There are at least four morphologically different ontogenetic phases, which are statistically distinguished by different combinations of principal components loadings (Table 4) and anatomically by their corresponding changes of landmark configurations.
Anatomical Evidence for Modularity
The basicranial shape changes associated with the principal components are shown as transparent 3D surfaces together with the corresponding landmark configurations of the reference shape (dark-red landmarks, most negative PC score) and target shape (light-blue landmarks, most positive PC-score) in Fig. 6. The most significant shape differences between these surfaces (and landmark configurations) together with the curved shape trajectory of Fig. 5 inform anatomically about modular development.
Upper part: 3D surfaces as they correspond to the shapes represented by the negative and positive extremes of a PC1, b PC2, and c PC3. Lower part shows the Procrustes registered landmarks of negative (dark/red) and positive (light/blue) PC scores and the transparent surface that corresponds to them. d PC1, e PC2 (the oval line indicates area of common displacement of lateral base landmarks relative to midline landmarks, f PC3 (the small oval indicates local displacement of petrosal landmarks relative to other landmarks)
Principal component 1 (Fig. 6a, d) reflects homogenous shape changes due to overall growth allometry, which occur simultaneously at many parts of the entire cranial base. The top view shows a relative increase of breadth of the cranial base. The lateral views indicate forwards shift of the greater sphenoid wings and an increase of cranial base flexion. The middle cranial fossa becomes relatively longer and posterior cranial fossa deeper.
The second principal component (Fig. 6b, e) describes local changes that are concentrated mainly at the lateral part of the basicranium. Towards the positive loadings the greater sphenoid wings together with the petrosals are shifted forwards and laterally with respect to midline cranial base structures, which decreases the basicranial angle and the relative spheno-occipital clivus length.
Principal component 3 (Fig. 6c, f) describes a relative elongation of the middle cranial fossa towards the negative scores which is due to a relatively stable antero-posterior position of the middle cranial fossa poles and marked changes at the petrosals. The latter become elongated and expanded postero-laterally. The floor of the posterior cranial fossa becomes shallow and the basicranial angle slightly retro-flexed.
Taking together shape trajectories and associated morphological changes clearly indicates modular development according to which different developmental stages are characterised by different morphologies and, more importantly, different ontogenetic patterns of shape changes.
Discussion
This paper investigated mosaic evolution of the basicranium in the genus Homo. In addition we looked at developmental modularity and integration during pre- and postnatal basicranial ontogeny to assess their potential implications in basicranial mosaicism.
Mosaic evolution was assumed on the hypothesis of Ross and Henneberg (1995) who reported relatively stable midline basicranial angles in human evolution since Australopithecus and early Homo. This hypothesized “stability” of basicranial flexion is interesting because relative cranial capacities - the main correlate of basicranial flexion in primate evolution (Ross and Ravosa 1993; Lieberman et al. 2000; Ross et al. 2004)—have increased considerably during human evolution (Ruff et al. 1997; Bruner et al. 2003; Rightmire 2004). This fact requires alternative processes leading to basicranial morphologies that are capable of spatially packing, supporting and protecting bigger brains within the neurobasicranial complex (Dabelow 1931; Hofer 1952; Biegert 1957; Gould 1977; Ross and Ravosa 1993; Ross and Henneberg 1995). Such alternative processes would then require a morphological dissociation (modularization) of basicranial flexion from other features of basicranial morphology (Bastir and Rosas 2005). Basicranial modularization could provide an example according to which modularity allows for evolvability (Cheverud 1982; Cheverud 1996; Wagner 1996; Wagner and Altenberg 1996; Winther 2001; Ackermann 2005; Klingenberg 2005).
However, so far mosaic evolution has not been addressed explicitly in this context and broadening of the comparative hominin context is necessary. Ross and Henneberg (1995) and Baba et al. (2003) have shown that values of midline basicranial flexion of some selected hominin fossils (Sts5, OH-9, SM4) are within the range of modern human variation. We added more fossil hominins (H. ergaster, H. heidelbergensis, s.l.; H. neanderthalensis) to this existing evidence. Our data show that basicranial flexion in evolution is indeed a key feature of midline cranial base variation because it appears as the most prominent feature of the first two principal components which explain about 75% of variance in the total sample (Fig. 4, Table 2). We also show that the range of basicranial flexion in modern humans encloses that of fossil hominins (Fig. 3a). While fossil hominins and modern humans completely overlap in the midline the lateral basicranial data suggests a clear evolutionary patterning in which fossil hominins differ from modern humans and both from chimpanzees (Fig. 3b). It has been shown elsewhere that this difference consists morphologically of a forward projection of the lateral middle cranial fossa poles beyond midline basicranial structures (Bastir et al. 2008) and has recently been added to other modern human autapomorphies (Stringer 2001; Lieberman et al. 2002; Bastir et al. 2008). Therefore, it is likely that basicranial modularity has facilitated relatively independent evolution of midline and lateral structures (Bastir et al. 2004; Bastir and Rosas 2005). This assumption becomes evident comparing evolutionary patterns of midline and lateral basicranial structures.
A second research question of this paper was: What is the 3D ontogenetic context of evolutionary processes of the midline and lateral basicranium? Is there evidence for corresponding developmental integration and modularity? We addressed this question looking at pre- and postnatal ontogeny of ossified basicrania of 71 human cranial bases. Modularity and integration were assessed on the combined evaluation of ontogenetic shape trajectories in principal component space and its associated shape changes in the landmark configurations (Cobb and O’Higgins 2004; Mitteroecker et al. 2005; Bulygina et al. 2006; O’Higgins et al. 2006).
Our findings reveal a clear evidence of a non-linear ontogenetic trajectory, the geometry of which—in a subspace of PC1, PC2 and PC3—appears similar to a “handle-bar” as indicated in Fig. 7. The shape of this ontogenetic trajectory implies four different developmental periods (Table 4, Fig. 7a). Non-linearity can be interpreted as evidence for modular basicranial development, an interpretation that is in line with other studies that addressed specific aspects of growth and development of the human cranial base (Sperber 1989; Enlow 1990; Bach-Petersen and Kjaer 1993; Bach-Petersen et al. 1995; Lieberman and McCarthy 1999; Jeffery and Spoor 2002; Bastir et al. 2006).
a Left: Schemes of shape trajectories as they indicate four different ontogenetic periods. PC1–PC2, PC1–PC3; the grey dash-dotted lines separate these different ontogenetic periods. Right: A 3D scheme of PC1, PC2, and PC3 together is drawn (note the “handle-bar” shape of the ontogenetic trajectory. Lower box: sequential pattern of integrated and modular growth and development. The four different periods are shown. The arrows on the 3D reconstructions indicate relative shifts of anatomical areas along ontogeny during the specific ontogenetic period. The schematic drawings below the 3D reconstructions illustrate the sequence of integration and modularity between the sphenoid (empty circle), petrosal pyramid (grey circle), lateral base (oval outline), midline base (rectangular outline). The grey lines indicate connectivity (solid lines → integration; dashed lines → modularity). b Integration (common development of all components), c modular development of petrosal, d integrated development of all components; e modular development of the lateral basicranium
In the first (smaller) half of the prenatal period, which corresponds roughly to the second prenatal trimester, the basicranium experiences integrated changes (Fig. 7b), in which all regions become simultaneously modified in size and shape. The lateral limits of the anterior and middle cranial fossae (ACF, MCF) and of the middle and posterior cranial fossae (PCF) become shifted anteriorly, while the midline base is retracted posteriorly. This produces a rounded basicranial outline in top view (Fig. 4b) similar to the findings of Plavcan and German (1995).
In the second (larger) half of the prenatal period, which corresponds approximately to the third prenatal trimester, modularization is observed within the lateral cranial floor (Fig. 6c). The link between the greater sphenoid wings and the petrosals becomes dissociated (Figs. 6c, f and 7c). The former become stabilized in position, while the petrosal pyramids experiment an elongation and backwards shift. This process enlarges the relative proportions of the middle and posterior cranial fossae. It is one ontogenetic process that may be involved in relative enlargement of the middle cranial fossa (Spoor et al. 1999; Lieberman et al. 2002; Bastir et al. 2008). This increase also fits with other observations, which assert that the overall effect of prenatal ontogeny produces more increase of antero-posterior dimensions than medio-lateral ones (Jeffery 2002b; Zumpano and Richtsmeier 2003; Morimoto et al. 2008).
The third phase between birth and M1 eruption can be considered a developmentally integrated period (Fig. 7d) because both midline cranial base and lateral cranial floor change shape simultaneously. The lateral floor is shifted forwards relative to the midline base (Figs. 6b and 7c), which flexes early and changes shape until about 8 years (Lieberman and McCarthy 1999; Bastir et al. 2006). The common shift of the lateral floor implies a re-integration of the greater sphenoid wings and the petrosals (previously dissociated) relative to the midline base. In addition, during this step an enormous size increase is observed (Fig. 5a, b), which is likely related to the fast brain growth in this period (Enlow 1990; Peña 2000; Richtsmeier et al. 2006). The consequence of this ontogenetic period is again a more rounded shape of the basicranial outline.
Another final change of the integrative pattern is indicated by a curve of the ontogenetic trajectory (Fig. 5a) after M1 eruption that initiates the fourth phase of basicranial ontogeny (Fig. 7a, e) characterised by a changes at the lateral basicranium relative to the midline. It is known that around M1 eruption midline base shape has become stable (Sperber 1989; Bastir et al. 2006) while the lateral basicranium continues ontogenetic change (Enlow 1990; Bastir et al. 2006).
Ontogenetic change of integration patterns have been detected in other regions of the hominoid skull (Ackermann 2005). This study shows evidence for the hypothesis of repeated (sequential) integration and modularisation of the human cranial base (Fig. 7b–d). Future study should test this hypothesis with complementary methods addressing quantitatively differences in patterns and degree of covariation (Bastir et al. 2005).
Basicranial Relations to Brain Evolution and Development
It is attractive to speculate that basicranial changes in evolution and ontogeny are related to changes in brain organization. With respect to human evolution two brain factors have been suggested: the evolution of the temporal lobes (Rilling and Seligman 2002; Rilling 2006) and of the parietal lobes (Holloway 1981, Holloway 1995; Bruner et al. 2003; Bruner 2004). While increase of the parietal lobes may spatially influence the position of the temporal lobes as well as of the basicranial flexure, the temporal lobes can theoretically expand without modifying basicranial flexure. Thus, joint consideration of the comparative anatomical evidence of stable midline and evolving MCF-anatomy (Fig. 3a, b) points to temporal lobe evolution as factor underlying mosaic changes at the lateral base (Spoor et al. 1999; Lieberman et al. 2002; Bastir et al. 2008). It is interesting to note that apparently a mosaic evolution of the human brain (parietal, temporal lobes) parallels a mosaic of the human basicranium (midline, lateral elements).
The interpretation of developmental shape changes of the basicranium and its relation to the ontogeny of the brain is more complex (Jeffery 2002a, b; Jeffery and Spoor 2002). Particularly cranial base flexion in the midline cannot be easily explained by brain expansion, at least in the second and third prenatal trimester (Jeffery and Spoor 2002). These authors found retro-flexion to accompany relative and absolute increase of brain size instead of the expected flexion. However, the unfused neurocranium of the fetus does not provide the spatial constraining neuro-basicranial context, on which the idea of brain increase and basicranial flexion as spatial packing mechanism has originally been developed (Hofer 1952; Biegert 1957; Gould 1977; Ross et al. 2004).
From a developmental point of view the correspondence of morphologies of the lateral basicranial floor and the inferior surface of the brain is explained by shared tissues and their origins (Sperber 1989; Richtsmeier et al. 2006). The developing ectomeninx consists of two layers, the inner of which differentiates in the dura mater, which covers the brain, while the outer layer gives rise to connective tissue, which ossifies into the precursors of the lateral basicranial floor (Sperber 1989). Definitively, more research is necessary to understand the underlying factors of midline and off-midline basicranial morphology in relation to development and evolution of the brain.
Finally, some aspects should be mentioned that may limit the present interpretations. One limiting factor is that the prenatal period is covered by fetuses of exclusively Asian origin. There is clear evidence for different basicranial architecture among adult populations from different geographic origin which is likely also present to some (unknown) degree in prenatal and immature data (Kuroe et al. 2004; Bastir and Rosas 2006; Rosas et al. 2008). However, there is no reason to assume different integration patterns among different populations of the same species although this is a question that needs to be tested. Also, comparison of the postnatal ontogenetic data of European origin and the adult sample, which is composed of similar numbers of Asian, African and European basicrania does not apparently reveal a bias on PC2 or PC3, which would invalidate our interpretations. In any case, inclusion of fetal data from other geographic regions should be analysed to clarify this problem.
A second limiting factor may be related to the medical imaging technique and reconstructions applied to obtain the prenatal shape data. 3D reconstructions of CT data of prenatal specimens that illustrate hard tissue morphology will not give information on cartilage morphologies due to threshold issues (Jeffery 2002b; Morimoto et al. 2008). Therefore, calculations of absolute dimensions on such reconstructions are biased. In our study the quantitative evaluation of absolute dimensions is not relevant. It is important, however, to note that ossification is likely a factor that influences the integration and modularization patterns discussed here. This should be borne in mind in the understanding of the relative increase of the posterior cranial fossa observed during the end of the prenatal period. Assuming that cartilage is not visualized in our 3D reconstructions it is possible that part of the posterior cranial fossa increase during the second phase is ossification of existing cartilage rather than growth. In terms of shape however, our findings are compatible to those obtained by hMRI (Jeffery 2002b) supporting our interpretation. Shape analysis of 3D reconstructions of hMRI could further refine the hypothesis of sequential integration and modularity.
Conclusions
The evolution of the human basicranium is characterized by a mosaic of midline and lateral basicranial elements and processes (Bastir and Rosas 2008). This mosaic is possibly based on the evolutionary modifications of modular structure of the basicranium which is known from postnatal ontogenetic (Enlow 1990; Sgouros et al. 1999; Bastir et al. 2006) and adult covariation patterns (Bastir and Rosas 2005; Bruner and Ripani 2008). In addition, the present 3D analysis of pre-and postnatal ontogeny suggests that modularity changes during development but this hypothesis requires further testing. Thus our results together with previous studies on basicranial growth and integration suggest that a sequential pattern of integration and modularisation describes human basicranial ontogeny in a way that is potentially important for the understanding of evolution in the genus Homo. Different phylogenetic modifications of this spatio-temporal pattern, morphogenesis, growth, and development of midline and lateral basicranial elements seem to underlie the mosaic evolution of the cranial base in hominins. They might relate to mosaic evolution of the brain (Holloway 1995; Bruner 2004). Future studies should focus on the causal relations between brain and basicranial prenatal ontogeny.
References
Ackermann, R. (2005). Ontogenetic integration of the hominoid face. Journal of Human Evolution, 48(2), 175–197. doi:10.1016/j.jhevol.2004.11.001.
Ackermann, R. R., & Cheverud, J. M. (2000). Phenotypic covariance structure in tamarins (genus Saguinus): A comparison of variation patterns using matrix correlation and common principal component analysis. American Journal of Physical Anthropology, 111(4), 489–501. doi:10.1002/(SICI)1096-8644(200004)111:4<489::AID-AJPA5>3.0.CO;2-U.
Baba, H., Aziz, F., Kaifu, Y., Suwa, G., Kono, R. T., & Jacob, T. (2003). Homo erectus Calvarium from the Pleistocene of Java. Science, 299(5611), 1384–1388. doi:10.1126/science.1081676.
Bach-Petersen, S., & Kjaer, I. (1993). Ossification of lateral components in the human prenatal cranial base. Journal of Craniofacial Genetics and Developmental Biology, 13, 76–82.
Bach-Petersen, S., Kjaer, I., & Fischer-Hansen, B. (1994). Prenatal development of the human osseous temporomandibular region. Journal of Craniofacial Genetics and Developmental Biology, 14(3), 135–143.
Bach-Petersen, S., Solow, B., Fischer-Hansen, B., & Kjaer, I. (1995). Growth in the lateral part of the human skull during the second trimester. Journal of Craniofacial Genetics and Developmental Biology, 15, 205–211.
Bastir, M. (2008). A systems-model for the morphological analysis of integration and modularity in human craniofacial evolution. Journal of Anthropological Sciences, 86 (in press).
Bastir, M., O’Higgins, P., & Rosas, A. (2007). Facial ontogeny in Neanderthals and modern humans. Proceedings of the Royal Society B: Biological Sciences, 274, 1125–1132.
Bastir, M., & Rosas, A. (2004). Comparative ontogeny in humans and chimpanzees: Similarities, differences and paradoxes in postnatal growth and development of the skull. Annals of Anatomy, 186(5–6), 503–509. doi:10.1016/S0940-9602(04)80096-7.
Bastir, M., & Rosas, A. (2005). The hierarchical nature of morphological integration and modularity in the human posterior face. American Journal of Physical Anthropology, 128(1), 26–34. doi:10.1002/ajpa.20191.
Bastir, M., & Rosas, A. (2006). Correlated variation between the lateral basicranium and the face: A geometric morphometric study in different human groups. Archives of Oral Biology, 51(9), 814–824. doi:10.1016/j.archoralbio.2006.03.009.
Bastir, M., & Rosas, A. (2008). Mosaic evolution, integration and modularity: Evolution of the human cranial base. American Journal of Physical Anthropology, S46, 65.
Bastir, M., Rosas, A., & Kuroe, K. (2004). Petrosal orientation and mandibular ramus breadth: Evidence of a developmental integrated petroso-mandibular unit. American Journal of Physical Anthropology, 123(4), 340–350. doi:10.1002/ajpa.10313.
Bastir, M., Rosas, A., Lieberman, D. E., & O’Higgins, P. (2008). Middle cranial fossa anatomy and the origins of modern humans. The Anatomical Record, 291(2), 130–140. doi:10.1002/ar.20636.
Bastir, M., Rosas, A., & O’Higgins, P. (2006). Craniofacial levels and the morphological maturation of the human skull. Journal of Anatomy, 209(5), 637–654. doi:10.1111/j.1469-7580.2006.00644.x.
Bastir, M., Rosas, A., & Sheets, D. H. (2005). The morphological integration of the hominoid skull: A Partial Least Squares and PC analysis with morphogenetic implications for European Mid-Pleistocene mandibles. In D. Slice (Ed.), Modern morphometrics in physical anthropology (pp. 265–284). New York: Kluwer Academic/Plenum Publishers.
Biegert, J. (1957). Der Formwandel des Primatenschädels und seine Beziehungen zur ontogenetischen Entwicklung und den phylogenetischen Spezialisationen der Kopforgane. Gegenbaurs Morphologisches Jahrbuch, 98, 77–199.
Biegert, J. (1963). The evaluation of characteristics of the skull, hands and feet for primate taxonomy. In S. L. Washburn (Ed.), Classification and human evolution (pp. 116–145). Chicago: Aldine.
Bookstein, F. L. (1991). Morphometric tools for landmark data. Cambridge: Cambridge University Press.
Bookstein, F. L., Gunz, P., Mitteroecker, P., Prossinger, H., Schaefer, K., & Seidler, H. (2003). Cranial integration in Homo: Singular warps analysis of the midsagittal plane in ontogeny and evolution. Journal of Human Evolution, 44(2), 167–187. doi:10.1016/S0047-2484(02)00201-4.
Bruner, E. (2004). Geometric morphometrics and paleoneurology: Brain shape evolution in the genus Homo. Journal of Human Evolution, 47(5), 279–303. doi:10.1016/j.jhevol.2004.03.009.
Bruner, E., Manzi, G., & Arsuaga, J.-L. (2003). Encephalization and allometric trajectories in the genus Homo. Evidence from the Neandertal and modern lineages. Proceedings of the National Academy of Sciences of the United States of America, 100(26), 15335–15340. doi:10.1073/pnas.2536671100.
Bruner, E., & Ripani, M. (2008). A quantitative and descriptive approach to morphological variation of the endocranial base in modern humans. American Journal of Physical Anthropology, 137(1), 30–40. doi:10.1002/ajpa.20837.
Bulygina, E., Mitteroecker, P., & Aiello, L. (2006). Ontogeny of facial dimorphism and patterns of individual development within one human population. American Journal of Physical Anthropology, 131(3), 432–443. doi:10.1002/ajpa.20317.
Chernoff, B., & Magwene, P. M. (1999). Afterword. In E. C. Olson & P. L. Miller (Eds.), Morphological integration (pp. 319–353). Chicago: University of Chicago.
Cheverud, J. M. (1982). Phenotypic, genetic, and environmental morphological integration in the cranium. Evolution; International Journal of Organic Evolution, 36(3), 499–516. doi:10.2307/2408096.
Cheverud, J. M. (1995). Morphological integration in the saddle-backed tamarin (Saguinis fuscicollis). American Naturalist, 145(4), 63–89. doi:10.1086/285728.
Cheverud, J. M. (1996). Developmental integration and the evolution of pleiotropy. American Zoologist, 36, 44–50.
Cobb, S., & O’Higgins, P. (2004). Hominins do not share a common postnatal facial ontogenetic shape trajectory. Journal of Experimental Zoology. Part B. Molecular and Developmental Evolution, 302B(3), 302–321. doi:10.1002/jez.b.21005.
Dabelow, A. (1931). Über Korrelationen in der phylogenetischen Entwicklung der Schädelform II. Die Beziehungen zwischen Gehirn und Schädelbasisform bei den Mammaliern. Gegenbaurs Morphologisches Jahrbuch, 67, 84–133.
Dean, M., & Wood, B. (2003). A digital radiographic atlas of great apes skull and dentition. In L. Bondioli & R. Macchiarelli (Eds.), Digital archives of human paleobiology. Milano: ADS Solutions.
Enlow, D. H. (1990). Facial growth. Philadelphia: W. B. Saunders Company.
Ford, E. H. R. (1958). Growth of the human cranial base. American Journal of Orthodontics, 44(7), 498–506. doi:10.1016/0002-9416(58)90082-4.
Goodrich, J. T. (2005). Skull base growth in craniosynostosis. Child’s Nervous System, 21(10), 871–879. doi:10.1007/s00381-004-1113-1.
Gould, S. J. (1977). Ontogeny and phylogeny. Cambridge, Massachusetts, London, England: Harvard University Press.
Hofer, H. (1952). Der Gestaltwandel des Schädels der Säugetiere und der Vögel, mit besonderer Berücksichtigung der Knickungstypen und der Schädelbasis. Verhandlungen der Anatomischen Gesellschaft, 99, 102–126.
Holloway, R. (1981). Exploring the dorsal surface of hominoid brain endocasts by stereoplotter and discriminant analysis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 292(1057), 155–166. doi:10.1098/rstb.1981.0024.
Holloway, R. (1995). Toward a synthetic theory of human brain evolution. In J. P. Changeaux & J. Chavaillon (Eds.), Origins of the human brain (pp. 42–54). Clarendon, Oxford: Clarendon Press.
Jeffery, N. (2002a). Differential regional brain growth and rotation of the prenatal human tentorium cerebelli. Journal of Anatomy, 200(2), 135–144. doi:10.1046/j.0021-8782.2001.00017.x.
Jeffery, N. (2002b). A high-resolution MRI study of linear growth of the human fetal skull base. Neuroradiology, 44(4), 358–366. doi:10.1007/s00234-001-0753-z.
Jeffery, N., & Spoor, F. (2002). Brain size and the human cranial base. American Journal of Physical Anthropology, 118, 324–340. doi:10.1002/ajpa.10040.
Klingenberg, C. P. (2005). Developmental constraints, modules, and evolvability. In B. Hallgrímsson & B. K. Hall (Eds.), Variation (pp. 219–247). San Diego: Academic Press.
Klingenberg, C. P., Mebus, K., & Auffray, J.-C. (2003). Developmental integration in a complex morphological structure: How distinct are the modules in the mouse mandible? Evolution & Development, 5(5), 522–531. doi:10.1046/j.1525-142X.2003.03057.x.
Kuroe, K., Rosas, A., & Molleson, T. (2004). Variation in the cranial base orientation and facial skeleton in dry skulls sampled from three major populations. European Journal of Orthodontics, 26(2), 201–207. doi:10.1093/ejo/26.2.201.
Larsen, W. J. (2001). Development of the head, the neck, the eyes and the ears. In L. S. Shermann, S. S. Potter, & W. J. Scott (Eds.), Human embryology. New York, Edinburgh, London, Philadelphia: Churchill Livingston.
Lieberman, D. E., Hallgrimsson, B., Liu, W., Parsons, T. E., & Jamniczky, H. A. (2008). Spatial packing, cranial base angulation, and craniofacial shape variation in the mammalian skull: Testing a new model using mice. Journal of Anatomy, 212(6), 720–735. doi:10.1111/j.1469-7580.2008.00900.x.
Lieberman, D. E., McBratney, B. M., & Krovitz, G. (2002). The evolution and development of cranial form in Homo sapiens. Proceedings of the National Academy of Sciences of the United States of America, 99(3), 1134–1139. doi:10.1073/pnas.022440799.
Lieberman, D. E., & McCarthy, R. C. (1999). The ontogeny of cranial base angulation in humans and chimpanzees and its implication for reconstructing pharyngeal dimensions. Journal of Human Evolution, 36, 487–517. doi:10.1006/jhev.1998.0287.
Lieberman, D. E., Ross, C., & Ravosa, M. J. (2000). The primate cranial base: Ontogeny, function, and integration. Yearbook of Physical Anthropology, 43, 117–169. doi:10.1002/1096-8644(2000)43:31+<117::AID-AJPA5>3.3.CO;2-9.
Marroig, G., Vivo, M., & Cheverud, J. M. (2004). Cranial evolution in sakis (Pithecia, Platyrrhini) II: Evolutionary processes and morphological integration. Journal of Evolutionary Biology, 17(1), 144–155. doi:10.1046/j.1420-9101.2003.00653.x.
Mitteroecker, P., & Bookstein, F. (2007). The conceptual and statistical relationship between modularity and morphological integration. Systematic Biology, 56(5), 818–836. doi:10.1080/10635150701648029.
Mitteroecker, P., & Bookstein, F. (2008). The evolutionary role of modularity and integration in the hominoid cranium. Evolution; International Journal of Organic Evolution, 62(4), 943–958. doi:10.1111/j.1558-5646.2008.00321.x.
Mitteroecker, P., Gunz, P., Bernhard, M., Schaefer, K., & Bookstein, F. L. (2004). Comparison of cranial ontogenetic trajectories among great apes and humans. Journal of Human Evolution, 46(6), 679–698. doi:10.1016/j.jhevol.2004.03.006.
Mitteroecker, P., Gunz, P., & Bookstein, F. L. (2005). Heterochrony and geometric morphometrics: A comparison of cranial growth in Pan paniscus versus Pan troglodytes. Evolution & Development, 7(3), 244–258. doi:10.1111/j.1525-142X.2005.05027.x.
Morimoto, N., Ogihara, N., Katayama, K., & Shiota, K. (2008). Three-dimensional ontogenetic shape changes in the human cranium during the fetal period. Journal of Anatomy, 212(5), 627–635. doi:10.1111/j.1469-7580.2008.00884.x.
Nemzek, W. R., Brodie, H. A., Hecht, S. T., Chong, B. W., Babcook, C. J., & Seibert, J. A. (2000). MR, CT, and plain film imaging of the developing skull base in fetal specimens. AJNR. American Journal of Neuroradiology, 21(9), 1699–1706.
O’Higgins, P. (2000). The study of morphological variation in the hominid fossil record: Biology, landmarks and geometry. Journal of Anatomy, 197, 103–120. doi:10.1046/j.1469-7580.2000.19710103.x.
O’Higgins, P., Bastir, M., & Kupczik, K. (2006). Shaping the human face. International Congress Series, 1296, 55–73. doi:10.1016/j.ics.2006.03.036.
O’Rahilly, R. G., & Gardner, E. (1972). The initial appearance of ossification in staged human embryos. The American Journal of Anatomy, 134, 291–308. doi:10.1002/aja.1001340303.
Olson, E. C., & Miller, R. L. (1958). Morphological integration. Chicago: The University of Chicago.
Peña, A. (2000). Development of human brain. Human Evolution, 15(1–2), 99–112. doi:10.1007/BF02436238.
Plavcan, J., & German, R. (1995). Quantitative evaluation of craniofacial growth in the third trimester human. The Cleft Palate-Craniofacial Journal, 32(5), 394–404. doi:10.1597/1545-1569(1995)032<0394:QEOCGI>2.3.CO;2.
Raff, R. A. (1996). The shape of life. Gene, development, and the evolution of animal form. Chicago and London: The University of Chicago Press.
Richtsmeier, J. T., Aldridge, K., DeLeon, V. B., Panchal, J., Kane, A. A., Marsh, J. L., et al. (2006). Phenotypic integration of neurocranium and brain. Journal of Experimental Zoology. Part B. Molecular and Developmental Evolution, 306(4), 360–378. doi:10.1002/jez.b.21092.
Riedl, R. (1975). Die Ordnung des Lebendigen. Systembedingungen der Evolution. Hamburg: Paul Parey Verlag.
Rightmire, G. (2004). Brain size and encephalization in early to Mid-Pleistocene Homo. American Journal of Physical Anthropology, 124(2), 109–123. doi:10.1002/ajpa.10346.
Rilling, J. K. (2006). Human and nonhuman primate brains: Are they allometrically scaled versions of the same design? Evolutionary anthropology: Issues. News Review (Melbourne), 15(2), 65–77.
Rilling, J. K., & Seligman, R. A. (2002). A quantitative morphometric comparative analysis of the primate temporal lobe. Journal of Human Evolution, 42(3), 1–29. doi:10.1006/jhev.2001.0544.
Rohlf, F. J., Loy, A., & Corti, M. (1996). Morphometric analysis of old world Talpidae (Mammalia, Insectivora) using partial warp scores. Systematic Biology, 45(3), 344–362. doi:10.2307/2413569.
Rohlf, F. J., & Slice, D. (1990). Extensions of the procrustes method for the optimal superimposition of landmarks. Systematic Zoology, 39(1), 40–59. doi:10.2307/2992207.
Rosas, A., & Bastir, M. (2004). Geometric morphometric analysis of allometric variation in the mandibular morphology from the hominids of Atapuerca, Sima de los Huesos Site. The Anatomical Record Part A, 278A, 551–560. doi:10.1002/ar.a.20049.
Rosas, A., Bastir, M., Alorcón, J. A., & Kuroe, K. (2008). Thin-plate spline analysis of the cranial base in African, Asian and European populations and its relationship with different malocclusions. Archives of Oral Biology, 53(9), 826–834.
Ross, C., & Henneberg, M. (1995). Basicranial flexion, relative brain size, and facial kyphosis in Homo sapiens and some fossil hominids. American Journal of Physical Anthropology, 98, 575–593. doi:10.1002/ajpa.1330980413.
Ross, C. F., Henneberg, M., Ravosa, M. J., & Richard, S. (2004). Curvilinear, geometric and phylogenetic modeling of basicranial flexion: Is it adaptive, is it constrained? Journal of Human Evolution, 46(2), 185–213. doi:10.1016/j.jhevol.2003.11.001.
Ross, C. F., & Ravosa, M. J. (1993). Basicranial flexion, relative brain size, and facial kyphosis in nonhuman primates. American Journal of Physical Anthropology, 91, 305–324. doi:10.1002/ajpa.1330910306.
Ruff, C. B., Trinkaus, E., & Holliday, T. W. (1997). Body mass and encephalization in Pleistocene Homo. Nature, 387(6629), 173–176. doi:10.1038/387173a0.
Sgouros, S., Natarajan, K., Hockley, A., Goldin, J. H., & Wake, M. (1999). Skull base growth in childhood. Pediatric Neurosurgery, 31, 259–268. doi:10.1159/000028873.
Slice, D. E. (1998). Morpheus et al.: Software for morphometric research. Revision 01-01-00. Version evision 01-01-00. New York: Department of Ecology and Evolution, State University, Stony Brook.
Sperber, G. H. (1989). Craniofacial embryology. London, Boston, Singapore, Sydney, Toronto, Wellington: Wright.
Spoor, F. (1997). Basicranial architecture and relative brain size of Sts5 (Australopithecus africanus) and other Plio-Pleistocene hominids. South African Journal of Science, 93, 182–186.
Spoor, F., O’Higgins, P., Dean, C., & Lieberman, D. E. (1999). Anterior sphenoid in modern humans. Nature, 397, 572. doi:10.1038/17505.
Stringer, C. (2001). Modern human origins: Progress and prospects. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 357, 563–579. doi:10.1098/rstb.2001.1057.
Virchow, R. (1857). Untersuchung über die Entwicklung des Schädelgrundes im gesunden und krankhaften Zustande und über den Einfluß derselben auf Schädelform, Gesichtsbildung und Gehirnbau. Berlin: Reimer, G.
von Dassow, G., & Munro, E. (1999). Modularity in animal development and evolution: Elements of a conceptual framework for EvoDevo. The Journal of Experimental Zoology, 285, 307–325. doi:10.1002/(SICI)1097-010X(19991215)285:4<307::AID-JEZ2>3.0.CO;2-V.
Wagner, G. (1996). Homologues, natural kinds and the evolution of modularity. American Zoologist, 36, 36–43.
Wagner, G. P. (1990). A comparative study if morphological integration in Apis mellifera (Insecta, Hymenoptera). Zeitschrift für Systematik und Evolutionsforschung, 28, 48–61.
Wagner, G. P., & Altenberg, L. (1996). Complex adaptation and the evolution of evolvability. Evolution; International Journal of Organic Evolution, 50(3), 967–976. doi:10.2307/2410639.
Weidenreich, F. (1941). The brain and its role in the phylogenetic transformation of the human skull. Transactions of the American Philosophical Society, 31, 321–442. doi:10.2307/1005610.
Winther, R. (2001). Varieties of modules: Kinds, levels, origins, and behaviors. Journal of experimental Zoology (Mol Dev Evol), 291, 116–129.
Zelditch, M. L. (1987). Evaluating models of developmental integration in the laboratory rat using confirmatory factor analysis. Systematic Zoology, 36(4), 368–380. doi:10.2307/2413401.
Zelditch, M. L., Swiderski, D. L., Sheets, H. D., & Fink, W. L. (2004). Geometric morphometrics for biologists: A primer. San Diego: Elsevier Academic Press.
Zumpano, M. P., & Richtsmeier, J. T. (2003). Growth-related shape changes in the fetal craniofacial complex of humans (Homo sapiens) and pigtailed macaques (Macaca nemestrina): A 3D-CT comparative analysis. American Journal of Physical Anthropology, 120(4), 339–351. doi:10.1002/ajpa.10125.
Acknowledgements
We thank Emma Mbua, Fred Spoor, Roberto Macchiarelli, Luca Bondioli, Chris Stringer, Rob Kruszynski, Gerhard Weber, Paul O’Higgins, Tom Schoenemann and Janet Monge, (ORSA), Dan Lieberman, and the NESPOS society (www.nespos.org) for access to data in their care and Katie Willmore and Campell Rolian for inviting us to the interesting symposium at the AAPA meetings in Columbus, Ohio. We thank two anonymous reviewers for their helpful comments on a previous version. This research is funded by projects CGL-2006-02131 (Spanish Ministry of Science) and MRTN-CT-2005-019564-EVAN (European Union).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bastir, M., Rosas, A. Mosaic Evolution of the Basicranium in Homo and its Relation to Modular Development. Evol Biol 36, 57–70 (2009). https://doi.org/10.1007/s11692-008-9037-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-008-9037-4