Abstract
Functional connectivity (FC) is fundamental to brain function and has been implicated in many neuropsychological and neuropsychiatric disorders. It is then of great scientific and clinical interest to find a non-invasive approach to modulate FC. Transcranial magnetic stimulation (TMS) is a non-invasive neuromodulational tool that can affect the target region and remote brain areas. While the distributed effects of TMS are postulated to be through either structural or functional connectivity, an understudied but of great scientific interest question is whether TMS can change the FC between these regions. The purpose of this study was to address this question in normal healthy brain using TMS with continuous theta burst stimulation (cTBS) pulses, which are known to have long-lasting inhibition function. FC was calculated from resting state fMRI before and after real and control (SHAM) stimulation. Compared to SHAM, the repetitive TMS (rTMS) reduces FC between the cTBS target: the left dorsolateral prefrontal cortex (lDLPFC) and brain regions within the default mode network (DMN), proving the effects of rTMS on FC. The reduction of FC might be the results of the inhibitory effects of cTBS rTMS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional connectivity (FC) refers to the temporal coherence of brain activity from spatially distributed brain regions (Friston 1994) and is now widely measured through functional magnetic resonance imaging (fMRI). As human brain is a highly integrated complex network, FC remains fundamental to brain functions and their alterations in brain disorders. Since the seminal work by Biswal and colleagues (Biswal et al. 1995, 1997), consistent FC patterns have been identified using fMRI in normal brain involving a few brain networks including the default mode network, the primary visual network, auditory network, and higher order cognitive networks (Biswal et al. 1997; Cordes et al. 2000, 2002; Damoiseaux et al. 2006; De Luca et al. 2005; Fox and Raichle 2007; Greicius et al. 2003; Lowe et al. 1998, 2000; van den Heuvel et al. 2008; Xiong et al. 1999). Meanwhile, numerous studies have shown alterations of FC in many neurological and psychiatric disorders (Greicius 2008; Zhang and Raichle 2010), raising a question of great scientific and clinical interest for whether FC can be non-invasively modulated.
One highly plausible approach to modulate brain FC is Transcranial Magnetic Stimulation (TMS), which has been increasingly used for modulating brain function in normal healthy state and diseased conditions especially depression (Barker et al. 1985; Verdon et al. 2004; Bolognini and Ro 2010; Fregni and Pascual-Leone 2007; Rossini and Rossi 2007). Through the varying magnetic field created with a rapidly changing electrical field, TMS induces weak electrical current in superficial cortical area underneath the coil, which subsequently interacts with the innate neuronal electrical activity and subsequently affects brain functions. Although TMS with single pulses may only have transient effects on cortical areas near the coil (Wagner et al. 2007; Kobayashi and Pascual-Leone 2003), repetitive TMS (rTMS) has been shown to have more sustained excitatory or inhibitory effects than single pulse or double pulse TMS (Maeda et al. 2000). rTMS can affect brain regions deeper than the stimulated site (Valero-Cabré et al. 2007; Valero-Cabré et al. 2005; Ruff et al. 2009; Siebner et al. 2009), which may be the main reason for its therapeutic effects on various complex psychiatric and neurological diseases, such as major depressive disorder, schizophrenia, and Parkinson’s disease (Fitzgerald et al. 2006; Wassermann and Lisanby 2001; Fitzgerald 2011). These remote effects are believed to be through the FC or network-wise interactions (Fox et al. 2012b), but it still remains understudied for whether rTMS can change FC rather than spreading its effects via FC.
The purpose of this study was to test the hypothesis that cortical rTMS can change FC from the target site to distributed brain regions. Resting-state fMRI (rs-fMRI) data were acquired from normal young subjects before and after receiving rTMS or control stimulation (SHAM). rTMS was applied to left dorsolateral prefrontal cortex (DLPFC) because of its critical role in various high level cognition including working memory and executive function (Curtis and D’Esposito 2003), attention (Fan et al. 2005), and decision making (Heekeren et al. 2006). The continuous theta burst TMS protocol (cTBS) (Huang et al. 2005; Oberman et al. 2011) was adopted to ensure that the effects can sustain long enough to be able to be detected by rs-fMRI after post-stimulation.
Methods
Participants
The study was approved by local internal review board in Hangzhou Normal University and adhered to the Declaration of Helsinki. Thirty-six healthy young adults (mean age: 22.86 ± 3.27 years, 15 males/21 females) gave written informed consent before participating in this study and were randomly divided into two groups: cTBS group (n = 18, mean age 22.67 ± 3.25 years, 9 males) and SHAM group (n = 18, mean age 23.06 ± 3.37 years, 6 males). Each subject completed a standard MRI safety screening form and a TMS safety screening questionnaire (Rossi et al. 2009). All subjects were right-handed and were naïve to TMS and had no history of psychiatric illness or neurological disorders. Subjects were instructed to refrain from caffeine, alcohol, nicotine or any kind of drugs for at least 24 h before the start of the experiment.
Experimental design
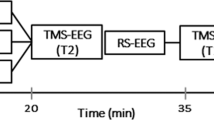
This was a randomized, single-blind, sham-controlled crossover study. Each subject received two sessions of Magnetic Resonance Imaging (MRI) scans which were performed on two separate days with 48 h apart using the same imaging protocol. Subjects received cTBS or SHAM stimulation in one session and no stimulation in the other one. The order of intervention was randomized and counterbalanced across subjects (Fig. 1). First, subjects were received a T1-weighted anatomical MRI scan. After that, the subjects went to a TMS room on the second floor to locate the target and receive stimulation. After the stimulation, the subjects immediately returned to the MRI control room. Resting-state fMRI and ASL scans were performed right after TBS (post-cTBS) or SHAM stimulation (post-SHAM) (within 15 min due to the pre-scan preparations) to capture the rTMS effects before they decline (Siebner et al. 2009). The 48 h interval between the two sessions was chosen to avoid any residual cTBS or SHAM effects from the preceding session if the stimulations were applied therein.
Experiment design. Each subject received two sessions of MRI scans which were performed on two separate days with 48 h apart using the same imaging protocol. In one session, the subject received cTBS or SHAM stimulation and no stimulation in the other session. The order of intervention was randomized and counterbalanced across subjects
rTMS
During cTBS or SHAM procedure, subjects were instructed to lie down in a chair with a headrest to support their heads. The cTBS or SHAM experiments were administrated using a Magstim TMS machine with a Figure-of-eight coil (Magstim Inc., Sheffield). The stimulation location was targeted via MRI using the BrainSight navigation system (Rogue Research Inc) which can dynamically visualize the TMS coil position on top of the individual subjects’ structural MRI. The coordinate of the target site in left DLPFC was set to be [−40, 26, 37] in the Montreal Neurological Institute (MNI) standard brain space. The coordinate was averaged on the various left DLPFC rTMS seed locations reported in the literature (Smittenaar et al. 2013; Mylius et al. 2013; Fox et al. 2012a, b; Feredoes et al. 2011). For cTBS, the coil was positioned tangentially to the skull surface above the left DLFPC site with the handle pointed backwards at a 45° angle. The left DLPFC was stimulated at an intensity of 80% of the individual resting motor threshold (RMT) applying a cTBS protocol with 3 pulses of stimulation given at 50 Hz, repeated every 200 ms. A train of 600 pulses was administrated which equals 40s of stimulation. RMT intensity was determined for each subject by stimulating the left primary motor cortex (M1) with single pulses and detecting corresponding muscle twitching of the relaxed contralateral first dorsal interosseous (FDI) muscle. The use of a subthreshold intensity avoided muscle twitches during cTBS. For SHAM cTBS, parameters were set to be the same as the cTBS experiments except that the coil was placed at an angle of 90 degrees to the skull. The target area was located at the same place for individual subjects in both cTBS and SHAM experiments, with the stimulation triggered on the platform of Magstim Rapid stimulator (Magstim Inc. Sheffield, UK).
MRI scans
MRI data were acquired twice from each subject using the same scanning protocols on a 3 T whole-body GE MR 750 Scanner (GE, Waukesha, USA) with a standard 8 channel receiver coil. During the scan, a comfortable and tight cushion was placed to immobilize the head and reduce motion. The participants were instructed to relax and remain still with their eyes close, not to fall asleep, and not to think about anything in particular. All subjects were monitored through the video camera in the scanner room and nobody was found to fall asleep during the scan, which was also confirmed by interview after the scan.
High-resolution T1-weighted anatomical MRI was acquired with a 3D spoiled gradient echo sequence (3D SPGR) with repetition time/echo time (TR/TE) = 8.1/3.39 ms, flip angle = 7°, field of view = 256 × 256 mm2, matrix = 256 × 256, 1.0mm3 isotropic voxels, 176 slices without interslice gap.
rs-fMRI was acquired with a T2*-weighted gradient-echo EPI pulse sequence with the following parameters: TR/TE = 2000/30 ms, flip angle = 90°, field of view = 220 × 220 mm2, matrix = 64 × 64, 3.4 mm3 isotropic spatial resolution, and 37 interleaved slices. 180 images were acquired in 6 mins.
To quantify regional brain function changes in response to rTMS, cerebral blood flow (CBF) was measured using a GE product pseudo continuous arterial spin labeling (pCASL) perfusion MRI sequence. Three interleaved images with and without labeling were obtained using a 3D fast-spin echo encoded spiral readout imaging sequence with 6 shots, 40 axial slices, TR/TE = 4690/10.9 ms, field of view =220 × 220 mm2, slice thickness = 3.4 mm3, labeling time = 1500 ms, post label delay = 1525 ms, bandwidth = 62.50Khz, matrix = 512 × 512.
Data analysis
Rs-fMRI data preprocessing
The rs-fMRI image preprocessing was done using SPM12 (Welcome Trust Centre for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm12). For each subject, the first 6 rs-fMRI volumes were discarded to allow the mean magnetization to reach steady state and the participants to get familiar to the MR scan environment. The remaining rs-fMRI data were then corrected for the acquisition time difference between the 2D image slices, and were realigned to the first volume to correct head-motions. All participants included in the study showed a maximum displacement of less than 2.5 mm at each axis and rotational motion of less than 2.5o. Next, the images were normalized to the MNI space using the EPI template. They were then smoothed with a Gaussian kernel with a full-width-at-half-maximum (FWHM) of 8 mm. Several nuisance signals (Friston 24 head motion parameters (Friston et al. 1996), mean rs-fMRI time series of white matter and cerebrospinal fluid) were regressed out of the rs-fMRI image series at each voxel. Finally, band pass filtering was performed (band pass: 0.01~0.1 Hz).
FC analysis
Seed-based FC analysis (Biswal et al. 1995) was performed to assess FC between the stimulated site and the rest of the brain. FC was calculated as the Pearson correlation coefficient. A sphere with a radius of 6 mm centered at the target site in left DLPFC was used as the seed. In order to improve the normality before performing group level analysis, the correlation coefficient map was converted to a z map using Fisher Z-transform. This work was done using Data Processing Assistant for Resting-State fMRI (Yan and Zang 2010) (DPARSF, www.restfmri.net).
CBF data analysis
ASL MRI images were processed using the SPM12 based batch scripts provided in ASLtbx (Wang et al. 2008). First of all, the origins of structural image, M0 map, and ASL images were set to be the center of the image matrix; Secondly, ASL CBF maps were calculated from the mean control-label difference map and M0 image and registered to the structural MR image for each subject; Next, CBF images were spatially normalized into the MNI space via each subject’s T1 weighted anatomical image; At last, CBF images were smoothed with a Gaussian kernel with a FWHM = 8 mm.
Resting state fMRI-based brain entropy mapping
Brain entropy (BEN) mapping provides a way to characterize the regional dynamic brain activity, which has been shown to be different from that captured by baseline CBF (Donghui Song et al. 2018). Recently, we have reported BEN reductions after applying short time excitatory high-frequency rTMS in left DLPFC in healthy human subjects (Song et al. 2018). To verify whether the inhibitory cTBS would increase BEN or change BEN at all, we calculated BEN using BENtbx (Wang et al. 2014a) from the pre-processed rsfMRI data. The details of BEN calculation can be found in (Song et al. 2018; Wang et al. 2014a, b).
The additional FC analysis based on CBF results
Additional FC analysis was performed using the suprathreshold cluster identified in the above CBF analysis as the seed. This analysis was to examine the FC between the seed region where rTMS had significant local functional effects to the rest of the brain. In other words, the analysis was to test whether regional rTMS effects are accompanied by a change to the FC from or to that region.
Statistic analysis
Group level statistical analyses were performed with SPM12. Two side paired-t tests were conducted to assess the FC difference, CBF difference, BEN changes caused by cTBS or SHAM separately (post-stimulation minus pre-stimulation). Two side two-sample t-tests were performed to assess cTBS vs SHAM induced changes to the FC, CBF, and BEN (post-pre stimulation difference).
Results
No significant abnormalities were observed in all subject’s MR images. Thus, all subjects were included in data analysis.
Figure 2 shows the FC analysis results when the seed was set to the left DLPFC (i.e. cTBS target site). No significant difference of the baseline FC was found between the cTBS group and SHAM group, so we only displayed the group level baseline FC patterns of the cTBS group in Fig. 2a, which was the one-sample t-test results of the FC of the pre-cTBS stimulation. The thresholded group level DLPFC-FC presented typical patterns frequently reported in the literature (Damoiseaux et al. 2006). Figure 2b, c shows the statistically thresholded pre- and post- modulation FC difference of the cTBS group and the SHAM group, respectively. Specifically, Fig. 2b shows that cTBS induced significant reduction of left DLPFC-FC in right parahippocampeal, left lingual and PCC/precuneus. No significant left DLPFC-FC change was observed after received SHAM stimulation (Fig. 2c). Figure 2d displays the time versus modulation (time means pre- or post- stimulation; modulation means SHAM or cTBS) interactions of left DLPFC-FC (identified by a two-sample t-test on the two groups’ post-pre FC differences). cTBS reduced left DLPFC-FC in PCC/precuneus as compared to SHAM. No FC increase was observed.
Left DLPFC FC comparison results. a the baseline left DLPFC-FC of the cTBS group (before applying cTBS), b left DLPFC-FC difference between post and pre-cTBS stimulation; c no significant left DLPFC-FC difference identified between post and pre-SHAM stimulation; d significant time vs stimulation type (time means pre or post, stimulation type means SHAM or cTBS) interaction effects on left DLPFC-FC: the statistical difference of the post-pre stimulation FC change between the cTBS group (b) and the SHAM group (c). Statistical significance level was defined by p < 0.005, cluster size>134 (corrected for multiple comparison using AlphaSim, alpha <0.005). Color bar indicates the display window for the t-values within the suprathreshold clusters. The cold color represents FC reduction. (L = left hemisphere, R = right hemisphere)
Figure 3 shows cTBS-induced CBF changes. Significant CBF increase was found on left parahippocampal, left hippocampus, left amygdala, left inferior temporal cortex, left inferior parietal cortex and left precuneus after cTBS (Fig. 3a, Table 1). By contrast, SHAM stimulation didn’t produce any significant CBF changes (Fig. 3b). After statistically controlling the placebo effects (due to SHAM) in the cTBS vs SHAM post-pre CBF difference two-sample t-test, no significant stimulation induced CBF change difference was observed (Fig. 3c).
cTBS induced change of CBF. a the post minus pre-cTBS stimulation CBF difference; b the post minus pre-SHAM stimulation CBF difference; c two sample t-test results of the cTBS group (a) and the SHAM group (b) (time vs stimulation interaction). Statistical significance level was defined by p < 0.005, cluster size>120 (corrected for multiple comparison using AlphaSim, alpha <0.005). Color bar indicates the display window for the t-value of the suprathreshold voxels. The warm color represents CBF increase. (L = left hemisphere, R = right hemisphere)
cTBS didn’t cause any significant BEN alterations. No significant BEN changes were found in both cTBS and the SHAM groups.
Figure 4 shows the additional FC analysis results when the seed was set to be left hippocampus cluster as revealed by the CBF analysis. Figure 4a shows that cTBS induced significant reduction of left hippocampus-FC in left pre/postcentral, bilateral inferior parietal cortex, bilateral superior parietal cortex and left precuneus after cTBS. No significant FC change was observed after received SHAM stimulation (Fig. 4b). After statistically controlling the SHAM effects, Fig. 4c demonstrated that part of the hippocampus-FC effects still exist in left postcentral, left superior parietal cortex, left inferior parietal cortex and left precuneus (Fig. 4c).
Left hippocampus FC comparison results. a FC difference between post and pre- cTBS stimulation; b post and pre-SHAM stimulation FC difference; c difference of the post-pre stimulation FC change between the cTBS group (a) and the SHAM group (b) (time vs stimulation). Statistical significance level was defined by p < 0.005, cluster size>191 (corrected for multiple comparison using AlphaSim, alpha <0.005). Color bar indicates the display window for the t-values of the suprathreshold voxels. The cold color represents FC reduction. (L = left hemisphere, R = right hemisphere)
Discussion
In the present study, we assessed effects of left DLPFC cTBS rTMS on inter-regional FC during the resting state. Two seed regions were examined: one was the TMS target, the left DLPFC; the other was hippocampus, a key region involved in resting state brain activity (Chan et al. 2017). Regarding the left DLPFC, significant FC decrease was found in right parahippocampal, left lingual and PCC/precuneus after cTBS rTMS; no significant changes were demonstrated in the SHAM group. After statistically subtracting out the SHAM-induced FC change from the cTBS-induced FC change, significant reduction of left DLPFC-FC was demonstrated in PCC/precuneus. Using the left hippocampus as the seed, we found significant FC reduction in left pre/postcentral, bilateral inferior parietal cortex, bilateral superior parietal cortex and left precuneus after cTBS rTMS. No significant FC change was observed after SHAM stimulation. The left hippocampus-FC reduction patterns in left post central, left superior parietal cortex, left inferior parietal cortex and left precuneus sustained even after statistically controlling the placebo effects (equivalent to the stimulation vs time interaction analysis in an Analysis of Variance model).
These data proved truth of our hypothesis of that rTMS can affect inter-regional FC. We found a reduction of FC between the target site and precuneus, consistent with the inhibitory effects of cTBS (Huang et al. 2005; Oberman et al. 2011). Interestingly, the FC reduction after cTBS was not accompanied by a change to the regional brain activity in both left DLPFC and precuneus measured by CBF and BEN. No regional change on the TMS target site might be caused by the large cross-subject variability as we demonstrated in studies based on two different brain activity measures (Gratton et al. 2014; Shang et al. 2018; Xue et al. 2017). Most likely the cross-subject variability was not coherently spread through the known DLPFC vs precuneus connectivity, leading to a reduced FC. Chen et al. (Chen et al. 2013) showed that the 1 Hz rTMS applied to the right posterior middle frontal gyrus (close to the contralateral site of the left DLPFC used in our study) shifted the DMN signal from low-frequency dominated to high-frequency dominated. Since having more higher frequency components often indicates reduced Pearson correlations to the same reference, their findings could be interpreted as a reduction of DMN vs the target middle frontal FC if the signal in the target region didn’t have a frequency shift after rTMS, but that was not reported. Nevertheless, the rTMS-induced change to the left DLFPC vs precuneus FC in the absence of significant focal changes to the connected areas suggests that rTMS can change the inter-regional coherence without having significant regional brain activity alterations and that inter-regional communication change might represent one of the underlying mechanisms for the remote effects of rTMS.
cTBS increased hippocampal CBF in the absence of FC changes between left DLPFC FC and hippocampus. Although the effects disappeared after controlling the placebo effects, the trend of CBF change in the absence of FC change provides additional evidence to the decoupling between the change of regional activity change and that of inter-regional FC as discussed above. Hippocampus has been shown to be pivotal to resting state brain activity in a recent optogenetic study (Chan et al. 2017), where the authors showed that the low-frequency hippocampal excitatory neural activity can propagate to the whole-brain and modulate the brain-wide FC. The trend of increased hippocampal CBF after DLPFC cTBS rTMS might reflect a mechanism to temporarily compensate the “lesion”-like effects of cTBS as suggested in previous low-frequency rTMS + CBF studies (Orosz et al. 2012).
Decreased hippocampus FC was found in parietal cortex, which might be partly contributed by the hippocampal CBF increase but without CBF change in parietal cortex since change to regional brain activity (here measured with CBF) on one area but not the other inevitably affects FC between the two regions. In concert with the reduced left DLPFC FC in precuneus, the reduced parietal-hippocampal FC suggest a reduced FC as a consequence of the inhibitory effects of cTBS rTMS. Reduced parietal-hippocampus FC by cTBS rTMS was consistent with the increased parietal hippocampus FC after receiving excitatory high frequency rTMS on parietal cortex (Wang et al. 2014a, b), but it is opposite to the findings in (Eldaief et al. 2011), where the authors found increased hippocampal FC to the left posterior inferior parietal lobule (lpIPL). The discrepancy may reflect the effects of different target site: we applied rTMS on left DLPFC but they applied to lpIPL, so while our results suggest that cTBS on left DLPFC reduced the within network FC as a consequence of its inhibition effects, the low-frequency inhibitory rTMS on lpIPL inhibits brain activity within the same network through an increase within network FC. Actually in the same study (Eldaief et al. 2011), the authors also reported the excitatory lpIPL high frequency rTMS increased FC from the lpIPL to posterior cingulate and precuneus, which matches the reduced left DLPFC FC to precuneus after receiving cTBS rTMS in this study if the aforementioned opposite effects of applying inhibitory rTMS to left DLPFC and lpIPL stand. Nevertheless, data from the same cohort with the same inhibitory rTMS applied to the two sites will be essential to verify that. We noticed that one study published by Gratton et al. (Gratton 2013) showed an overall increased FC after applying cTBS rTMS to multiple regions separately, which seems to be contradictory to the inhibitory effects of cTBS. As no control stimulation was performed, it would need further experiments with SHAM stimulation to fully delineate the findings.
One common feature of these FC reductions is that the observed FC changes were all within the default mode network (DMN). Since the target site, the left DLPFC, is also part of DMN (Greicius et al. 2003; Raichle et al. 2001), the within-DMN FC changes suggest that the short-term rTMS effects are constrained by the brain network in which the target site locates. This postulation has been implicated in our recent high-frequency rTMS-fMRI studies (Shang et al. 2018; Song et al. 2018; Xue et al. 2017) where the left DLPFC high-frequency rTMS-induced regional or inter-regional effects were found to be within the DMN. It is worth to note that we didn’t test other TMS targets from outside of DMN, so whether short-term rTMS targeting other brain network spots would still promote predominately a within-network effect remains unknown. Regardless of the apparent network-wise constraint, another reason for short-term rTMS only affecting DMN might be related to the role of DMN, which is the most prominent network presenting coherent activity during the resting state (Greicius et al. 2003; Raichle et al. 2001). It is possible that any short-term neuromodulations on resting state brain activity as performed in this study would affect DMN the most though the effects on other networks could simply disappeared at the time of data acquisition. Nevertheless, the apparent network constraint suggests that rTMS target should be chosen to be within the same brain network as the treatment target area to have desirable modulation effects.
Several limitations exist in this study. One was the lack of behavior measures, making it impossible to relate the imaging findings to behavioral consequences though short-term TMS may not be able to produce effects strong enough to be observable at the behavioral level as reported in (Orosz et al. 2012). Another is the across-subject design, which may contribute large inter-subject variations. The long interval between stimulation and scan represents a third limitation, which may partly account for the lack of focal resting brain activity changes in the target site. Future study with interleaved rTMS and fMRI acquisition should enhance the capability for a more comprehensive rTMS neuromechanism investigation. In addition, by turning the coil orthogonal to the scalp may not be sufficient to achieve a full control condition. Fully solving this problem needs a control coil to perform the SHAM stimulation.
Conclusions
In conclusion, we showed that cTBS rTMS over the left DLPFC changed resting state FC from the target site to remote brain regions in the default mode network, as well as the hippocampal-cortical FC in the absence of regional resting state activity change in those areas. Changes of FC to DMN might represent one mechanism of the remote effects of cortical rTMS.
References
Barker, A. T., Jalinous, R., & Freeston, I. L. (1985). Non-invasive magnetic stimulation of human motor cortex. The Lancet, 325(8437), 1106–1107.
Biswal, B. B., Van Kylen, J., & Hyde, J. S. (1997). Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR in Biomedicine, 10(4–5), 165–170.
Biswal, B., Yetkin, F. Z., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541.
Bolognini, N., & Ro, T. (2010). Transcranial magnetic stimulation: Disrupting neural activity to Alter and Assess brain function. Journal of Neuroscience, 30(29), 9647–9650.
Chan, R. W., Leong, A. T. L., Ho, L. C., Gao, P. P., Wong, E. C., Dong, C. M., et al. (2017). Low-frequency hippocampal-cortical activity drives brain-wide resting-state functional MRI connectivity. Proceedings of the National Academy of Sciences, 201703309.
Chen, A. C., Oathes, D. J., Chang, C., Bradley, T., Zhou, Z.-W., Williams, L. M., Glover, G. H., Deisseroth, K., & Etkin, A. (2013). Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proceedings of the National Academy of Sciences, 110(49), 19944–19949.
Cordes, D., Haughton, V., Carew, J. D., Arfanakis, K., & Maravilla, K. (2002). Hierarchical clustering to measure connectivity in fMRI resting-state data. Magnetic Resonance Imaging, 20(4), 305–317.
Cordes, D., Haughton, V. M., Arfanakis, K., Wendt, G. J., Turski, P. A., Moritz, C. H., et al. (2000). Mapping functionally related regions of brain with functional connectivity MR imaging. American Journal of Neuroradiology, 21(9), 1636–1644.
Curtis, C. E., & D’Esposito, M. (2003). Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences, 7(9), 415–423.
Damoiseaux, J. S., Rombouts, S. A. R. B., Barkhof, F., Scheltens, P., Stam, C. J., Smith, S. M., & Beckmann, C. F. (2006). Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences, 103(37), 13848–13853.
De Luca, M., Smith, S., De Stefano, N., Federico, A., & Matthews, P. M. (2005). Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Experimental Brain Research, 167(4), 587–594.
Donghui Song, D. C., Zhang, J., Ge, Q., Zang, Y.-F., & Ze, W. (2018). Associations of brain entropy (BEN) to cerebral blood flow and fractional amplitude of low-frequency fluctuations in the resting brain. Brain imaging and behavior.
Eldaief, M. C., Halko, M. A., Buckner, R. L., & Pascual-Leone, A. (2011). Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proceedings of the National Academy of Sciences, 108(52), 21229–21234.
Fan, J., McCandliss, B. D., Fossella, J., Flombaum, J. I., & Posner, M. I. (2005). The activation of attentional networks. NeuroImage, 26(2), 471–479.
Feredoes, E., Heinen, K., Weiskopf, N., Ruff, C., & Driver, J. (2011). Causal evidence for frontal involvement in memory target maintenance by posterior brain areas during distracter interference of visual working memory. Proceedings of the National Academy of Sciences, 108(42), 17510–17515.
Fitzgerald. (2011). The effects of repetitive transcranial magnetic stimulation in the treatment of depression. Expert Review of Medical Devices, 8(1), 85–95.
Fitzgerald, P. B., Fountain, S., & Daskalakis, Z. J. (2006). A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clinical Neurophysiology, 117(12), 2584–2596.
Fox, M. D., Buckner, R. L., White, M. P., Greicius, M. D., & Pascual-Leone, A. (2012a). Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biological Psychiatry, 72(7), 595–603.
Fox, M. D., & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8(9), 700–711.
Fox, M., Halko, M., Eldaief, M., & Pascual-Leone, A. (2012b). Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation. Neuroimage, 62(4), 2232–2243.
Fregni, F., & Pascual-Leone, A. (2007). Technology insight: Noninvasive brain stimulation in neurology - perspectives on the therapeutic potential of rTMS and tDCS. Nature Clinical Practice Neurology, 3(7), 383–393.
Friston, K. J. (1994). Functional and effective connectivity in neuroimaging:A synthesis. Human Brain Mapping, 2(2), 56–78.
Friston, K. J., Williams, S., Howard, R., & Frackowiak, R. S. J. (1996). Movement-Related Effects in fMRI Time-Series.
Gratton, T. G. L. (2013). E. M. N. and M. D. Modelling Complex Networks: Cameo Graphs And Transport Processes, 7(Dec), 1–14.
Gratton, C., Lee, T. G., Nomura, E. M., & D’Esposito, M. (2014). Perfusion MRI indexes variability in the functional brain effects of theta-burst transcranial magnetic stimulation. PLoS One, 9(7).
Greicius, M. D. (2008). Resting-state functional connectivity in neuropsychiatric disorders. Current Opinion in Neurology, 21(4), 424–430.
Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences, 100(1), 253–258.
Heekeren, H. R., Marrett, S., Ruff, D. A., Bandettini, P. A., & Ungerleider, L. G. (2006). Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proceedings of the National Academy of Sciences, 103(26), 10023–10028.
Huang, Y. Z., Edwards, M. J., Rounis, E., Bhatia, K. P., & Rothwell, J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron, 45(2), 201–206.
Kobayashi, M., & Pascual-Leone, A. (2003). Transcranial magnetic stimulation in neurology. The Lancet Neurology, 2(3), 145–156.
Lowe, M. J., Dzemidzic, M., Lurito, J. T., Mathews, V. P., & Phillips, M. D. (2000). Correlations in low-frequency BOLD fluctuations reflect cortico-cortical connections. NeuroImage, 12(5), 582–587.
Lowe, M. J., Mock, B. J., & Sorenson, J. A. (1998). Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage, 7(2), 119–132.
Maeda, F., Keenan, J. P., Tormos, J. M., Topka, H., & Pascual-Leone, A. (2000). Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clinical Neurophysiology, 111, 800–805.
Mylius, V., Ayache, S. S., Ahdab, R., Farhat, W. H., Zouari, H. G., Belke, M., Brugières, P., Wehrmann, E., Krakow, K., Timmesfeld, N., Schmidt, S., Oertel, W. H., Knake, S., & Lefaucheur, J. P. (2013). Definition of DLPFC and M1 according to anatomical landmarks for navigated brain stimulation: Inter-rater reliability, accuracy, and influence of gender and age. NeuroImage, 78, 224–232.
Oberman, L., Edwards, D., Eldaief, M., & Pascual-Leone, A. (2011). Safety of theta burst transcranial magnetic stimulation: A systematic review of the literature. Journal of Clinical Neurophysiology, 28(1), 67–74.
Orosz, A., Jann, K., Wirth, M., Wiest, R., Dierks, T., & Federspiel, A. (2012). Theta burst TMS increases cerebral blood flow in the primary motor cortex during motor performance as assessed by arterial spin labeling (ASL). NeuroImage, 61(3), 599–605.
Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98(2), 676–682.
Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A., Avanzini, G., Bestmann, S., et al. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–2039.
Rossini, P. M., & Rossi, S. (2007). Transcranial magnetic stimulation: Diagnostic, therapeutic, and research potential. Neurology, 68(7), 484–488.
Ruff, C. C., Driver, J., & Bestmann, S. (2009). Combining TMS and fMRI: From “virtual lesions” to functional-network accounts of cognition. Cortex, 45(9), 1043–1049.
Shang, Y.-Q., Xie, J., Peng, W., Zhang, J., Chang, D., & Wang, Z. (2018). Network-wise cerebral blood flow redistribution after 20 Hz rTMS on left dorso-lateral prefrontal cortex. European Journal of Radiology, 101, 144–148.
Siebner, H. R., Bergmann, T. O., Bestmann, S., Massimini, M., Johansen-Berg, H., Mochizuki, H., Bohning, D. E., Boorman, E. D., Groppa, S., Miniussi, C., Pascual-Leone, A., Huber, R., Taylor, P. C. J., Ilmoniemi, R. J., de Gennaro, L., Strafella, A. P., Kähkönen, S., Klöppel, S., Frisoni, G. B., George, M. S., Hallett, M., Brandt, S. A., Rushworth, M. F., Ziemann, U., Rothwell, J. C., Ward, N., Cohen, L. G., Baudewig, J., Paus, T., Ugawa, Y., & Rossini, P. M. (2009). Consensus paper: Combining transcranial stimulation with neuroimaging. Brain Stimulation, 2(2), 58–80.
Smittenaar, P., FitzGerald, T. H. B., Romei, V., Wright, N. D., & Dolan, R. J. (2013). Disruption of dorsolateral prefrontal cortex decreases model-based in favor of model-free control in humans. Neuron, 80(4), 914–919.
Song, D., Chang, D., Zhang, J., Peng, W., Shang, Y., Gao, X., & Wang, Z. (2018). Reduced brain entropy by repetitive transcranial magnetic stimulation on the left dorsolateral prefrontal cortex in healthy young adults. Brain Imaging and Behavior.
Valero-Cabré, A., Payne, B. R., & Pascual-Leone, A. (2007). Opposite impact on 14C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Experimental Brain Research, 176(4), 603–615.
Valero-Cabré, A., Payne, B. R., Rushmore, J., Lomber, S. G., & Pascual-Leone, A. (2005). Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity: A 14C-2DG tracing study in the cat. Experimental Brain Research, 163(1), 1–12.
van den Heuvel, M., Mandl, R., & Pol, H. H. (2008). Normalized cut group clustering of resting-state fMRI data. PLoS One, 3(4).
Verdon, C.M., Saba, G. & Januel, D., (2004). Stimulation magnétique transcrânienne et fonctions cognitives. L'Encéphale, 30(4), 363–368.
Wagner, T., Valero-Cabre, A., & Pascual-Leone, A. (2007). Noninvasive human brain stimulation. Annual Review of Biomedical Engineering, 9(1), 527–565.
Wang, J. X., Rogers, L. M., Gross, E. Z., Ryals, A. J., Dokucu, M. E., Brandstatt, K. L., . . . Voss, J. L. (2014b). Targeted enhancement of cortical-hippocampal brain networks and associative memory Jane X. Wang, 1054.
Wang, Z., Aguirre, G. K., Rao, H., Wang, J., Fernández-Seara, M. A., Childress, A. R., & Detre, J. A. (2008). Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magnetic Resonance Imaging, 26(2), 261–269.
Wang, Z., Li, Y., Childress, A. R., & Detre, J. A. (2014a). Brain entropy mapping using fMRI. PLoS One, 9(3), 1–8.
Wassermann, E. M., & Lisanby, S. H. (2001). Therapeutic application of repetitive transcranial magnetic stimulation: A review. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology, 112, 1367–1377.
Xiong, J. H., Parsons, L. M., Gao, J. H., & Fox, P. T. (1999). Interregional connectivity to primary motor cortex revealed using MRI resting state images. Human Brain Mapping, 8(2–3), 151–156.
Xue, S. W., Guo, Y., Peng, W., Zhang, J., Chang, D., Zang, Y. F., & Wang, Z. (2017). Increased low-frequency resting-state brain activity by high-frequency repetitive TMS on the left dorsolateral prefrontal cortex. Frontiers in Psychology, 8(Dec), 1–8.
Yan, C.-G., & Zang, Y.-F. (2010). DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Frontiers in System Neuroscience, 4(May), 1–7.
Zhang, D., & Raichle, M. E. (2010). Disease and the brain’s dark energy. Nature Reviews Neurology, 6(1), 15–28.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 61671198), the Youth 1000 Talent Program of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared no conflict of interest regarding the study reported in this paper.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animalsperformed by any of the authors.
Informed consent
Informed written consents were obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shang, Y., Chang, D., Zhang, J. et al. Theta-burst transcranial magnetic stimulation induced functional connectivity changes between dorsolateral prefrontal cortex and default-mode-network. Brain Imaging and Behavior 14, 1955–1963 (2020). https://doi.org/10.1007/s11682-019-00139-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-019-00139-y