Abstract
Studies that investigated neurobiological parameters subtended to impulsivity trait found their relationship with structural and functional brain alterations. No studies investigated the white matter microstructural attributes of impulsivity in a large sample of healthy subjects. In the present study 1007 subjects from Human Connectome Project public dataset were divided in two groups, impulsive and not impulsive, basing on Delay Discounting task score. For both groups brain morphometric and microstructural characteristics were investigated. A t-test (correct for multiple comparisons) was performed for each brain parcel and impulsivity measure. Magnetic resonance diffusion images were pre-processed and selected to perform a voxelwise analysis on the fractional anisotropy (FA) maps between impulsive and not impulsive groups. Group analysis showed significant differences in morphometric brain data mainly for temporal and frontal lobes. The impulsive group presented higher FA values in four regions: bilateral medial lemniscus and midbrain reticular formation, right superior longitudinal fasciculus, left forceps major, right corticospinal tract. Not impulsive group showed higher FA values in two significant regions: right and left anterior thalamus radiation. Concluding, macroscopic and microstructural brain alterations were assessed, identifying new neuroanatomical substrates for multidimensional impulsivity construct in a large sample of healthy subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impulsivity is a multi-dimensional construct referring to several self-regulatory capabilities that can be measured through different ways. Impulsivity main components are: personality trait, that means the ability in controlling prevailing aptitude and is measured by self-report questionnaires; response inhibition, that refers to faculty in inhibiting a dominant response in experimental tasks; decision making also measured by experimental tasks; motor impulsivity, reflecting outsourcing behaviors (Mackillop et al. 2016; Gray et al. 2018).
One of the most widely used indices of decision-making factor of impulsivity is the delay discounting task, a simple but effective paradigm where subject is requested to choose between a greater delayed reward and an immediate lesser reward (Madden and Bickel 2009; Amlung et al. 2017).
Several studies have investigated neuroanatomical pathways subtending impulsivity demonstrating that delay discounting mainly involves prefrontal cortex, primarily ventromedial division, orbitofrontal cortex and striatum (Kable and Glimcher 2007; Bartra et al. 2013; for a review see Peters and Buchel 2011). Differently, the preference for the immediate or delayed gains is more linked to activity in areas afferent to temporal pole and limbic system as insula, cingulate cortex and amygdala.
In healthy subjects, available studies on brain structural aspects of delay discounting have been conducted on relatively small samples, not providing unitary results. Wang et al. (2017) hypothesized correlations between delay discounting and smaller gray matter volume in dorsolateral prefrontal cortex and medial orbitofrontal cortices, parahippocampal and precentral gyri. Similarly, Pehlivanova et al. (2018) found that impulsive choice was associated with reduced cortical thickness in two networks including prefrontal and orbitofrontal cortices, core structures in reward-related decision-making.
Microstructural brain characteristics linked to impulsivity are poorly investigated in healthy subjects. Usually, diffusion tensor imaging (DTI) is suitable for detecting white matter microstructural differences (Basser et al. 1994; Alexander et al. 2007) and specifically the fractional anisotropy (FA) is the index that estimates fibers integrity. Ikuta et al. (2018) used probabilistic tractography to investigate the association between the accumbofrontal tract integrity and impulsive tendencies (measured by the Urgency Premeditation Perseverance and Sensation Seeking impulsive behavior total score) in a sample of healthy subjects. They found that impulsive behavior is predicted by accumbofrontal tract integrity.
In several types of abuse/dependence disorders structural, microstructural, functional and electrophysiological alterations, have been demonstrated. For example, in methamphetamine users Uhlmann et al. (2016) reported that high levels of self-report impulsivity were significantly associated to higher frontal white matter integrity and Andres et al. (2016) observed that white matter alterations in the striatum were positively related to greater impulsivity (measured by the Barratt Impulsiveness Scale); in cocaine abuser negative correlations between impulsivity and corpus callosum WM integrity (Moeller et al. 2005) and with inferior frontal WM (Romero et al. 2010) have been demonstrated. Horn et al. (2003) reported functional activation of paralimbic areas during response inhibition in impulsive subjects. Regarding electrophysiological alteration, a positive relationship between impulsive personality trait and motor system excitability during the preparation of self-initiated movements was found by Rossi et al. (2018).
The association between impulsivity, alcohol dependence and brain alterations has been well investigated. Herting et al. (2010) reported a negative correlation of FA values in the inferior longitudinal fasciculus and the optic radiation with greater impulsivity assessed by a delay discounting task in youth with family history of alcohol abuse, which suggests that WM integrity may act as an intrinsic risk factor for alcohol use disorder (for a review see O'Halloran et al. 2017). Burnette et al. (2019) observed that functional neural activation in frontostriatal regions in response to alcohol cues was related with a measure of impulsivity trait. Increased blood oxygen level dependent signal in the mesolimbic reward system (Vollstädt-Klein et al. 2010), and increased spontaneous brain activity in the beta frequency band (Herrera-Diaz et al. 2016), were also described in alcohol dependent patients. Wang et al. (2016) in alcohol dependent patients have demonstrated both grey matter and white matter alterations in the mesocorticolimbic system also linked with abnormal impulsivity.
In adolescents with internet gaming disorder Du et al. (2017) demonstrated positive correlation between WM integrity and impulsivity, consistently with studies on delay discounting in healthy young subjects (Olson et al. 2009).
To the best of our knowledge, no studies specifically investigated the white matter abnormalities related to high impulsivity, as assessed by an objective test, on a large sample of healthy participants. Therefore, the present study aims to investigate brain structural and microstructural abnormalities between high-scoring and low-scoring impulsivity groups, assessed by a Delay Discounting task examining the interplay between reward processing and temporal processing, in a large cohort of healthy subjects.
Materials and methods
Subjects
The Human Connectome Project (HCP) public is a dataset that includes high-resolution Magnetic Resonance Imaging (MRI) scans from healthy adults. Pre-processed structural and diffusion MRI as well as demographic, clinical and personality data from 1206 participants from the ‘S1200 Subjects release’ were obtained from the HCP public repository (https://www.humanconnectome.org/study/hcp-young-adult/document/1200-subjects-data-release), informed consent was obtained for all participants (consent procedure detailed in Van Essen et al. 2013). All subjects were young and healthy adults (n: 1206; 656 females; mean age: 28.8 ± 3.5; age-range: 22–36). Sample size was filtered for left handedness, completion delay discounting test, and completion full structural and diffusion MRI sessions, determining a sample size of 1007 subjects (553 females, mean age 28.9 ± 3.6; 22–36 age-range). Impulsive and non-impulsive subjects have been defined according to their behavioral measures, as described in statistical analyses section.
MRI scanning and pre-processing
HCP structural MRIs were collected from a 3-Tesla Siemens Skyra unit (housed at Washington University in St. Louis) using an axial 3D-T1-weighted sequence (TR = 2400 ms, TE = 2.14 ms, flip angle = 8°, voxel-size 0.7 × 0.7 × 0.7 mm). Diffusion MRI (dMRI) was performed using a Spin-echo EPI sequence (TR = 5520 ms, TE = 89.5 ms, flip angle = 78°, voxel-size 1.25 × 1.25 × 1.25 mm, b-values = 1000, 2000 and 3000 s/mm2). Structural images were also reviewed for incidental brain abnormalities by a neuroradiologist. All MRI data were pre-processed using the Version 3 of the pre-processing pipelines. These pipelines are freely available from HCP public repository and Connectome Workbench image analysis suites that are discussed in detail in Glasser et al. (2013) and in the HCP Reference Manual, Chapter 4: HCP Processing Pipeline (Jenkinson et al. 2012; Glasser et al. 2013).
Brain morphometry
Brain morphological parameters including surface area and cortical volume were calculated using pre-processed and pre-segmented HCP data. To map all subjects’ brains to a common space, reconstructed surfaces were registered to Desikan-Killiany atlas using a non-linear procedure that optimally aligned sulcal and gyral features across subjects (Fischl et al. 1999).
Brain diffusion
As a marker for white matter (WM) integrity, fractional anisotropy (FA) is a useful quantity to compare across subjects as it is computable voxelwise and is a scalar value that is independent of the local fibre orientation. For each subject, dMRI data were collected with both L/R and R/L phase encodings using the same gradient table, which were then merged into a single copy of dMRI data after the correction of distortions with the HCP pre-processing pipeline (Glasser et al. 2013). Diffusion tensor for the calculation of FA metric has been estimated using iteratively reweighted linear least squares (Veraart et al. 2013) implemented in MRTrix suite, version 3.0. All the brain diffusion data were normalized in MNI space as estimated within the freesurfer pipeline; FA maps with b-value 1000 were chosen as a default measure for every subject.
Behavioral measures
The HCP collects many behavioral measures developed for the NIH Toolbox Assessment of Neurological and Behavioral function and several additional measures to assess domains not covered by the NIH Toolbox. Delay Discounting test was selected, that describes the undervaluing of rewards that are delayed in time. A version of the discounting task that identifies ‘indifference points’ at which a person is equally likely to choose a smaller reward (e.g., $100) sooner versus a larger reward later (e.g., $200 in 3 years) was used. Based on the work of Green and Myerson (Estle et al. 2006; Green et al. 2007), they use an adjusting-amount approach, in which delays are fixed and reward amounts are adjusted on a trial -by-trial basis based on participants’ choices. This approach has been repeatedly validated to provide reliable estimates of delay discounting (Estle et al. 2006). As a summary measure, we use an area-under-the-curve discounting measure (AUC) that provides a valid and reliable index of how steeply an individual discount delayed rewards (Myerson et al. 2001). AUC provides a score based on how quickly a subject switches to immediate smaller rewards as opposed to delayed larger rewards; the lower scores reflect an impulsive choice and the higher scores reflect non-impulsive choice. HCP database provides delay discounting test with two rewards: 40.000$ and 200$.
Statistical analyses
A Shapiro-Wilk test was performed to assess normal distribution of the subjects in the two conditions; Only for 40.000$ reward condition, subjects were normally distributed, and were chosen for the analyses in order to obtain homogeneous groups. In order to determine two groups of subjects, 40.000$ test scores were progressively ordered from lower to higher (0–1), identifying impulsive group in the first quartile (0–0.25; n = 224), and not impulsive group in the third quartile (0.75–1; n = 274).
A Pearson correlation analysis was performed between the task score in all subjects with age, gender, brain surface areas and grey matter volumes. Furthermore, a two tailed two samples t-test, corrected for Bonferroni multiple comparisons (significant p value<0.0004) was performed to compare brain morphological parameters in both groups. HCP database provides brain morphological parameters in pre-segmented parcel derived from freesurfer analysis, so in order to keep an original data from raw HCP database, no further voxel-wise analysis was performed with morphological parameters.
Regarding spatially normalized diffusion data, a voxel-wise analysis, two-sample t-test, corrected for family-wise error rate (FWE) (significant FWE p value <0.05) was performed, with SPM toolbox (https://www.fil.ion.ucl.ac.uk/spm/), on FA maps derived from impulsive vs not impulsive group. A white matter mask containing voxels with WM probability greater than 0.3 was used to exclude voxels belonging to gray matter. The population-based, DTI cerebral WM tract atlas developed in John Hopkins University and distributed with FSL (Wakana et al. 2004) was used to anatomically localize significant clusters along major WM tracts.
Results
Brain morphometry & behavioral measures
In 40.000$ condition, group analysis showed significant lower morphometric brain data in the impulsive compared to the not impulsive group: areas that survived at multiple comparisons test were left lingual, left and right middle temporal, left precentral, left and right superior parietal, right entorhinal, right inferior temporal; significantly decreased volumes were left and right entorhinal, left and right lingual left and right middle temporal, left postcentral, right banks of superior temporal sulcus, right fusiform, right inferior temporal, right lateral orbitofrontal, right parahippocampal, right superior parietal (Table 1). No significant correlation was found between age, gender, area and volumetric data with task scores.
Brain diffusion
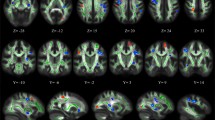
In 40.000$ conditions significant differences were found in FA values voxelwise analysis between impulsive and not impulsive group. Impulsive group presents higher FA values in four significant clusters: brain stem, corresponding to bilateral medial lemniscus and midbrain reticular formation (p = 1.2E-09); right superior longitudinal fasciculus (p = 0,008); left forceps major (p = 0.005); right corticospinal tract (p = 0.024) (Table 2; Fig. 1). On the other hand, not impulsive group has higher FA values in two significant clusters: right and left anterior thalamus radiation (p = 2.5E-07 and 0.005) (Table 2; Fig. 2).
Discussion
The aim of the present study was to investigate morphometric and microstructural characteristics between high- and low-scoring impulsivity in a large sample of healthy subjects. According to the Delay Discounting task response participants were differentiated in impulsive and not impulsive groups. Similarly to previous research, age, gender and neuroanatomical measures produced no differences in impulsivity tendencies (Steward et al. 2017).
Differences in brain areas and volumes between the two samples of subjects were detected. Several previous studies have been conducted for investigating neurofunctional bases of impulsivity, but discordant results were presented. Both reduced gray matter volume (GMV) in prefrontal cortex (Bjork et al. 2009), superior frontal gyrus (Schwartz et al. 2010) and putamen (Dombrovski et al. 2012; Cho et al. 2013) and higher GMV in the ventral striatum, superior frontal gyrus (Schwartz et al. 2010), prefrontal cortex, and its medial portion, and anterior cingulate cortex (Cho et al. 2013; Wang et al. 2017) were reported in highly impulsive subjects. Here, more impulsive preference, as indexed by higher discounting, was associated with diminished brain areas and volumes of several regions. Relative to previous reports on brain structural volumes, the effects observed here were more widespread across the cortex, the use of larger sample of subjects could account for this heterogeneous and larger effect.
Impulsivity and decision making are complex processes involving interaction among multiple subsystems governed by different parameters. In particular, impulsive decision-making suggests not properly considering actions consequences, reflecting a process based on emotional and not on rational evaluation (Moeller et al. 2001). Decision-making has been found impaired in some pathologies as addiction (Hester and Garavan 2004), gambling (Cavedini et al. 2002) and some psychiatric disorders (Moeller et al. 2001; Alicata et al. 2009; Lederer et al. 2016).
We found reduced area and volume in impulsive subjects for the orbitofrontal cortex (OFC), one of the core structures in impulsivity, being site, together with the striatum, of mental representation of the incentive values of different kinds of reward (Kable and Glimcher 2009; Chib et al. 2009; Peters and Buchel 2010). The OFC plays a crucial role in reward and punishment processing (O’Doherty et al. 2001; O’Doherty 2004) and in top-down control (Elliott and Deakin 2005) and its alteration might be a shared neural substrate underlying impulsivity (Blair, 2004). This finding, in line with previous data that reported association between the OFC and delayed reward discounting (Jiang et al. 2015; Bjork et al. 2009; Mohammadi et al. 2015; Wang et al. 2017) confirms the pivotal role in controlling impulsive behavior and in monitoring and updating the representation of the expected rewards. Indeed, OFC reduced volume could be responsible for a less accurate evaluation of reward.
We also found that parahippocampal volume was reduced in impulsive subjects relative to not impulsive subjects. Recent evidences by Gupta et al. (2010) reported impaired decision-making following hippocampal damage and stated that hippocampal-prefrontal cortex interaction results in a prospection network (Peters and Buchel 2010). The diminished volume of both regions in impulsive subjects sustains the idea about a responsibility for impaired evaluation of future-rewards and difficulty in making a more conservative choice. These data support that striatal interactions with the prefrontal cortex and the hippocampus are central to impulsivity. OFC and hippocampus (among ventral striatum posterior cingulate) are part of those areas more activated by choices with immediate rewards according to McClure et al. (2004). The authors hypothesized that short-run impatience is driven by the limbic system which preferentially responds to immediate rewards and is less sensitive to the value of future rewards. Conversely, long-run patience is mediated by the lateral prefrontal cortex and associated structures, able to evaluate trade-offs between rewards, including those in the more distant future. Results of Jiang et al. (2015) indicated that cortical thickness of the OFC, fusiform and parahippocampal gyri was associated with higher level of impulsivity.
The ability to postpone rewards in a more distant future is expression of cognitive control, a process based on goal-directed behavior and supported by brain interactions as fronto-parietal network, related with decision-making processing (Sanfey et al. 2006). Reduced bilateral superior parietal volume evidenced in the present study probably contribute to the difficulty in inhibiting the impulse to choose smaller more immediate rewards instead of a greater later one.
Owens et al. (2017) reported in delayed reward discounting decision making atrophy in precentral and postcentral regions, entorhinal cortex, lingual and fusiform gyri. Jiang et al. (2015) found that callous-unemotional personality trait was inversely related with fusiform and lingual gyri, they found diminished volume of the inferior temporal cortex including the fusiform gyrus as well as the precentral area. Also, Kubera et al. (2018) in a sample of non-demented patients with Parkinson disease reported that cortical thickness in precentral area was significantly associated with the Barratt Impulsiveness Scale, probably due to impaired inhibitory cognitive control that leads to a proactive impulse control. A recent study by Gavazzi et al. (2019) explored with fMRI the “readiness” period (subjects were waiting and preparing a motor response) during a GoNoGo task. They found a positive correlation between motor impulsivity scores and the activation of motor cortices. This result indicates that motor impulsivity trait might be associated with a disinhibition of the motor system in impulsive people.
Aberrant cortical motor regions, like the precentral cortex, may weaken the control-motor circuit, thereby resulting in a poor regulation of impulsive behavior. As for microstructural analysis, impulsive subjects displayed higher FA values in midbrain regions, including ascending reticular formation and bilateral medial lemniscus tracts. Ascending reticular activating system also known as extrathalamic control modulatory system, is crucial to maintain behavioral arousal and consciousness, mediating various levels of alertness (Jones 2008). Although its role has not clearly been identified, midbrain reticular formation dysregulation has been postulated in many neurological and psychiatric diseases characterized by arousal disturbances (GarciaRill 1997). Moreover, compulsive behavior seems linked to excessive stimulation of arousal and reticular formation (Mills 1985; Siegel and Victoroff 2009). The highly significant alteration detected in microstructural properties of midbrain reticular formation can sustain an altered communication within impulsivity network circuitry. Instead, medial lemniscus tract is involved in somatosensory perception and responsible of transferring sensory information of proprioception (Navarro-Orozco and Bollu 2019). These data, together with higher FA values in impulsive subjects also in the corona radiate, part of cortico-spinal tract, and probably with the reduced grey matter in the primary motor and sensory cortices, could reflect the motor hyperactivity manifestation of impulsivity trait. Correlation between impulsivity and FA values of the cortico-spinal tract has also been observed in adolescents with internet gaming disorder, that could reflect potential WM microstructural changes associated to greater impulsivity in this disorder (Du et al. 2017).
In addition to the sensory and motor systems involvement, also visuo-spatial abilities seem to be involved, since higher FA values were found in the superior longitudinal fasciculus and in the left forceps that connects visual occipital areas. By contrast, not impulsive subjects had higher FA values in the anterior thalamic radiation projecting to frontal lobes, that could reflect greater ability to reduce action triggered by impulsive stimuli; this was in agreement with a previous paper on the correlation between accumbo-frontal projections and impulsivity trait (Ikuta et al. 2018). Our data contribute to address the question about if the maturation of prefrontal-striatal white matter connections contributes to predict future oriented choices across development. Investigating white matter, Achterberg et al. (2016) suggested that stronger prefrontal-striatal interaction can mediate and predict the ability in delay gratification, leading to less impulsive choices. Since higher fronto-striatal WM integrity is associated with increased preference for delayed rewards in adults (Peper et al. 2013) and adolescents (van den Bos et al. 2015), our results support the idea that an alteration of this white matter tract integrity could result in the degeneration of ability in preferring delayed rewards.
The present study has several limitations. Firstly, impulsivity is a multi-faced construct and here we investigated only impulsive decision-making measured through the delay discounting task. Therefore, our findings cannot be generalized to encompass all impulsivity components. Nevertheless, neuroimaging studies have investigated the neural bases of the other components, demonstrating neural correlates of motor impulsivity (Hampton et al. 2017), response inhibition (Bari and Robbins 2013) and impulsivity trait (McDonald et al. 2017). Secondly, literature provides a poor characterization of brainstem areas and functions, probably due to the limited MR spatial resolution for these very small and few outlined structures, mainly for functional studies, thus limiting our interpretation results. Furthermore, the present study evaluates specifically microstructural data, not providing functional results. Even if this could be considered a limitation, it should be taken into account that functional analyses are more frequently used, in fact, a lot of studies provide functional data instead of those that investigate microstructural alterations. In order to a better understanding of how neurofunctional mechanisms subtend to impulsivity, it could be useful to focus future studies on the correlation between functional and structural data, together with behavioral information. Among structural analyses, it could be interesting to use the tractography, in order to study potential alterations of connections across the different impulsivity-related disorders.
Concluding, magnetic resonance imaging data support the hypothesis that impulsivity manifestations, namely behavioral, motor and cognitive, result from an alteration of different cortical and brainstem areas. These differences include macroscopic and microstructural brain modifications, identifying new neuroanatomical substrates for multidimensional impulsivity construct in a large sample of healthy subjects.
References
Achterberg, M., Peper, J. S., van Duijvenvoorde, A. C., Mandl, R. C., & Crone, E. A. (2016). Frontostriatal white matter integrity predicts development of delay of gratification: A longitudinal study. Journal of Neuroscience, 10(6), 1954–1961. https://doi.org/10.1523/JNEUROSCI.3459-15.2016.
Alexander, A. L., Lee, J. E., Lazar, M., & Field, A. S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics, 4, 316–329.
Alicata, D., Chang, L., Cloak, C., Abe, K., & Ernst, T. (2009). Higher diffusion in striatum and lower fractional anisotropy in white matter of methamphetamine users. Psychiatry Research, 174, 1–8.
Amlung, M., Vedelago, L., Acker, J., Balodis, I., & Mackillop, J. (2017). Steep delay discounting and addictive behavior: A meta-analysis of continuous associations. Addiction, 112(1), 51–62.
Andres, T., Ernst, T., Oishi, K., Greenstein, D., Nakama, H., & Chang, L. (2016). Brain microstructure and impulsivity differ between current and past methamphetamine users. Journal of Neuroimmune Pharmacology, 11(3), 531–541.
Bari, A., & Robbins, T. W. (2013). Inhibition and impulsivity: Behavioral and neural basis of response control. Progress in Neurobiology, 108, 44–79.
Bartra, O., McGuire, J. T., & Kable, J. W. (2013). The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage, 76, 412–427.
Basser, P. J., Mattiello, J., & LeBihan, D. (1994). MR diffusion tensor spectroscopy and imaging. Biophysical Journal, 66(1), 259–267.
Bjork, J. M., Momenan, R., & Hommer, D. W. (2009). Delay discounting correlates with proportional lateral frontal cortex volumes. Biological Psychiatry, 65, 710–713.
van den Bos, W., Rodriguez, C.A., Schweitzer, J.B., McClure, S.M. (2015), Adolescent impatience decreases with increased frontostriatal connectivity. Proceedings of the National Academy of Sciences USA, 112:E3765–E3774.
Blair RJ. (2004). The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn, 55(1), 198–208.
Burnette, E. M., Grodin, E. N., Lim, A. C., MacKillop, J., Karno, M. P., & Ray, L. A. (2019). Association between impulsivity and neural activation to alcohol cues in heavy drinkers. Psychiatry Research: Neuroimaging, 30(293), 110986. https://doi.org/10.1016/j.pscychresns.2019.110986.
Cavedini P., Riboldi G., Keller R., D'Annucci A., Bellodi L. (2002). Frontal lobe dysfunction in pathological gambling patients. Biol Psychiatry, 51(4), 334–41.
Chib, V. S., Rangel, A., Shimojo, S., & O’Doherty, J. P. (2009). Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. Journal of Neuroscience, 29, 12315–12320.
Cho, S. S., Pellecchia, G., Aminian, K., Ray, N., Segura, B., Obeso, I., & Strafella, A. P. (2013). Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topography, 26, 479–487.
Dombrovski, A. Y., Siegle, G. J., Szanto, K., Clark, L., Reynolds, C. F., & Aizenstein, H. (2012). The temptation of suicide: Striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychological Medicine, 42(6), 1203–1215.
Du, X., Liu, L., Yang, Y., Qi, X., Gao, P., Zhang, Y., Zhu, J., Du, G., Dai, S., Li, X., & Zhang, Q. (2017). Diffusion tensor imaging of the structural integrity of white matter correlates with impulsivity in adolescents with internet gaming disorder. Brain and Behavior, 21(8), e00753. https://doi.org/10.1002/brb3.753.
Elliott, R., & Deakin, B. (2005). Role of the orbitofrontal cortex in reinforcement processing and inhibitory control: Evidence from functional magnetic resonance imaging studies in healthy human subjects. International Review of Neurobiology, 715(65), 89–116.
Estle, S. J., Green, L., Myerson, J., & Holt, D. D. (2006). Differential effects of amount on temporal and probability discounting of gains and losses. Memory & Cognition, 34, 914.
Fischl, B., Sereno, M. I., Tootell, R. B., & Dale, A. M. (1999). High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping, 8, 272–284.
GarciaRill, E. (1997). Disorders of the reticular activating system. Medical Hypotheses., 49(5), 379–387.
Gavazzi, G., Rossi, A., Orsolini, S., Diciotti, S., Giovannelli, F., Salvadori, E., Pantoni, L., Mascalchi, M., & Viggiano, M. P. (2019). Impulsivity trait and proactive cognitive control: An fMRI study. European Journal of Neuroscience, 49(9), 1171–1179. https://doi.org/10.1111/ejn.14301.
Glasser, M. F., Sotiropoulos, S. N., Wilson, J. A., Coalson, T. S., Fischl, B., Andersson, J. L., Xu, J., Jbabdi, S., Webster, M., Polimeni, J. R., Van Essen, D. C., & Jenkinson, M. (2013). The minimal preprocessing pipelines for the human Connectome project. Neuroimage, 80, 105–124.
Gray, J. C., MacKillop, J., Weafer, J., Hernandez, K. M., Gao, J., Palmer, A. A., & de Wit, H. (2018). Genetic analysis of impulsive personality traits: Examination of a priori candidates and genome-wide variation. Psychiatry Research, 259, 398–404.
Green, L., Myerson, J., Shah, A. K., Estle, S. J., & Holt, D. D. (2007). Do adjusting-amount and adjusting-delay procedures produce equivalent estimates of subjective value in pigeons? Journal of the Experimental Analysis of Behavior, 87, 337–347.
Gupta, R., Koscik, T. R., Bechara, A., & Tranel, D. (2010). The amygdala and decision-making. Neuropsychologia, 49(4), 760–766.
Hampton, W. H., Alm, K. H., Venkatraman, V., Nugiel, T., & Olson, I. R. (2017). Dissociable frontostriatal white matter connectivity underlies reward and motor impulsivity. NeuroImage, 1590, 336–343.
Herting, M. M., Schwartz, D., Mitchell, S. H., & Nagel, B. J. (2010). Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcoholism, Clinical and Experimental Research, 34, 1590–1602.
Herrera-Díaz A., Mendoza-Quiñones R., Melie-Garcia L., Martínez-Montes E., Sanabria-Diaz G., Romero-Quintana Y., Salazar-Guerra I., Carballoso-Acosta M., Caballero-Moreno A. (2016). Functional Connectivity and Quantitative EEG in Women with Alcohol Use Disorders: A Resting-State Study. Brain Topogr, 29(3):368–81. https://doi.org/10.1007/s10548-015-0467-x.
Hester R., Garavan H. (2004). Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci, 24(49), 11017–22.
Horn, N. R., Dolan, M., Elliott, R., Deakin, J. F. W., & Woodruff, P. W. R. (2003). Response Inhibition and Impulsivity: An FMRI Study. Neuropsychologia, 41(n.14), 1959–1966. https://doi.org/10.1016/S0028-3932(03)00077-0.
Ikuta, T., del Arco, A., & Karlsgodt, K. H. (2018). White matter integrity in the fronto-striatal accumbofrontal tract predicts impulsivity. Brain Imaging and Behavior, 12(5), 1524–1528. https://doi.org/10.1007/s11682-017-9820-x.
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., & Smith, S. M. (2012). FSL. Neuroimage, 62, 782–790.
Jiang, Y., Guo, X., Zhang, J., Gao, J., Wang, X., Situ, W., Yi, J., Zhang, X., Zhu, X., Yao, S., & Huang, B. (2015). Abnormalities of cortical structures in adolescent-onset conduct disorder. Psychological Medicine, 45(16), 3467–3479.
Jones, B. E. (2008). Modulation of cortical activation and behavioral arousal by cholinergic and orexinergic systems. Annals of the New York Academy of Sciences, 1129(1), 26–34.
Kable, J. W., & Glimcher, P. W. (2007). The neural correlates of subjective value during intertemporal choice. Nature Neuroscience, 10, 1625–1633.
Kable, J. W., & Glimcher, P. W. (2009). The neurobiology of decision: Consensus and controversy. Neuron, 63, 733–745.
Kubera, K. M., Schmitgen, M. M., Nagel, S., Hess, K., Herweh, C., Hirjak, D., Sambataro, F., & Wolf, R. C. (2018). A search for cortical correlates of trait impulsivity in Parkinson’s disease. Behavioral Brain Research, 13(369), 111911.
Lederer, K., Fouche, J. P., Wilson, D., Stein, D. J., & Uhlmann, A. (2016). Frontal white matter changes and aggression in methamphetamine dependence. Metabolic Brain Disease, 31, 53–62.
Mackillop, J., Weafer, J., Gray, J. C., Oshri, A., Palmer, A., & Wit, H. D. (2016). The latent structure of impulsivity: Impulsive choice, impulsive action, and impulsive personality traits. Psychopharmacology, 3361–3370.
Madden, G., & Bickel, W. K. (Eds.). (2009). Impulsivity: The behavioural and neurological science of discounting. Washington, D.C.: American Psychological Association.
McClure, S. M., Laibson, D. I., Loewenstein, G., & Cohen, J. D. (2004). Separate neural systems value immediate and delayed monetary rewards. Science, 15(5695), 503–507.
McDonald, V., Hauner, K. K., Chau, A., Krueger, F., & Grafman, J. (2017). Networks underlying trait impulsivity: Evidence from voxel-based lesion-symptoms mapping. Human Brain Mapping, 38(2), 656–665.
Mills, I. H. (1985). The neuronal basis of compulsive behavior in anorexia nervosa. Journal of Psychiatric Research, 19(2–3), 231–235.
Moeller, F. G., Dougherty, D. M., Barratt, E. S., Schmitz, J. M., Swann, A. C., & Grabowsky, J. (2001). The impact of impulsivity on cocaine use and retention in treatment. Journal of Substance Abuse Treatment, 21(4), 193–198.
Moeller, F. G., Hasan, K. M., Steinberg, J. L., Kramer, L. A., Dougherty, D. M., Santos, R. M., Valdes, I., Swann, A. C., Barratt, E. S., & Narayana, P. A. (2005). Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: Diffusion tensor imaging. Neuropsychopharmacology, 30(3), 610–617.
Mohammadi, B., Hammer, A., Miedl, S. F., Wiswede, D., Marco-Pallares, J., Herrmann, M., & Munte, T. F. (2015). Intertemporal choice behavior is constrained by brain structure in healthy participants and pathological gamblers. Brain Structure and Function, 3157–3170.
Myerson, J., Green, L., & Warusawitharana, M. (2001). Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior, 76(2), 235–243.
Navarro-Orozco, D., & Bollu, P. C. (2019). Neuroanatomy, Medial Lemniscus (Reils band, Reils ribbon). StatPearls. Treasure Island (FL): StatPearls Publishing.
O’Doherty, J. P. (2004). Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Current Opinion in Neurobiology, 14, 769–776.
O’Doherty, J., Kringelbach, M. L., Rolls, E. T., Hornak, J., & Andrews, C. (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience, 4(840), 95–102.
O'Halloran, L., Nymberg, C., Jollans, L., Garavan, H., & Whelan, R. (2017). The potential of neuroimaging for identifying predictors of adolescent alcohol use initiation and misuse. Addiction, 112(4), 719–726. https://doi.org/10.1111/add.13629 Epub 2016 Dec 5.
Olson, E. A., Collins, P. F., Hooper, C. J., Muetzel, R., Lim, K. O., & Luciana, M. (2009). White matter integrity predicts delay discounting behavior in 9-to 23-year- olds: A diffusion tensor imaging study. Journal of Cognitive Neuroscience, 21, 1406–1421.
Owens, M.M., Gray, J.C., Amlung, M.T., Oshri, A., Sweet, L.H., & MacKillop, J. (2017). Neuroanatomical foundations of delayed reward discounting decision making. Neuroimage, 1;161:261-270.
Pehlivanova, M., Wolf, D. H., Sotiras, A., Kaczkurkin, A. N., Moore, T. M., Ciric, R., Cook, P. A., Garcia de La Garza, A., Rosen, A. F. G., Ruparel, K., Sharma, A., Shinohara, R. T., Roalf, D. R., Gur, R. C., Davatzikos, C., Gur, R. E., Kable, J. W., & Satterthwaite, T. D. (2018). Diminished Cortical Thickness Is Associated with Impulsive Choice in Adolescence. Journal of Neuroscience, 7(10), 2471–2481.
Peper, J. S., Mandl, R. C., Braams, B. R., de Water, E., Heijboer, A. C., Koolschijn, P. C., & Crone, E. A. (2013). Delay discounting and frontostriatal fiber tracts: A combined DTI and MTR study on impulsive choices in healthy young adults. Cerebral Cortex, 23, 1695–1702.
Peters, J., & Buchel, C. (2010). Neural representations of subjective reward value. Behavioral Brain Research, 213, 135–141.
Peters, J., & Buchel, C. (2011). The neural mechanism of inter-temporal decision-making: Understanding variability. Trends in Cognitive Sciences, 15, 227–239.
Romero, M. J., Asensio, S., Palau, C., Sanchez, A., & Romero, F. J. (2010). Cocaine addiction: Diffusion tensor imaging study of the inferior frontal and anterior cingulate white matter. Psychiatry Research, 30(1), 57–63. https://doi.org/10.1016/j.pscychresns.2009.07.004.
Rossi, A., F. Giovannelli, G. Gavazzi, S. Righi, M. Cincotta, e M. P. Viggiano. «Electrophysiological activity prior to self-initiated movements is related to impulsive personality traits». Neuroscience 372 (2018): 266–272. https://doi.org/10.1016/j.neuroscience.2018.01.011.
Sanfey, A. G., Loewenstein, G., McClure, S. M., & Cohen, J. D. (2006). Neuroeconomics: Cross-currents in research on decision-making. Trends in Cognitive Sciences, 10(3), 108–116.
Schwartz, D. L., Mitchell, A. D., Lahna, D. L., Luber, H. S., Huckans, M. S., Mitchell, S. H., & Hoffman, W. F. (2010). Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage, 50, 1392–1401.
Siegel, A., & Victoroff, J. (2009). Understanding human aggression: New insights from neuroscience. International Journal of Law Psychiatry., 32(4), 209–215.
Steward, T., Mestre-Bach, G., Fernàndez-Aranda, F., Granero, R., Perales, J. C., Navas, J. F., Soriano-Mas, C., Bano, M., Fernàndez-Formoso, J. A., Martìn-Romera, V., Mnechòn, J. M., & Jiménez-Murcia, S. (2017). Delay discounting and impulsivity traits in young and older gambling disorder patients. Addcitive Behaviors, 71, 96–103.
Uhlmann, A., Fouche, J. P., Lederer, K., Meintjes, E. M., Wilson, D., & Stein, D. J. (2016). White matter microstructure and impulsivity in methamphetamine dependence with and without a history of psychosis. Human Brain Mapping, 37(6), 2055–2067.
Van Essen, D. C., Smith, S., Barch, D., Behrens, T. E. J., Yacoub, E., & Ugurbil, K. (2013). The WU-Minn human Connectome project: An overview. Special issue Mapping the Connectome: NeuroImage.
Veraart, J., Sijbers, J., Sunaert, S., Leemans, A., & Jeurissen, B. (2013). Weighted linear least squares estimation of diffusion MRI parameters: Strengths, limitations, and pitfalls. NeuroImage, 81, 335–346.
Vollstädt-Klein S1, Hermann D, Rabinstein J, Wichert S, Klein O, Ende G, Mann K. (2010). Increased activation of the ACC during a spatial working memory task in alcohol-dependence versus heavy social drinking. Alcohol Clin Exp Res, 34(5), 771–6. https://doi.org/10.1111/j.1530-0277.2010.01149.x.
Wakana, S., Jiang, H., Nagae-Poetscher, L. M., van Zijl, P. C., & Mori, S. (2004). Fiber tract-based atlas of human white matter anatomy. Radiology, 230, 77–87.
Wang, J., Fan, Y., Dong, Y., Ma, M., Ma, Y., Dong, Y., Niu, Y., Jiang, Y., Wang, H., Wang, Z., Wu, L., Sun, H., & Cui, C. (2016). Alterations in brain structure and functional connectivity in alcohol dependent patients and possible association with impulsivity. PLoS One, 30(8), e0161956. https://doi.org/10.1371/journal.pone.0161956.
Wang, S., Kong, F., Zhou, M., Chen, T., Yang, X., Chen, G., & Gong, Q. (2017). Brain structure linking delay discounting and academic performance. Human Brain Mapping, 38, 3917–3926.
Acknowledgments
These data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and KamilUgurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University in St. Louis. The authors are deeply appreciative to the Human Connectome Project for open access to its data.
This work was supported by RC projects of the Italian MOH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they had no financial or non-financial conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alfano, V., Longarzo, M., Aiello, M. et al. Cerebral microstructural abnormalities in impulsivity: a magnetic resonance study. Brain Imaging and Behavior 15, 346–354 (2021). https://doi.org/10.1007/s11682-020-00261-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-020-00261-2