Abstract
Purpose
Malaria, which is a vector-borne disease caused by Plasmodium sp., continue to become a serious threat, causing more than 600,000 deaths annually, especially in developing countries. Due to the lack of a long-term, and effective vaccine, and an increasing resistance to antimalarials, new strategies are needed for prevention and treatment of malaria. Recently, the impact of microbiota on development and transmission of Plasmodium, and the severity of malaria has only begun to emerge, although its contribution to homeostasis and a wide variety of disorders is well-understood. Further evidence has shown that microbiota of both mosquito and human host play important roles in transmission, progression, and clearance of Plasmodium infection. Furthermore, Plasmodium can cause significant alterations in the host and mosquito gut microbiota, affecting the clinical outcome of malaria.
Methodology
In this review, we attempt to summarize results from published studies on the influence of the host microbiota on the outcome of Plasmodium infections in both arthropods and mammalian hosts.
Conclusion
Modifications of microbiota may be an important potential strategy in blocking Plasmodium transmission in vectors and in the diagnosis, treatment, and prevention of malaria in humans in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vector-borne diseases (VBD) are a major cause of morbidity and mortality worldwide especially in the tropical and subtropical regions. In fact, according to a World Health Organization (WHO) report, approximately 80% of the world’s population is at risk of one or more vector-borne disease. Annually, over 700, 000 deaths are reported from VBDs, such as malaria, leishmaniasis, chagas disease, yellow fever, human African trypanosomiasis, dengue, Japanese encephalitis, and onchocerciasis [1]. Due to global warming, there has been increased distribution of vectors to regions that were previously inhabitable, thus increasing the risk of VBDs. This has increased a sense of urgency in the research and public health communities to develop novel effective vector control strategies to prevent a potential future vector-borne disease outbreak. One of vector control strategy is the symbiotic control, which employs the identification of suitable microbes that can spread among vector populations. Through paratransgenesis, these microbes are bioengineered to reduce or induce the fecundity and vector competence of insects [2,3,4]. Paratransgenic microbes can also be applied in development of transmission-blocking strategies, thus reducing disease transmissions while maintaining disease-free arthropods that maybe important in ecosystem food chains [5]. Identification of best candidates for paratransgenesis requires better understanding of composition, diversity, function, and role of host microbiota in vector–parasite interactions.
Many protozoan parasites that cause important human VBDs are heteroxenous i.e., during one stage of their lifecycle they live in the vertebrates and during other stages they live in the gut of bloodsucking insects. Therefore, there is a high possibility of interactions between parasite and microbiota of the host. Microbiota refers to the collective microorganisms (bacteria, archaea, viruses, fungi, and protozoans) that inhabit a host, while microbiome describes the entire genomic elements of microbiota [6]. Microbiota of important VBD vectors such as mosquitoes, sand fly, tsetse fly, triatomines and ticks have been previously well reviewed [3, 7,8,9]. Microbiota influence vector’s innate immunity, reproduction, feeding and behavior, ultimately affecting vectorial capacity of various arthropod vectors. Furthermore, microbiota is also significant in determining the pathogenicity and virulence of parasites such as Plasmodium sp., Leishmania sp., and Trypanosoma cruzi in mammalian hosts [10,11,12]. However, the mechanisms of interactions between parasite and microbiota, which play a role in the development and transmission of infections, are not fully clear.
To understand the effects and possible mechanisms of parasite-microbiota interactions on the development and transmission of malaria, we reviewed published articles on Plasmodium and microbiota interactions in both mammalian and mosquito hosts.
Vector-Borne Parasite and Microbiota Interactions
Humans and arthropods are colonized with a variety of bacteria, fungi, viruses, and protozoans that play important role in homeostatic balance of the host. Now, it has been revealed that the formation and severity of diseases are associated with microbial diversity in humans, as well as the roles of microbiota in food digestion, vitamin production and regulation of the immune system [13]. Several studies have reported the influence of gut microbiota in vector competence of mosquito, tsetse fly, and sand fly [14,15,16]. Regarding Anopheles mosquitoes, presence of some bacterial species increases the chances of Plasmodium to develop into transmissible stages while others are associated with negative growth of parasites in arthropod hosts [17,18,19].
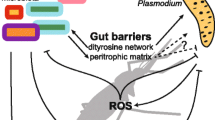
Mosquitoes, sand flies, tsetse flies and triatomines have different biology, behavior, and ecologies, therefore, they have different core microbiota. Due to this microbial community, the interactions among microbiota, parasite, and host systems can significantly vary. However, outcomes of parasite- microbiota interactions can be summarized into three types: (i) perturbation of gut microbiota composition reduce\increases the survival of parasite and severity of disease in host; (ii) parasite infection reshapes microbial composition in the host; and (iii) microbiota and parasite influence on host’s metabolism and immune system (Table 1). These outcomes can result from competition in the use of resources, production of antimicrobial and antiparasitic metabolites, and modulation of host’s immune system by both the microbiota and parasite (Fig. 1).
Mechanisms involved in host microbiota and Plasmodium interactions. Microbiota and parasite can directly influence each other, independent of the host, via mechanisms that they induce individually (A). Furthermore, microbiota and parasite can indirectly influence each other via host modulated mechanisms such as immune response and metabolism (B). (Created with BioRender.com)
Microbiota Analyzes
Accurate identification of host microbiota is the basis for investigating possible symbiont candidates for paratransgenesis and mechanisms involved in parasite-microbiota interaction. Currently culture-dependent and\or culture-independent techniques have been used in host microbiota studies [20, 21]. Although culture-dependent techniques are widely applied in microbial studies, they are limited to identification of culturable microbes. Consequently, various culture-independent methods such as gene sequencing have been developed for identification of all microorganisms. Recently, with the further development of sequencing technologies and data analysis methods, the composition and functions of the microbiota has been successfully revealed [21,22,23].
The most widely used method in microbiome analysis is marker gene (16 s rRNA) sequencing [20, 23]. Sequencing of the hypervariable regions of the 16 s rRNA gene, which is found in all bacteria and is highly conserved, enables the identification of bacterial species and taxonomic prediction [21, 24]. However, this technique can cause inaccuracies in species-level classification and only analyzes for bacterial species, ignoring other members of the community [20,21,22].
Another method, whole metagenome sequencing, examines unbiasedly at the full genomic content of the microbiota, rather than at a single gene in bacteria [22, 23], and functionally evaluates the genetic contribution of each member of the community [23, 25]. For this, shotgun metagenomic sequencing, in which long DNA molecules are randomly fragmented and then sequenced, is the most suitable strategy [23]. It allows for the identification of the full genome as well as a more accurate of species-level classification, and detection of less abundant taxa [22, 23]. It can also reveal the metabolic potential of these communities by providing information about functional genes [22, 26].
Plasmodium sp.–Microbiota Interactions
Plasmodium spp. are apicomplexan protozoan parasites that are transmitted by anopheline mosquitoes, causing malaria. WHO estimates that there are 219 million cases of malaria globally and more than 400, 000 deaths from the disease reported every year. Severity of malaria infection is dependent on many factors including Plasmodium species, age, genetic factors, nutrition status, immune status, duration of exposure, and history of previous exposure [27, 28]. In addition, recently, the role of composition of human gut microbiota in severity of malaria has been investigated. Yooseph et al. [10] performed a microbial analysis in the stool of subjects with lower and higher risk of P. falciparum infection and reported distinct microbiota profiles. After taking into account age and other potential co-features, the authors reported that higher proportion of Bifidobacterium, Streptococcus, Enterobacteriaceae, Escherichia, and Shigella in lower risk subjects compared to those at higher risk of P. falciparum infection. These results suggest possibility of modulation of gut microbiota through probiotics to supplement microbes such as Bifidobacterium to reduce severity of P. falciparum infection. Interestingly, Yilmaz et al. [29] reported that anti-α-gal antibodies produced against human gut E. coli 0.86: B7 expressed α-gal protected humans from malaria transmission. In addition, infection of α1,3GT- deficient mice with E. coli 0.86: B7 induced production of anti- α-gal antibodies that targeted Plasmodium sporozoites inoculated by Anopheles mosquitoes [29]. In the future it will be interesting to investigate the association of this bacteria’s α-gal expression with risk of P. falciparum infection in humans.
Changes in gut bacteria significantly affects severity of malaria in mammalian hosts. Mandal et al. [30] observed varying severity of P. yoelii infection in mice obtained from the same commercial vendor at different shipping times. Mice shipped in 2016 were able to clear P. yoelii infection faster than mice shipped in 2017, which had high malaria severity. Indeed, microbiota analysis of fecal samples from these two groups of mice showed a difference in bacteria abundance and composition. This result suggested that there was shift in microbiota in the microbiota of mice grown in the same environment over different time periods, thus, leading to a significant change in malaria severity. This result was validated by transferring fecal samples from both groups of mice to germ-free mice. Germ-free mice colonized with cecal contents from 2017 mice showed higher numbers of the parasite than those from 2016 mice [30]. Therefore, there are some difficulties in reproducing outcomes in experimental mouse models, even when using genetically identical mice ordered from the same production suite and vendor. In addition, it has been previously reported that mice from different vendors have different gut microbiota composition, thus showing significant varying levels of pathology upon Plasmodium infection [31]. Therefore, researchers should consider the impact of shift in gut microbiota during set up experiments and interpretation of outcomes of murine disease model systems. Particularly, researchers should include the vendor and shipping details of mice used in experiment at the time the results of publication to enable experimental reproducibility by other researchers/labs.
Mosquito first acquires its microbiota via trans-stadial transmission from the mother, and from breeding sites of its larva and pupa. Later, adult mosquitoes encounter microbes during feeding or mating. Specially, blood meal induces changes in gut microbiota community dynamics in mosquitoes [32, 33]. The shift in gut microbiota composition of the mosquito has profound effect on Plasmodium infection. For instance, introduction of P. putida, Pantoea sp., and S. marcescens resulted in strong inhibition of P. falciparum infection in An. gambiae [17]. A negative impact on numbers of P. falciparum oocyst has also been reported when infected An. gambiae mosquitoes were challenged by bacteria, such as Escherichia coli, Serratia marcescens, Pseudomonas stutzeri, Enterobacter spp., Acinetobacter septicus, Comamonas spp., and Bacillus pumilus [34]. E. coli, S. marcescens, and P. stutzeri had the highest effect on oocyst intensity. A study reported that S. marcescens increased mortality of P. vivax oocyst 13 times more than other bacteria in An. albimanus [35]. These studies highlight the significance of screening specific bacteria isolated from mosquitoes for anti-Plasmodium effects. They can be the basis of identifying bacteria candidates for vector control and transmission-blocking strategies. For instance, studies have shown that presence of Enterobacter bacterium renders mosquitoes 99% resistance to P. falciparum infection, it was then further investigated to determine specific anti-Plasmodium mechanism employed by the bacteria. The results show that bacteria inhibit the parasite infection via generation of reactive oxygen species (ROS) [36]. Other gut bacteria that have been investigated for inhibition of Plasmodium sp. infection in anopheles mosquitoes include Wolbachia (wAlb strain), Wickerhamomyces anomalus (WaF17.12 strain), paratransgenic Asaia, E. coli (351 and 444 strains), E. cloacae and Chromobacterium sp. (isolate Csp-P), [37,38,39,40,41,42,43]. Suggested mechanisms and outcomes of Plasmodium-microbiota interactions have been summarized in Table 1.
Experimental elimination of gut microbiota via antibiotic treatment have led to mosquitoes that are highly susceptible to Plasmodium spp. infection [44,45,46,47,48]. Antibiotic-treated mosquitoes are deprived of gut bacteria that play important role in antiparasitic activity in the insect. For instance, Gao et al. [48] reported that antibiotic treatment of mosquitoes inhibited the activation of Peptidoglycan Recognition Proteins LA (PGRP-LA), which is involved in antiparasitic defenses, and that gut microbiota played important role in the formation and integrity of the peritrophic membrane (PM), which inhibits Plasmodium development [48]. Song et al. [47] also showed that when the treatment of An. stephensi with antibiotics led to increased vector competence. Recolonization of the gut with Enterobacter sp. restored PM integrity and reduced susceptibility of the mosquitoes to P. berghei infection [47]. These two studies suggest that gut microbiota can also influence parasite establishment in the vector via PM formation and integrity. So, it will be interesting to research in detail, which mechanisms and specific microorganisms are involved in the interaction between gut microbiota and PM for development of transmission-blocking strategies.
Microbiota-mediated parasite control may be a consequence of microbiota influence on mosquito’s immune system and metabolism. Various transcriptomic analysis studies have shown activation of immune system in mosquito challenged by both parasite and microorganisms. When An. stephensi females were challenged with Serratia Y1 strain bacterium isolated from An. sinensis, an increase in the expression of some immunogenic genes such as C-type lectins, CLIP serine proteases, thioester-containing protein 1 (TEP1), fibrinogen immunolectin (FBN9), and leocine rich repeat protein (LRRD7), was detected [49]. These immune effectors have anti-plasmodium effect, as they rendered mosquitoes colonized with Serratia Y1 resistant to P. berghei infection. In addition, other immune effector molecules, such as anti-microbial peptide cecropin (CEC1), defensin (DEF1), and C-type lectin 4 (CTL4) have been reported to be involved in reducing Plasmodium infection in mosquitoes [50,51,52,53,54].
Although adaptive immunity has not been reported in insects there is a fair amount of specific immune response in mosquitoes. For instance, challenge of both An. stephensi and An. gambiae with Asaia bacterium showed upregulation of TEP1 in An. stephensi and not the latter [55]. Interestingly, there was a high reduction in Plasmodium replication in An. stephensi compared to An. gambiae. On the other hand, Bai et al. [49] also reported that Serratia J1 strain did not activate immune response in An. stephensi in contrast to Serratia Y1 strain. Notably, specific immune response in mosquitoes could be related to the ability of the bacteria to colonize the mosquito’s gut with dominant symbionts showing quick and consistent activation of host’s immune system [55]. Certainly, further studies are needed to confirm these hypotheses. However, it is also important to note that not all microorganisms enhance the immune response of mosquitoes to Plasmodium infection. For instance, Angleró-Rodríguez et al. [56] reported that the presence of Penicillium chrysogenum suppressed the immune response of An. gambiae rendering the mosquitoes susceptible to Plasmodium infections. According to the authors, the fungi secreted a heat-stable secondary metabolite that compromised mosquito defense mechanisms. Also, ingestion of Pe. chrysogenum resulted in upregulation of ornithine decarboxylase (ODC) gene, which limits the availability of L-arginine important for nitric oxide (NO) production [56]. Ultimately, specific microorganism-mosquito combinations need further research to identify “good” and “bad” symbionts in relation to Plasmodium infections. Identification of symbionts that enable parasite infections in mosquitoes may offer new opportunities to control transmission cycle via elimination of these symbionts.
In addition to feeding, the gut microbiota composition and diversity of mosquito are shaped by Plasmodium. Parasite infection may change the ecology of the gut via production of antimicrobials, competition for nutrients, and modification of the host physiology. As a result, some symbionts are eliminated while others gain dominance. Boissie`re et al. [18] reported a positive correlation between P. falciparum infection status and the prevalence of Enterobacteriaceae [18]. In addition, a study of dynamics of bacterial community in field-caught mosquitoes reported high abundance of Serratia and Methylobacterium in flies infected with P. falciparum compared to non-infected ones [57]. These studies suggest that the presence of certain bacteria is required for parasite development and transmission. However, within the scope of these Plasmodium-microorganisms cooperation, it remains unclear to decipher, which organism enables the existence of the other possible and the mechanisms involved.
Conclusion
Parasite-microbiota interactions are crucial in development, transmission, and severity of malaria. Inside the vector, the parasite must not only suppress the gut digestives enzymes and immune response, but also compete with the microbiota and protect themselves from antimicrobial effects of their metabolites. In addition, human microbiota plays a critical role in pathogenicity and virulence of the infecting parasite. Both microbiota and parasite can modulate the human immunity to control or exacerbate parasitic infections. Moreover, microbiota via production of antimicrobial metabolites, agglutination, modulation of nutrients and osmotic conditions, and formation of biofilms on parasite surfaces can kill or inhibit parasite growth. However, it remains unclear which exact mechanisms are directly employed by parasites to modulate microbiota composition and diversity. Further studies to determine the microbiota in vectors with high and low transmission capacity for malaria may provide new data for the development of molecules that target parasite-supporting bacteria and inhibit parasite-transmission in the vector. Moreover, investigation of microbiota in human with malaria may provide data for the development of new diagnostic, therapeutic and prevention strategies for plasmodium infections.
References
World Health Organization. (2017). Global vector control response 2017–2030. Geneva. Retrieved from https://apps.who.int/iris/handle/10665/259002. Accessed 05 May 2022
Kariithi H, Meki I, Schneider D, De Vooght L, Khamis F, Geiger A et al (2018) Enhancing vector refractoriness to trypanosome infection: achievements, challenges and perspectives. BMC Microbiol. https://doi.org/10.1186/s12866-018-1280-y
Gao H, Cui C, Wang L, Jacobs-Lorena M, Wang S (2020) Mosquito microbiota and implications for disease control. Trends Parasitol 36(2):98–111. https://doi.org/10.1016/j.pt.2019.12.001
Wijerathna T, Gunathunga S, Gunathilaka N (2020) Recent developments and future directions in the paratransgenesis based control of Leishmania transmission. Biol Control 145:104260. https://doi.org/10.1016/j.biocontrol.2020.104260
Lefèvre T, Sauvion N, Almeida R, Fournet F, Alout H (2022) The ecological significance of arthropod vectors of plant, animal, and human pathogens. Trends Parasitol 38(5):404–418. https://doi.org/10.1016/j.pt.2022.01.004
Dekaboruah E, Suryavanshi M, Chettri D, Verma A (2020) Human microbiome: an academic update on human body site specific surveillance and its possible role. Arch Microbiol 202(8):2147–2167. https://doi.org/10.1007/s00203-020-01931-x
Omondi Z, Demir S (2020) Bacteria composition and diversity in the gut of sand fly: impact on Leishmania and sand fly development. Int J Trop Insect Sci 41(1):25–32. https://doi.org/10.1007/s42690-020-00184-x
Hamidou Soumana I, Simo G, Njiokou F, Tchicaya B, Abd-Alla A, Cuny G, Geiger A (2013) The bacterial flora of tsetse fly midgut and its effect on trypanosome transmission. J Invertebr Pathol 112:S89–S93. https://doi.org/10.1016/j.jip.2012.03.029
Salcedo-Porras N, Umaña-Diaz C, de Oliveira Barbosa Bitencourt R, Lowenberger C (2020) The role of bacterial symbionts in triatomines: an evolutionary perspective. Microorganisms 8(9):1438. https://doi.org/10.3390/microorganisms8091438
Yooseph S, Kirkness E, Tran T, Harkins D, Jones M, Torralba M et al (2015) Stool microbiota composition is associated with the prospective risk of Plasmodium falciparum infection. BMC Genom. https://doi.org/10.1186/s12864-015-1819-3
Lappan R, Classon C, Kumar S, Singh O, de Almeida R, Chakravarty J et al (2019) Meta-taxonomic analysis of prokaryotic and eukaryotic gut flora in stool samples from visceral leishmaniasis cases and endemic controls in Bihar State India. PLoS Negl Trop Dis 13(9):e0007444. https://doi.org/10.1371/journal.pntd.0007444
de Souza-Basqueira M, Ribeiro R, de Oliveira L, Moreira C, Martins R, Franco D et al (2020) Gut dysbiosis in chagas disease a possible link to the pathogenesis. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2020.00402
de Vos W, Tilg H, Van Hul M, Cani P (2022) Gut microbiome and health: mechanistic insights. Gut 71(5):1020–1032. https://doi.org/10.1136/gutjnl-2021-326789
Romoli O, Gendrin M (2018) The tripartite interactions between the mosquito, its microbiota and Plasmodium. Parasit Vectors. https://doi.org/10.1186/s13071-018-2784-x
Louradour I, Monteiro C, Inbar E, Ghosh K, Merkhofer R, Lawyer P et al (2017) The midgut microbiota plays an essential role in sand fly vector competence for Leishmania major. Cell Microbiol 19(10):e12755. https://doi.org/10.1111/cmi.12755
Dale C, Welburn S (2001) The endosymbionts of tsetse flies: manipulating host–parasite interactions. Int J Parasitol 31(5–6):628–631. https://doi.org/10.1016/s0020-7519(01)00151-5
Bahia A, Dong Y, Blumberg B, Mlambo G, Tripathi A, Ben Marzouk-Hidalgo O et al (2014) Exploring Anopheles gut bacteria for Plasmodium blocking activity. Environ Microbiol 16(9):2980–2994. https://doi.org/10.1111/1462-2920.12381
Boissière A, Tchioffo M, Bachar D, Abate L, Marie A, Nsango S et al (2012) Midgut microbiota of the malaria mosquito vector Anopheles gambiae and Interactions with Plasmodium falciparum Infection. PLoS Pathog 8(5):e1002742. https://doi.org/10.1371/journal.ppat.1002742
Dennison N, Saraiva R, Cirimotich C, Mlambo G, Mongodin E, Dimopoulos G (2016) Functional genomic analyses of Enterobacter, Anopheles and Plasmodium reciprocal interactions that impact vector competence. Malaria J. https://doi.org/10.1186/s12936-016-1468-2
Rajendhran J, Gunasekaran P (2011) Microbial phylogeny and diversity: small subunit ribosomal RNA sequence analysis and beyond. Microbiol Res 166(2):99–110. https://doi.org/10.1016/j.micres.2010.02.003
Claesson M, Clooney A, O’Toole P (2017) A clinician’s guide to microbiome analysis. Nat Rev Gastroenterol Hepatol 14(10):585–595. https://doi.org/10.1038/nrgastro.2017.97
Iyer N (2016) Methods in microbiome research. Lab. Animal 45(9):323–326. https://doi.org/10.1038/laban.1093
Durazzi F, Sala C, Castellani G, Manfreda G, Remondini D, De Cesare A (2021) Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci Rep. https://doi.org/10.1038/s41598-021-82726-y
Choi KY, Lee TK, Sul WJ (2015) Metagenomic analysis of chicken gut microbiota for improving metabolism and health of chickens—a review. Asian Australas J Anim Sci 28(9):1217–1225. https://doi.org/10.5713/ajas.15.0026
Scholz M, Lo C, Chain P (2012) Next generation sequencing and bioinformatic bottlenecks: the current state of metagenomic data analysis. Curr Opin Biotechnol 23(1):9–15. https://doi.org/10.1016/j.copbio.2011.11.013
Laudadio I, Fulci V, Palone F, Stronati L, Cucchiara S, Carissimi C (2018) Quantitative assessment of shotgun metagenomics and 16S rDNA amplicon sequencing in the study of human gut microbiome. OMICS J Integr Biol. 22(4):248–254. https://doi.org/10.1089/omi.2018.0013
Phillips A, Bassett P, Zeki S, Newman S, Pasvol G (2009) Risk factors for severe disease in adults with falciparum malaria. Clin Infect Dis 48(7):871–878. https://doi.org/10.1086/597258
Khuu D, Eberhard M, Bristow B, Javanbakht M, Ash L, Shafir S, Sorvillo F (2018) Risk factors for severe malaria among hospitalized patients in the United States, 2000–2014. Infect Dis Health 23(2):93–106. https://doi.org/10.1016/j.idh.2018.01.002
Yilmaz B, Portugal S, Tran T, Gozzelino R, Ramos S, Gomes J et al (2014) Gut microbiota elicits a protective immune response against malaria transmission. Cell 159(6):1277–1289. https://doi.org/10.1016/j.cell.2014.10.053
Mandal R, Denny J, Waide M, Li Q, Bhutiani N, Anderson C et al (2020) Temporospatial shifts within commercial laboratory mouse gut microbiota impact experimental reproducibility. BMC Biol. https://doi.org/10.1186/s12915-020-00810-7
Villarino N, LeCleir G, Denny J, Dearth S, Harding C, Sloan S et al (2016) Composition of the gut microbiota modulates the severity of malaria. Proc Natl Acad Sci 113(8):2235–2240. https://doi.org/10.1073/pnas.1504887113
Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C (2010) A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science 327(5973):1644–1648. https://doi.org/10.1126/science.1184008
Muturi E, Njoroge T, Dunlap C, Cáceres C (2021) Blood meal source and mixed blood-feeding influence gut bacterial community composition in Aedes aegypti. Parasit Vectors. https://doi.org/10.1186/s13071-021-04579-8
Tchioffo M, Boissière A, Churcher T, Abate L, Gimonneau G, Nsango S et al (2013) Modulation of malaria infection in Anopheles gambiae mosquitoes exposed to natural midgut bacteria. PLoS ONE 8(12):e81663. https://doi.org/10.1371/journal.pone.0081663
Gonzalez-Ceron L, Santillan F, Rodriguez M, Mendez D, Hernandez-Avila J (2003) Bacteria in midguts of field-collected Anopheles albimanus block Plasmodium vivax sporogonic development. J Med Entomol 40(3):371–374. https://doi.org/10.1603/0022-2585-40.3.371
Cirimotich C, Dong Y, Clayton A, Sandiford S, Souza-Neto J, Mulenga M, Dimopoulos G (2011) Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 332(6031):855–858. https://doi.org/10.1126/science.1201618
Bian G, Joshi D, Dong Y, Lu P, Zhou G, Pan X et al (2013) Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium Infection. Science 340(6133):748–751. https://doi.org/10.1126/science.1236192
Eappen A, Smith R, Jacobs-Lorena M (2013) Enterobacter-activated mosquito immune responses to Plasmodium involve activation of SRPN6 in Anopheles stephensi. PLoS ONE 8(5):e62937. https://doi.org/10.1371/journal.pone.0062937.g005
Ramirez J, Short S, Bahia A, Saraiva R, Dong Y, Kang S et al (2014) Chromobacterium Csp_P reduces malaria and dengue infection in vector mosquitoes and has entomopathogenic and in vitro anti-pathogen activities. PLoS Pathog 10(10):e1004398. https://doi.org/10.1371/journal.ppat.1004398
Bongio N, Lampe D (2015) Inhibition of Plasmodium berghei development in mosquitoes by effector proteins secreted from Asaia sp. bacteria using a novel native secretion signal. PLoS ONE 10(12):e0143541. https://doi.org/10.1371/journal.pone.0143541
Valzano M, Cecarini V, Cappelli A, Capone A, Bozic J, Cuccioloni M et al (2016) A yeast strain associated to Anopheles mosquitoes produces a toxin able to kill malaria parasites. Malaria J. https://doi.org/10.1186/s12936-015-1059-7
Joshi D, Pan X, McFadden M, Bevins D, Liang X, Lu P et al (2017) The maternally inheritable wolbachia wAlbB induces refractoriness to Plasmodium berghei in Anopheles stephensi. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00366
Cappelli A, Valzano M, Cecarini V, Bozic J, Rossi P, Mensah P et al (2019) Killer yeasts exert anti-plasmodial activities against the malaria parasite Plasmodium berghei in the vector mosquito Anopheles stephensi and in mice. Parasit Vectors. https://doi.org/10.1186/s13071-019-3587-4
Dong Y, Manfredini F, Dimopoulos G (2009) Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog 5(5):e1000423. https://doi.org/10.1371/journal.ppat.1000423
Sharma A, Dhayal D, Singh O, Adak T, Bhatnagar R (2013) Gut microbes influence fitness and malaria transmission potential of Asian malaria vector Anopheles stephensi. Acta Trop 128(1):41–47. https://doi.org/10.1016/j.actatropica.2013.06.008
Gendrin M, Rodgers F, Yerbanga R, Ouédraogo J, Basáñez M, Cohuet A, Christophides G (2015) Antibiotics in ingested human blood affect the mosquito microbiota and capacity to transmit malaria. Nat Commun. https://doi.org/10.1038/ncomms6921
Song X, Wang M, Dong L, Zhu H, Wang J (2018) PGRP-LD mediates A. stephensi vector competency by regulating homeostasis of microbiota-induced peritrophic matrix synthesis. PLoS Pathog 14(2):1006899. https://doi.org/10.1371/journal.ppat.1006899
Gao L, Song X, Wang J (2020) Gut microbiota is essential in PGRP-LA regulated immune protection against Plasmodium berghei infection. Parasit Vectors. https://doi.org/10.1186/s13071-019-3876-y
Bai L, Wang L, Vega-Rodríguez J, Wang G, Wang S (2019) A gut symbiotic bacterium Serratia marcescens renders mosquito resistance to Plasmodium infection through activation of mosquito immune responses. Front Microbiol. https://doi.org/10.3389/fmicb.2019.01580
Blandin S, Shiao S, Moita L, Janse C, Waters A, Kafatos F, Levashina E (2004) Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116(5):661–670. https://doi.org/10.1016/s0092-8674(04)00173-4
Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, Dimopoulos G (2006) Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog 2(6):e52. https://doi.org/10.1371/journal.ppat.0020052
Hillyer J (2010) Mosquito immunity. Adv Exp Med Biol. https://doi.org/10.1007/978-1-4419-8059-5_12
Wang Y, Wang Y, Zhang J, Xu W, Zhang J, Huang F (2013) Ability of TEP1 in intestinal flora to modulate natural resistance of Anopheles dirus. Exp Parasitol 134(4):460–465. https://doi.org/10.1016/j.exppara.2013.04.003
Pang X, Xiao X, Liu Y, Zhang R, Liu J, Liu Q et al (2016) Mosquito C-type lectins maintain gut microbiome homeostasis. Nat Microbiol. https://doi.org/10.1038/nmicrobiol.2016.23
Cappelli A, Damiani C, Mancini M, Valzano M, Rossi P, Serrao A et al (2019) Asaia activates immune genes in mosquito eliciting an anti-Plasmodium response: implications in malaria control. Front Genet. https://doi.org/10.3389/fgene.2019.00836
Angleró-Rodríguez Y, Blumberg B, Dong Y, Sandiford S, Pike A, Clayton A, Dimopoulos G (2016) A natural Anopheles-associated Penicillium chrysogenum enhances mosquito susceptibility to Plasmodium infection. Sci Rep. https://doi.org/10.1038/srep34084
Tchioffo M, Boissière A, Abate L, Nsango S, Bayibéki A, Awono-Ambéné P et al (2016) Dynamics of bacterial community composition in the malaria mosquito’s epithelia. Front Microbiol. https://doi.org/10.3389/fmicb.2015.01500
Tchioffo M, Abate L, Boissière A, Nsango S, Gimonneau G, Berry A et al (2016) An epidemiologically successful Escherichia coli sequence type modulates Plasmodium falciparum infection in the mosquito midgut. Infect Genet Evol 43:22–30. https://doi.org/10.1016/j.meegid.2016.05.002
Meister S, Agianian B, Turlure F, Relógio A, Morlais I, Kafatos F, Christophides G (2009) Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites. PLoS Pathog 5(8):e1000542. https://doi.org/10.1371/journal.ppat.1000542
Wang S, Dos-Santos A, Huang W, Liu K, Oshaghi M, Wei G et al (2017) Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science 357(6358):1399–1402. https://doi.org/10.1126/science.aan5478
Pumpuni C, Kent M, Davis J, Beier J, Demaio J (1996) Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. Am J Trop Med Hyg 54(2):214–218. https://doi.org/10.4269/ajtmh.1996.54.214
Martínez-de la Puente J, Gutiérrez-López R, Díez-Fernández A, Soriguer R, Moreno-Indias I, Figuerola J (2021) Effects of mosquito microbiota on the survival cost and development success of avian Plasmodium. Front Microbiol. https://doi.org/10.3389/fmicb.2020.562220
Wang M, An Y, Gao L, Dong S, Zhou X, Feng Y et al (2021) Glucose-mediated proliferation of a gut commensal bacterium promotes Plasmodium infection by increasing mosquito midgut pH. Cell Rep 35(3):108992. https://doi.org/10.1016/j.celrep.2021.108992
Waide M, Polidoro R, Powell W, Denny J, Kos J, Tieri D et al (2020) Gut microbiota composition modulates the magnitude and quality of germinal centers during Plasmodium infections. Cell Rep 33(11):108503. https://doi.org/10.1016/j.celrep.2020.108503
Rodrigues J, Brayner F, Alves L, Dixit R, Barillas-Mury C (2010) Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science 329(5997):1353–1355. https://doi.org/10.1126/science.1190689
Habtewold T, Groom Z, Christophides G (2017) Immune resistance and tolerance strategies in malaria vector and non-vector mosquitoes. Parasit Vectors. https://doi.org/10.1186/s13071-017-2109-5
Noden B, Vaughan J, Pumpuni C, Beier J (2011) Mosquito ingestion of antibodies against mosquito midgut microbiota improves conversion of ookinetes to oocysts for Plasmodium falciparum, but not P. yoelii. Parasitol Int 60(4):440–446. https://doi.org/10.1016/j.parint.2011.07.007
Barletta A, Trisnadi N, Ramirez J, Barillas-Mury C (2019) Mosquito midgut prostaglandin release establishes systemic immune priming. Iscience 19:54–62. https://doi.org/10.1016/j.isci.2019.07.012
Pumpuni C, Beier M, Nataro J, Guers L, Davis J (1993) Plasmodium falciparum: inhibition of sporogonic development in anopheles stephensi by gram-negative bacteria. Exp Parasitol 77(2):195–199. https://doi.org/10.1006/expr.1993.1076
Riehle M, Moreira C, Lampe D, Lauzon C, Jacobs-Lorena M (2007) Using bacteria to express and display anti-Plasmodium molecules in the mosquito midgut. Int J Parasitol 37(6):595–603. https://doi.org/10.1016/j.ijpara.2006.12.002
Hughes G, Koga R, Xue P, Fukatsu T, Rasgon J (2011) Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium Falciparum in Anopheles gambiae. PLoS Pathog 7(5):e1002043. https://doi.org/10.1371/journal.ppat.1002043
Kwon H, Smith R (2022) Anopheles gambiae actively metabolizes uric acid following Plasmodium infection to limit malaria parasite survival. Front Physiol. https://doi.org/10.3389/fphys.2021.821869
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Literature search and analysis were performed by ZNO. The first draft of the manuscript was written by ZNO and AC, and both authors commented on previous versions of the manuscript. Figure prepared by ZNO. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Omondi, Z.N., Caner, A. An Overview on the Impact of Microbiota on Malaria Transmission and Severity: Plasmodium–Vector–Host Axis. Acta Parasit. 67, 1471–1486 (2022). https://doi.org/10.1007/s11686-022-00631-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-022-00631-4