Abstract
Purpose
Haematophagous Diptera, such as mosquitoes (Culicidae), biting midges (Ceratopogonidae), and black flies (Simuliidae), are important insects for public and animal health due to their capacity to bite and transmit pathogens. Outdoor recreation areas are usually affected by biting species and provide suitable habitats to both adult and immature stages. This study aimed to determine the species diversity and larval sites of these Diptera groups in two golf courses.
Methods
A multi-method collection approach using ultraviolet-CDC traps, human landing catches, collection in breeding sites, and ovitraps was implemented during summer 2020 in northern Spain. Insects were determined by morphological features accompanied by DNA barcoding.
Results
A total of ten native mosquito species were recorded either as adults or as larval stages. The invasive species Aedes japonicus was collected only at egg or pupa stage in ovitraps. Culex pipiens s.l. and Culex torrentium were both common mosquito species accounting for 47.9% of the total larval site collections and their larvae might be found in a wide range of natural and artificial sites. Culiseta longiareolata specimens were also prominent (30.1% of the total) and occurred exclusively in man-made water-filled containers. A total of 13 Culicoides species were identified, 10 of which were captured by ultraviolet-CDC traps, particularly members of the Obsoletus complex (Culicoides obsoletus/Culicoides scoticus, 74.9%) and seven species by emergence traps, being the two most abundant C. kibunensis (44.8%) and C. festivipennis (34.9%). Simulium cryophilum was also collected hovering around the operator under field sampling.
Conclusion
A comprehensive representation of the blood-sucking Diptera fauna and their larval sites was obtained by the multi-method approach in two Spanish golf courses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Outdoor activities have many benefits for human welfare and socialisation [1], so safe and healthy environments are essential for quality of life. Golfing is a significant outdoor recreation activity in which people spend long periods of time in the open air. In Spain, ca. 400 golf courses exist and the total annual economic impact of golfing is greater than 2,050,000,000 € with more than 11,000 direct employees [2].
Whereas this sector generates relevant local income to residents [3], from a public health point of view, human welfare relies on the absence of pests as the annoyance caused by bites of haematophagous species can turn pleasure time into an endurance course, thus negatively affecting all the industry behind it. However, there are only few scientific studies about nuisance and breeding sites of dipteran pests over outdoor recreation areas. Immature mosquitoes can develop in a wide range of aquatic habitats, from small and highly ephemeral sites to large and permanent natural and artificial saline or fresh water bodies. Floodwater mosquito species (i.e. Aedes caspius and Aedes cinereus) and flooded woodland species (i.e. Aedes cantans/Aedes annulipes, Aedes rusticus and Aedes detritus) have caused major problems in several aquatic environments and recreation areas of Europe [4]. Within midges, pre-imaginal forms of Culicoides develop either in aquatic and semi-aquatic habitats and the most annoying nuisance in Europe is caused by the biting midge Culicoides impunctatus in the Highlands of Scotland, which is well known to harm all kinds of outdoor activities, such as tourism, recreation, forestry and agriculture [5]. In Spain, there is no clear evidence of biting nuisance caused by Culicoides species, but other ceratopogonid midges from the genus Leptoconops (L. noei and L. bezzi) have been reported causing marginal discomfort in residential areas [6, 7]. Black flies are found in running watercourses and are also known to cause significant biting nuisance in places near rivers and streams in Spain, specifically the anthropophilic species Simulium erythrocephalum [8]. More information is available from the Americas, where large numbers of biting Diptera (mosquitoes, biting midges, and black flies) as well as non-biting as Chironomids emerging from tidal salt marshes, ponds, lakes and other water collections can cause nuisance and economic losses in recreation areas, outdoor sports, picnics and vacation [9,10,11,12].
Some of the aforementioned haematophagous groups (i.e. mosquitoes) are also efficient vectors of human pathogens, so that they should be monitored and controlled. The recent discovery of the presence of the invasive Aedes albopictus [13] and Aedes japonicus in the north of Spain [14, 15] is increasing concern from public health authorities. In fact, Aedes. albopictus has been implicated in chikungunya, dengue and Zika outbreaks in Europe and also recent autochthonous outbreaks of dengue in Spain [16,17,18]. Although Ae. japonicus has low vectorial competence for several viruses (i.e. West Nile-WN, usutu, dengue, Zika, and chikungunya), this species should not be underestimated in Europe [19,20,21,22]. Native mosquito species, such as Culex pipiens s.l. (biotypes and hybrids) and Culex perexiguus, are also increasing their epidemiological relevance as vectors of WN virus in several parts of Europe [23], particularly in southwest Spain, where a recent epidemic of this arboviral disease led to 77 cases and 7 human deaths across one of the most suitable geographical areas for these species [24]. In Europe, biting midges and black flies besides being a major nuisance for humans in certain regions [8, 25], have also an impact on animal health. Biting midges are important vectors of viral diseases associated with livestock (i.e. bluetongue and Schmallenberg viruses) [26] and black flies are primarily known to cause significant nuisance to livestock [27]. Both groups play also a pivotal role in the transmission of several pathogens to birds [28].
After the identification of the involved pest species, monitoring larval habitats must be stepped up for Integrated Vector Management (IVM) programs as they provide valuable information to carried out cost-efficient and environmentally sound control actions. Therefore, this study aimed to investigate on larval habitats, species richness, relative abundance and early detection of invasive species of mosquitoes and other blood-feeding dipteran groups using a multi-sourced study carried out in 2020 in two golf courses from northern Spain.
Materials and Methods
Study Area
Two golf courses located in the south of the Basque Country (northern Spain) in the Álava province were selected for this study (Fig. 1). Golf 1 (coordinates: 42,968,426–2,618,128; 570 masl) is around 60 ha surface and receives an average of 120 players per day during the summer season. This recreation area is located between two large swamps and surrounded mainly by Monterey pines (Pinus radiata) woodlands and broad meadows for cattle pasture. There is a permanent human settlement of approximately 150 inhabitants living in apartments and semidetached houses in and around the perimeter of the golf course. There is no application of insecticides, or very occasionally to control ornamental pests. Golf 2 (coordinates: 42,661,596– 2,511,232; 740 masl) is around 75 ha surface and receives an average of 80 players per day during the summer season. It is located in the Izki Natural Park, one of the Europe´s largest Pyrenean oak (Quercus pyrenaica) forests with more than 3500 ha. Other species, such as Fagus sylvatica and Ilex aquifolium, are also common in this area. There is an important cattle farming activity in the surroundings and the nearest village (located 400 m away) hosts ca. 70 inhabitants. Adulticide application is conducted by ultra-low volume fogging on vegetated areas from May to August to control high densities of the zoophilic fruit fly Phortica variegata, which are very annoying flies to players. Personal protection against mosquitoes and horse flies is recommended.
A, B Map of the study area in the Basque Country region, Northern Spain. Depiction of the multiple-sampling study implemented in the two golf courses labelled as 1 and 2 during the summer of 2020. C, D Location of the sampling stations. − OVI AJ Ovitrap negative to Ae. japonicus, + OVI AJ Ovitrap positive to Ae. japonicus, − BS MOS Breeding site negative to native mosquitoes, + BS MOS Breeding site positive to native mosquitoes, − BS CUL Breeding site negative to Culicoides, + BS CUL Breeding site positive to Culicoides, UV-CDC Ultraviolet-CDC trap, HLC Human landing catches. Car parking locations are surrounded by a hyphenated white line
The following climatic conditions were registered during the sampling period (1 June–30 September 2020) in golf 1 and 2, respectively: average mean temperature of 17.7 and 16.5 °C; average maximum temperature of 25.5 and 24.1ºC, average minimum temperature of 9.1 and 9.7 °C; total rainfall of 144 and 160 mm; and average relative humidity of 68.1 and 70.1%. The climate is classified as oceanic (cfb) with cold winters and mild summers. Climatic data were obtained from the closest weather stations from each golf course (located no more than 5–8 km away) [29].
Sampling Methods
We carried out a multi-sourced sampling effort, which consisted in trapping with ultraviolet (UV)-suction traps and ovitraps, collection of immature stages in larval sites or using emergence traps, and performing opportunistic human landing captures to collect individuals from three families of haematophagous dipterans in different locations of the study area.
UV-CDC Traps
UV-CDC-miniature portable traps (model 512, Bioquip, USA) were used to collect mosquitoes and biting midges in both golf courses during two sampling sessions (15–17 June and 14–16 September 2020). These traps were equipped with an UV-LED array light (390 nm) and were set in suitable places at 1.5 m above ground level, and working for 48 h (16:00 pm–16:00 pm) using four D-cell 1.5 V batteries fitted into a holder. Collected individuals were killed by a cold shock (− 20 °C). All biting midges and mosquito males were preserved in ethanol, whereas mosquito females were kept dry at − 20 °C to perform genetic analysis.
Ovitraps for Aedes Invasive Mosquito Sampling
Ovitraps consisted of a black pot (12 cm height, 10 cm diameter at the opening and 6 cm diameter at the base) filled with 500 ml of tap water containing a rectangular wooden stick (14 cm height × 3 cm width). Eight of such devices were set up in each golf course from 1 June to 30 September 2020. The ovitraps were placed in shaded, humid and safe places, particularly under bushes and at the corners of the car parking lot in each golf course following the ECDC recommendations [30]. Both the wooden stick and water of the ovitraps were replaced bi-weekly.
Following the detection of eggs consistent with invasive Aedes species at both golf courses, eight extra ovitraps were added in places near households, toilets, forest patches, under trees, and human structures within a radius of 1 km from the car parking in each golf course (Fig. 1). If the water-filled ovitraps contained immature stages of mosquitoes, these were collected and processed as explained below. In the laboratory, each labelled wooden stick was observed under a stereoscopic microscope (20×) and the paddles containing eggs with a morphology compatible with invasive Aedes species were separated. Wooden sticks with eggs were kept in a humid chamber (with wet cotton) at 25 ± 8 °C, 50–70% RH over three consecutive days for embryogenesis (usually two to three days are needed for full development) [31]. Immediately after, they were transferred to plastic trays (30 cm × 15 cm × 5 cm) containing 1 L of dechlorinated water for eggs hatching with 1 g of food (50% Tetramin and 50% yeast). After 3 days, wooden sticks were removed from the water and allowed to dry, a procedure that was repeated up to three times to simulate natural conditions inducing hatching. The resulting larvae were counted and reared until adult emergence together with already hatched larvae found in ovitraps at collection time. Mortality of immature stages was not recorded since it was insignificant.
Collection of Mosquito Immature Stages
The golf courses and their surroundings were searched for potential water-filled mosquito larval habitats (Fig. 1). These places (n = 27) were checked once at the end of each month (June, July, August and September) for the collection of immature mosquito species. Immature stages were collected with a large pipette (30 mL volume) driven by visual observation during a 10 min sampling effort per site. The individuals were brought to the lab in mosquito breeders (Bioquip, USA), reared there until adult emergence, killed and preserved at − 20 °C for identification.
Collection of Biting Midge Immature Stages
As immature stages of biting midges are difficult to locate, we employed commercially available emergence traps (tent-shaped, Bioquip, USA) to cover a ground surface area of about 1 m2 on each of three humid or semi-aquatic substrates: river shore muddy areas, margins of ponds, and over leaf litter in the nearby forest. The three emergence traps were placed over two consecutive months (June and July) in each golf course. After two months, the emergence traps were relocated to adjacent areas (2 m apart) for the next two sampling months (August and September). A polyethylene collecting jar filled with a mix of 150 mL ethanol and 10 mL glycerine was placed in the upper part of the tent-shaped emergence trap. The collection jar was replaced every month and midges were stored in ethanol (70%) for further analysis.
Human Landing Catches
Blood-sucking Diptera that were landing or attempting to feed on the human operator (always the same person wearing short-sleeved shirts and shorts) during the field work, were collected using a mouth aspirator and preserved in dry for further analysis. Catches were conducted two times per month in the evening (18:00–21:00 pm) from June to September. Similarly, individuals hovering over the operator were also captured using a sweep net, and stored in 70% ethanol.
Morphological and Molecular Identification
All the collected specimens were separated by taxonomical groups, sexed and counted. Mosquitoes, biting midges, and black flies were identified with the appropriate identification keys for western European fauna [32, 34]. All mosquito males, black flies (both sexes) and biting midge specimens with plain/similar-looking wings (both sexes) were dissected, and specific diagnosis body structures were mounted on slides with permanent media (Hoyer´s medium). Males of Cx. pipiens s.l. and Cx. torrentium were separated based on male genitalia [33] and closely related females were pooled and labelled as Cx. pipiens s.l./Cx. torrentium. Members of the Obsoletus complex (Culicoides obsoletus and Culicoides scoticus) were separated based on male genitalia while morphologically indistinguishable females were pooled [35,36,37]. Black flies and damaged mosquito females captured by human landing were determined molecularly by the cytochrome c oxidase I (COI) gene, after DNA extraction, PCR amplification, purification and sequencing procedures already described in Ruiz-Arrondo et al. [38,39,40]. Additionally, one specimen of Ae. japonicus from each golf course was also molecularly determined for species confirmation. Detailed mosquito records and sequence information were submitted to GenBank under the accession numbers: MZ444611–MZ444612 (black flies) and MZ444613–MZ444617 (mosquitoes). Aedes japonicus specimens were photographed with a Canon 450D + 100 mm 2.8f (Fig. 2).
Results
A total of 11 mosquito species, 13 biting midge species, and one black fly species were recorded by the described approach in two golf courses along the summer of 2020 (Tables 1, 2, 3 and 4). One mosquito (Culex modestus) and black fly species (Simulium cryophilum) are reported for the first time to the fauna of the Basque Country. Molecular identification (DNA barcoding) confirmed the identity of Ae. cantans (n = 1 with 99.85% identity with individual MN413933.1 from Spain), Ae. japonicus (n = 2, with 100% identity with individual KF211480.1 from Germany and MW509626.1 from Romania), Aedes rusticus (n = 2, with 99.84 and 99.79% identity with individual MK403533.1 from UK), and S. cryophilum (n = 3, with 99.85% identity with individual KP861185.1 from UK).
UV-CDC-Traps Collection
Whereas only seven specimens of two mosquito species (Cx. pipiens s.l./Cx. torrentium and Cs. longiareolata) were captured by UV-CDC traps, a total of ten Culicoides species (n = 122) were collected by suction traps (Table 1). The sibling species C. obsoletus and C. scoticus were the most abundant species (74.6% of the total collections) followed by C. punctatus (10.6%). According to males, both species (C. scoticus and C. obsoletus) were present in similar numbers. Eight remaining Culicoides species accounted for less than 15% of the total collections (Table 2).
Ovitraps for Invasive Aedes Species Collection
The invasive species Ae. japonicus was recorded either as eggs (280 eggs) or immature stages (two pupae) in two and one ovitraps placed on the car parkings of golf 1 and 2, respectively (Table 3). Eggs of Ae. japonicus were not uniformly collected along the four-month sampling period (Table 3). No individuals of this species were found in ovitraps placed outside of the parking lots, neither in the monitored potential breeding sites nor in the UV-CDC traps. The cumulative hatching curve showed that 64% of the Ae. japonicus eggs hatched within the two first immersions, 5% in the third immersion, and the remaining 31% were unhatched eggs considered as not functional. During the study, three more mosquito species Cx. torrentium (two times), Cx. hortensis (one time) and Anopheles claviger s.l. (two times) were identified from emerged specimens in ovitraps in cohabitation with Ae. japonicus.
Mosquito Immature Stages Collection
A total of 481 (288 females and 193 males) specimens were collected from 17 out of the 27 water-filled potential mosquito larval sites inspected (Fig. 1; Table S1). Seven mosquito species were found developing in 11 artificial larval sites, whereas six mosquito species were found in six natural/semi-natural larval sites (Fig. 3). Culex pipiens s.l. and Cx. torrentium were the most abundant and well-distributed group of species (n = 230; 47.9% of the total catches; 8/17 larval sites) (Table S1). Based on males, we determined that Cx. torrentium was mainly found in small water-filled sites (mainly ovitraps and plastic/tube containers), whereas the developmental sites of Cx. pipiens s.l. also included water-filled containers of larger volume (1–100 L) and several natural habitats (tree hole, puddle and pond) (Fig. 3; Table S1). Culiseta longiareolata specimens (n = 151; 31.4%; 7/17) were mostly found in artificial sites (Fig. 3).
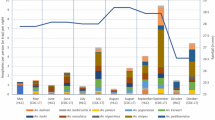
Relative abundance of mosquito species collected in larval sites during the summer of 2020 in northern Spain. Artificial larval sites: Container ≤ 1 L = Ovitraps, plastic tube, and plastic container; Container 1–10 L = Metallic container, plastic bucket, drinking trough, and drainage roof drain; Container 10–100 L = Artificial pond, drainage channel, and drinking rough; Container ≥ 100 L = Swimming pool; Tree-hole (Acer sp.); Ditch (with vegetation on the edges); Puddle (muddy bed); Pool (deep hollow rich in organic matter); Pond (semi-natural herbaceous pond). The relative abundance is calculated by diving the number of mosquito specimens of each species by the total number specimens in each larval site category
Biting Midges’ Collection
A total of 190 Culicoides specimens were obtained from emergence traps (Table 4). The three most abundant species were C. kibunensis (45.2%), C. festivipennis (35.2%), and C. punctatus (13.5%). Culicoides festivipennis and C. punctatus were the predominant species in the muddy margins of the ponds, C. kibunensis in the muddy edges of the rivers, and C. obsoletus s.s. and C. kibunensis in the soil rich in organic matter of forest patches (Table 4).
Human Landing Catches
Aedes cantans (n = 1), Ae. rusticus (n = 2), and Cx. modestus (n = 2) were aspirated when trying to land on the collector. Simulium cryophilum (n = 7) was recorded by sweeping flying around the collector though not biting. Some biting midges were also captured hovering around the collector but none belonged to the genus Culicoides or Leptoconops.
Discussion
To the best of our best knowledge, this paper represents the first attempt at a systematic study of biting insects in golf courses of Europe. The multiple-trapping methodology employed allowed to perform a more comprehensive characterization of the biodiversity and larval habitats in these areas, including many aquatic and semi-aquatic substrates, such as ponds, lakes, and ditches, as typical landscape obstacles for golf players, which also could provide suitable habitats for mosquitoes and biting midges. On the other hand, only one black fly species was recorded, despite the fact that suitable habitats (i.e. rivers and streams) were also available, perhaps because no specific trapping method was employed for this family. Interestingly, only three species of mosquitoes were attracted to the collector whereas the Simulium and Culicoides species showed limited or no attraction to human hosts, respectively. However, the presence of potential vectors of arboviruses (i.e. Cx. pipiens s.l.) and malaria parasites (i.e. Anopheles plumbeus) deserves major attention for public health as expected and likewise many other areas in Spain.
From the five invasive Aedes mosquito species known to be introduced or established in Europe [41], two of them (Ae. albopictus and Ae. japonicus) have been detected in Spain. While Ae. albopictus is widely extended over several parts of mainland Spain, Mediterranean coast, and the Balearic Islands [42], the distribution of its congeneric Ae. japonicus is still restricted to the Cantabrian cornice [15, 16]. A number of studies have been published on Ae. japonicus in Europe suggesting repeated importation, e.g. through the used tyre trade and/or by quick autonomous dispersal [43]. One of the results in our study was the record of this exotic invasive species in a few ovitraps located within the car parkings, thus supporting the use of these devices as an effective tool for the early detection of this invasive species as opposed to other works where ovitraps are reported as low or fair efficacy tools [31]. Our observations suggest a recent arrival of the species to these spots, based on the absence of host-seeking adults in traps or around humans, failure in detecting larvae in breeding sites, absence of eggs in any other of the ovitraps scattered across the golf course and absence of constant catches along the sampling period. A road introduction of Ae. japonicus either by vehicles or by gardening commodities has been proposed by Koban et al. [43]. However, due to the relatively isolated area of the sampling sites located in the middle of broad areas of forest patches in dead-end unique roads, thus the natural dispersal capacity of the species through the forest might also deserve further research. Although golf courses are not commonly used in surveillance programs [44], we found a number of natural and artificial permanently water-filled structures could serve as larval sites for this exotic invasive species. However, we did not find Ae. japonicus immatures breeding in natural or artificial sources so we cannot comment on the original breeding sites for these local populations.
In this study, the breeding site preferences of Cx. pipiens s.l. were wider and more versatile compared to Cx. torrentium. The latter preferred artificial containers and particularly those with small water amounts, whereas Cx. pipiens s.l. was found in a wide range of artificial, natural/semi-natural breeding sites, including tree-holes as observed elsewhere [45]. The identification of the larval sites of these species is of paramount importance given their status as WN virus vectors [46]. In accordance to other studies, a wide range of man-made containers were also the most favourable breeding sites for the development of other native Culex species (Cx. hortensis and Cx. territans) [47]. The generalist Cs. longiareolata was also prominent in medium to high water volume artificial containers in accordance with other studies [39, 48,49,50]. Anopheles plumbeus, a suspected vector of cryptic malaria transmission in central–western Europe [51, 52], was the only species found at larval stages breeding in margins of vegetated ditches. Anopheles plumbeus is originally considered as a tree hole breeder, but larvae have also been found exploiting a wide range of other larval sites [52, 53]. With lower epidemiological concern, Anopheles claviger s.l. was found breeding both in natural and artificial breeding sites, in accordance to other studies [47]. The three species captured by human landing (Ae. cantans, Ae. rusticus and Cx. modestus) are known as aggressive mammalophilic daily biters [33], though they seem to be present in low numbers according to the field sampling.
No anthropophilic species of Simuliidae were recorded in the area. Whereas some individuals of S. cryophilum exhibited some degree of interest to humans, this species is considered as ornithophilic [54] and is widely distributed throughout the Spanish geography [55]. Despite black flies are commonly found in rivers and streams from the Basque Country, no biological or faunistic studies have been published yet. Therefore, our finding of S. cryophilum is presented as the first record of the species in the region. The use of suction traps baited with carbon dioxide would improve the monitoring and surveillance not only of black flies but also of mosquitoes.
The knowledge about the ecology and biology of Culicoides has improved substantially over the last years in Europe. One of the most predominant taxa according to UV-CDC-traps was the Obsoletus complex (C. obsoletus and C. scoticus). These two sibling species are frequently reported across Europe as the most abundant Culicoides species in farm and stable holdings associated mainly with cattle, horses, sheep and goats [56, 57]. The proximity of the golf courses to cattle farms might account for the predominance of these species, although C. obsoletus s.l. is a widespread species also found in prominent numbers at several other settings [58,59,60,61]. Emergence traps were productive in collecting C. kibunensis in river edges in accordance to González et al. [62] and Werner et al. [63]. The muddy margins of the ponds were used as larval development sites by several other Culicoides species, particularly C. punctatus and C. festivipennis. These semi-aquatic micro-habitats are known to be very productive in terms of diversity of Culicoides species [64, 65]. Fallen leaves (moist decaying leaves) on forest soils were used by C. obsoletus s.s. as favourable breeding sites in accordance with other studies [62, 66]. Note that the type of trapping implemented and devoted efforts were greater for mosquito fauna than for biting midges and black flies, so this might explain their lower capture rate in this study relative to mosquitoes. In addition, the sampling effort might be considered unbalanced as some methodologies (i.e. collection of immature stages) were performed on a regular basis as opposed to UV-suction traps. In any case, this fact does not influence our results as we have not used these data for comparison, only to generate a broader picture of the species diversity at these sites.
Some points of interest are highlighted in this study. First, the importance is stressed of using multi-source surveillance methods in conjunction with molecular tools (i.e. barcoding) to adequately characterize the fauna of an unexplored area. Second, the relevance of an accurate identification of the eggs from invasive Aedes species under the current scenario with up to four exotic Aedes mosquito species occurring in Europe [67]. It is strongly recommended to rear larvae of invasive Aedes species until L3–L4 for identification. Although authors suggest that identification of eggs of Ae. japonicus is feasible based on the colour, size and shape of chorionic cells [68], non-trained entomologists might find this task tricky needing powerful optical devices.
Conclusion
This work highlights the biodiversity of haematophagous Diptera collected by the multi-method approach implemented. Recreation areas as golf courses offer several natural and artificial larval sites to support native and exotic mosquitoes and biodiversity of biting midges. As in other regions in Europe as well as in Spain, it seems that Ae. japonicus remains quite unnoticed; thus, it would be needed to keep the surveillance on these places by the next years to assess its future population dynamics. The autochthonous Culicidae species captured do not play a relevant role in generating nuisance, except some anthropophilic species that were marginally collected. One of the common vectors for WN virus, Cx. pipiens s.l. was also collected in large numbers as expected since this is a ubiquitous species. The study also shows that Culicoides bluetongue vectors belonged to the Obsoletus complex are common in these recreation areas.
Availability of Data and Material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Eigenschenk B, Thomann A, Mcclure M, Davies L, Gregory M, Dettweiler U et al (2019) Benefits of outdoor sports for society. A systematic literature review and reflections on evidence. Int J Environ Res Public Health 16:937. https://doi.org/10.3390/ijerph16060937
Aymerich F, Anabitarte J (2016) El impacto económico del golf en España. http://www.rfegolf.es/ArtculosDocumento/Turismo%20e%20impacto%20econ%C3%B3mico/Turismo%20e%20impacto%20econ%C3%B3mico%202016/2016%20impacto%20econ%C3%B3mico%20del%20golf%20en%20Espa%C3%B1a.pdf. Accessed 25 June 2021
Donaldson J, Kazmierski B, Marcouiller D (2011) Local economic impacts of golfing: a case study of the Luck Golf Course in Polk County, Wisconsin. https://dpla.wisc.edu/wp-content/uploads/sites/1021/2017/06/11-01.pdf. Accessed 15 May 2021
Medlock JM, Vaux AG (2015) Seasonal dynamics and habitat specificity of mosquitoes in an English wetland: implications for UK wetland management and restoration. J Vector Ecol 40:90–106. https://doi.org/10.1111/jvec.12137
Hendry G (2011) Midges in Scotland, 5th edn. Bell & Bain Ltd, Glasgow
González MA, López S, Goldarazena A (2013) New record of the biting midge Leptoconops noei in northern Spain: notes on its seasonal abundance and flying height preference. J Insect Sci 13:45. https://doi.org/10.1673/031.013.4501
Obregón R, Flores E, Jordano D (2019) First report of the Asian tiger mosquito, Aedes (Stegomyia) albopictus Skuse, 1984 (Diptera, Culicidae) in Cordoba (southern Spain). New challenges for the administration and citizens of Cordoba. J Euro Mosq Control Assoc 37:29–33
Ruiz-arrondo I, Garza-hernández JA, Reyes-villanueva F, Lucientes-curdi J, Rodríguez-pérez MA (2017) Human-landing rate, gonotrophic cycle length, survivorship, and public health importance of Simulium erythrocephalum in Zaragoza, northeastern Spain. Parasit Vectors 10:175. https://doi.org/10.1186/s13071-017-2115-7
Lothrop BB, Mulla MS (1995) Mode of existence and seasonality of midge larvae (Diptera: Chironomidae) in man-made lakes in the Coachella Valley, southern California. J Am Mosq Control Assoc 11:77–85
Gray EW, Adler PH, Noblet R (1996) Economic impact of black flies (Diptera:Simuliidae) in South Carolina and development of a localized suppression program. J Am Mosq Control Assoc 12:676–678
Breidenbaugh MS, Clark JW, Brodeur RM, de Szalay FA (2009) Seasonal and diel patterns of biting midges (Ceratopogonidae) and mosquitoes (Culicidae) on the Parris Island marine corps recruit depot. J Vector Ecol 34:129–140. https://doi.org/10.3376/038.034.0116
Adler PH, Currie DC, Wood M, Idema RM (2004) The black flies (Simuliidae) of North America. Comstock Publ, Ontario
Goiri F, González MA, Goikolea J, Oribe M, Castro V, Delacour S et al (2020) Progressive invasion of Aedes albopictus in Northern Spain in the period 2013–2018 and a possible association with the increase in insect bites. Int J Environ Res Public Health 17:1678. https://doi.org/10.3390/ijerph17051678
Eritja R, Ruiz-arrondo I, Delacour-Estrella S, Schaffner F, Álvarez-chachero J, Bengoa M et al (2019) First detection of Aedes japonicus in Spain: an unexpected finding triggered by citizen science. Parasit Vectors 12:53. https://doi.org/10.1186/s13071-019-3317-y
Eritja R, Delacour-Estrella S, Ruiz-Arrondo I, González MA, Barceló C, García-Pérez AL et al (2021) At the tip of an iceberg: citizen science and active surveillance collaborating to broaden the known distribution of Aedes japonicus in Spain. Parasite Vectors 14:375. https://doi.org/10.1186/s13071-021-04874-4
Tomasello D, Schlagenhauf P (2013) Chikungunya and dengue autochthonous cases in Europe, 2007–2012. Travel Med Infect Dis 11:274–284. https://doi.org/10.1016/j.tmaid.2013.07.006
Aranda C, Martinez MJ, Montalvo T, Corbella I, Bigas E, Barrabeig I et al (2018) Arbovirus surveillance: first dengue virus detection in local Aedes albopictus mosquitoes in Europe, Catalonia, Spain, 2015. Euro Surveill 23:1700837. https://doi.org/10.2807/1560-7917
ECDC 2019. Epidemiological update: third case of locally acquired Zika virus disease in Hyères F. https://www.ecdc.europa.eu/en/news-events/epidemiological-update-third-case-locally-acquired-zika-virus-disease-hyeres-france. Accessed 13 Dec 2019
Schaffner F, Vazeille M, Kaufmann C, Failloux A, Schaffner F, Vazeille M et al (2011) Vector competence of Aedes japonicus for chikungunya and dengue viruses. Euro Mos Bull 29:141–142
Veronesi E, Paslaru A, Silaghi C, Tobler K, Glavinic U, Torgerson P et al (2018) Experimental evaluation of infection, dissemination, and transmission rates for two West Nile virus strains in European Aedes japonicus under a fluctuating temperature regime. Parasitol Res 117:1925–1932. https://doi.org/10.1007/s00436-018-5886-7
Abbo SR, Visser TM, Wang H, Göertz GP, Fros JJ, Abma-Henkens M et al (2020) The invasive Asian bush mosquito Aedes japonicus found in the Netherlands can experimentally transmit Zika virus and Usutu virus. PLoS Negl Trop Dis 14:e0008217. https://doi.org/10.1371/journal.pntd.0008217
Glavinic U, Varga J, Paslaru AI, Hauri J, Torgerson P, Schaffner F et al (2020) Assessing the role of two populations of Aedes japonicus japonicus for Zika virus transmission under a constant and a fluctuating temperature regime. Parasites Vectors 13:479. https://doi.org/10.1186/s13071-020-04361-2
Vogels CBF, Göertz GP, Pijlman GP, Koenraadt CJM (2017) Vector competence of European mosquitoes for west Nile virus. Emerg Microbes Infect 6:1–13. https://doi.org/10.1038/emi.2017.82
ECDC (2020) Weekly updates: 2020 West Nile virus transmission season. https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc. Accessed 25 June 2021.
Carpenter S, Groschup MH, Garros C, Felippe-Bauer ML, Purse BV (2013) Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res 100:102–113. https://doi.org/10.1016/j.antiviral.2013.07.020
Koenraadt CJM, Balenghien T, Carpenter S, Ducheyne E, Elbers ARW et al (2014) Bluetongue, Schmallenberg—what is next? Culicoides-borne viral diseases in the 21st century. BMC Vet Res 10:77
Figueras L, Lucientes J, Ruiz Arrondo I, Ramos Antón JJ et al (2011) Caso clínico. Ataque de simúlidos en rumiantes. Veterinaria Independiente 147:22
Santiago-Alarcón D, Palinauskas V, Schaefer HM (2012) Diptera vectors of avian Haemosporidian parasites: untangling parasite life cycles. Biol Rev Camb Philos Soc 87:928–864. https://doi.org/10.1111/j.1469-185X.2012.00234.x
Basque Institute of Meteorological Data (2020) Euskalmet. Meteorological data. https://apps.euskadi.eus/s07-5853x/es/meteorologia/graficos.apl?e=5. Accessed 15 Jan 2021
ECDC (2012) Technical report. Guidelines for the surveillance of invasive mosquitoes in Europe. https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/TER-Mosquito-surveillance-guidelines.pdf. Accessed 15 May 2021
Farnesi LC, Martins AJ, Valle D, Rezende GL (2009) Embryonic development of Aedes aegypti (Diptera: Culicidae): influence of different constant temperatures. Mem Inst Oswaldo Cruz 104:124–126. https://doi.org/10.1590/s0074-02762009000100020
Rivosecchi L, Addonisio, M, Maiolini B (2007) Ditteri Simulidi: nuove chiavi dicotomiche per l’identificazione delle specie italiane con brevi note bio-tassonomiche. Mus Trident di Sci Nat, Italy.
Becker N, Petric D, Zgomba M, Boase C, Madon MDC (2010) Mosquitoes and their control, 2nd edn. Springer, Berlin
Mathieu B, Cêtre-Sossah C, Garros C, Chavernac D, Balenghien T, Carpenter S et al (2012) Development and validation of IIKC: an interactive identification key for Culicoides (Diptera: Ceratopogonidae) females from the Western Palaearctic region. Parasit Vectors 5:1–11. https://doi.org/10.1186/1756-3305-5-137
Augot D, Sauvage F, Jouet D, Simphal E, Veuille M, Couloux A et al (2010) Discrimination of Culicoides obsoletus and Culicoides scoticus, potential bluetongue vectors, by morphometrical and mitochondrial cytochrome oxidase subunit I analysis. Infect Genet Evol 10:629–637. https://doi.org/10.1016/j.meegid.2010.03.016
Nielsen SA, Kristensen M (2011) Morphological and molecular identification of species of the Obsoletus group Diptera: Ceratopogonidae) in Scandinavia. Parasitol Res 109:1133–1141. https://doi.org/10.1007/s00436-011-2357-9
González M, Goldarazena A (2011) El Género Culicoides en el País Vasco: Guía Práctica para su Identificación y Control. Servicio Central de Publicaciones del Gobierno Vasco, Vitoria-Gasteiz
Ruiz-arrondo I, Hernández-triana LM, Ignjatovi A, Nikolova N, Garza-hernández JA, Rodríguez-pérez MA et al (2018) DNA barcoding of blackflies (Diptera: Simuliidae) as a tool for species identification and detection of hidden diversity in the eastern regions of Spain. Parasit Vectors 11:463. https://doi.org/10.1186/s13071-018-3046-7
Ruiz-Arrondo I, McMahon BJ, Hernández-Triana LM, Santibañez P, Portillo A, Oteo JA (2019) Surveillance of mosquitoes (Diptera, Culicidae) in a northern central region of Spain: implications for the medical community. Front Vet Sci 6:86. https://doi.org/10.3389/fvets.2019.00086
Ruiz-Arrondo I, Hernández-Triana LM, Nikolova NI, Fooks AR, Oteo JA (2020) Integrated approaches in support of taxonomic identification of mosquitoes (Diptera: Culicidae) in vector surveillance in Spain. Vector Borne Zoonotic Dis 20:831–842. https://doi.org/10.1089/vbz.2020.2662
Schaffner F, Medlock JM, Van Bortel W (2013) Public health significance of invasive mosquitoes in Europe. Clin Microbiol Infect 19:685–692. https://doi.org/10.1111/1469-
Collantes F, Delacour S, Delgado JA, Bengoa M, Torrell-Sorio A, Guinea H et al (2016) Updating the known distribution of Aedes albopictus in (Skuse, 1894)Spain. Acta Trop 164:64–68. https://doi.org/10.1016/j.actatropica.2016.08.023
Koban MB, Kampen H, Scheuch DE, Frueh L, Kuhlisch C, Janssen N et al (2019) The Asian bush mosquito Aedes japonicus japonicus (Diptera: Culicidae) in Europe, 17 years after its first detection, with a focus on monitoring methods. Parasit Vectors 12:109. https://doi.org/10.1186/s13071-019-3349-3
Marabuto E, Rebelo MT (2018) The Asian tiger mosquito, Aedes albopictus (Skuse, 1894), a vector of dengue, chikungunya and zika, reaches Portugal. Zootaxa 413:97–200. https://doi.org/10.11646/zootaxa.4413.1.10
Zittra C, Flechl E, Kothmayer M, Vitecek S, Rossiter H, Zechmeister T (2016) Ecological characterization and molecular differentiation of Culex pipiens complex taxa and Culex torrentium in eastern Austria. Parasit Vectors 9(9):7. https://doi.org/10.1186/s13071-016-1495-4
Brugman VA, Hern LM, Medlock JM, Fooks AR, Carpenter S, Johnson N (2018) The role of Culex pipiens L. (Diptera: Culicidae) in virus transmission in Europe. Int J Environ Res Public Health 15:389. https://doi.org/10.3390/ijerph15020389
Bueno Marí R (2010) Bioecologia, diversidad e interés epidemiológico de los culícidos mediterráneos (Diptera:Culicidae). Dissertation, University of Valencia.
Seidel B, Nowotny N, Duh D, Indra A, Hufnagl P, Allerberger F (2012) First records of the thermophilic mosquito Culiseta longiareolata (Macquart, 1838) in Austria, 2012, and in Slovenia, 2013. J Am Mosq Control Assoc 31:17–20
Becker N, Hoffmann D (2011) First record of Culiseta longiareolata (Macquart) for Germany. J Am Mosq Control Assoc 29:143–150
Roiz D, Eritja R, Escosa R, Lucientes J, Marquès E, Ruiz S (2017) A survey of mosquitoes breeding in used tires in Spain for the detection of imported potential vector species. J Vector Ecol 32:10–15. https://doi.org/10.3376/1081-1710
Schaffner F, Thiéry I, Kaufmann C, Zettor A, Lengeler C (2012) Anopheles plumbeus (Diptera : Culicidae) in Europe: a mere nuisance mosquito or potential malaria vector ? Malar J 11:393. https://doi.org/10.1186/1475-2875-11-393
Tagliapietra V, Arnoldi D, Luca MD, Toma L, Rizzoli A (2019) Investigation on potential malaria vectors (Anopheles spp.) in the Province of Trento, Italy. Malar J 18:151. https://doi.org/10.1186/s12936-019-2785-z
Bueno Marí R, Jiménez-Peydró R (2010) New anopheline records from the Valencian Autonomous Region of Eastern Spain (Diptera: Culicidae: Anophelinae). J Eur Mosq Control Assoc 28:148–156
Chakarov N, Kampen H, Wiegmann A, Werner D, Bensch S (2020) Blood parasites in vectors reveal a united blackfly community in the upper canopy. Parasit Vectors 13:309. https://doi.org/10.1186/s13071-020-04177-0
López-Peña D, Jiménez Peydró R (2017) Updated checklist and distribution maps of blackflies (Diptera: Simuliidae) of Spain. Simuliid Bull 48(supplement):1–45
Cuéllar AC, Kjær LJ, Kirkeby C, Skovgard H, Nielsen SA, Stockmarr A et al (2018) Spatial and temporal variation in the abundance of Culicoides biting midges (Diptera: Ceratopogonidae) in nine European countries. Parasit Vectors 11:112. https://doi.org/10.1186/s13071-018-2706-y
Mignotte A, Garros C, Gardès L, Balenghien T, Duhayon M, Rakotoarivony I et al (2020) The tree that hides the forest:cryptic diversity and phylogenetic relationships in the Palaearctic vector Obsoletus/Scoticus Complex (Diptera: Ceratopogonidae) at the European level. Parasit Vectors 13:265. https://doi.org/10.1186/s13071-020-04114-1
Zimmer JY, Smeets F, Simonon G, Fagot J, Haubruge E, Francis F et al (2013) Are bogs reservoirs for emerging disease vectors? Evaluation of Culicoides populations in the Hautes Fagnes Nature Reserve (Belgium). PLoS ONE 8:e66893. https://doi.org/10.1371/journal.pone.0066893
Möhlmann TWR, Bekendam AM, van Kemenade I, Wennergren U, Favia G, Takken W et al (2019) Latitudinal diversity of biting midge species within the Obsoletus group across three habitats in Europe. Med Vet Entomol 33:420–426. https://doi.org/10.1111/mve.12379
England ME, Kelly PP, Brugman VA, King S, Gubbins S, Sach F et al (2020) Culicoides species composition and molecular identification of host blood meals at two zoos in the UK. Parasit Vectors 13:139. https://doi.org/10.1186/s13071-020-04018-0
González MA, Goiri F, Barandika JF, García-Pérez AL (2020) Culicoides biting midges and mosquito fauna at three dog and cat shelters in rural and periurban areas in Northern Spain. Med Vet Entomol 35:79–87. https://doi.org/10.1111/mve.12471
González MA, López S, Mullens BA, Baldet T, Goldarazena A (2013) A survey of Culicoides developmental sites on a farm in northern Spain, with a brief review of immature habitats of European species. Vet Parasitol 191:81–93. https://doi.org/10.1016/j.vetpar.2012.08.025
Werner D, Groschupp S, Bauer C, Kampen H (2020) Breeding habitat preferences of major Culicoides species (Diptera: Ceratopogonidae) in Germany. Int J Environ Res Public Health 17:5000. https://doi.org/10.3390/ijerph17145000
Uslu U, Dik B (2017) Description of breeding sites of Culicoides species (Diptera: Ceratopogonidae) in Turkey. Parasite 4:173–177. https://doi.org/10.1051/parasite/2007142173
Foxi C, Delrio G (2010) Larval habitats and seasonal abundance of Culicoides biting midges found in association with sheep in northern Sardinia, Italy. Med Vet Entomol 24:199–209. https://doi.org/10.1111/j.1365-2915.2010.00861.x
Harrup L, Affairs R, Purse BV, Mellor PS, Carpenter S (2013) Larval development and emergence sites of farm-associated Culicoides in the United Kingdom. Med Vet Entomol 27:441–449. https://doi.org/10.1111/mve.12006
Montarsi F, Martini S, Michelutti A, Rold GD, Mazzucato M, Qualizza D et al (2019) The invasive mosquito Aedes japonicus japonicus is spreading in northeastern Italy. Parasit Vectors 12:120. https://doi.org/10.1186/s13071-019-3387-x
Bova AJ, Paulson S, Paulson G (2016) Morphological differentiation of the eggs of North American container-inhabiting Aedes mosquitoes. J Am Mosq Control Assoc 32:244–246. https://doi.org/10.2987/15-6535.1
Acknowledgements
We thank the managers of the golf clubs for allowing us to conduct this study. The first author of this study (MAG) has carried out the investigation without any financing. The author wants to claim the difficulty to make science in a country that invests very little in I + D.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Data collection and analysis were performed by MAG, MB, CB, SD and RBM. The first draft of the manuscript was written by MAG, RE and IRA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
González, M.A., Delacour-Estrella, S., Bengoa, M. et al. A Survey on Native and Invasive Mosquitoes and Other Biting Dipterans in Northern Spain. Acta Parasit. 67, 867–877 (2022). https://doi.org/10.1007/s11686-022-00529-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-022-00529-1