Abstract
Impairment in cognitive control in alcohol use disorder (AUD) contributes to difficulty controlling alcohol use and, in many populations, difficulties with emotion regulation. However, the most reliable and robust marker of clinically-relevant deficits in cognitive control in AUD is unclear. Our aims were to measure relationships between BOLD signal during a Stroop task and AUD severity and change in BOLD signal and change in drinking over three weeks. We also aimed to explore the relationships between BOLD signal and subjective negative affect. Thirty-three individuals with AUD underwent a multisensory Stroop task during functional magnetic resonance imaging (fMRI), as well as a battery of neuropsychological tests and self-report assessments of negative affect and AUD severity. Greater activation in temporal gyrus and cerebellum during incongruent trials compared to congruent trials was observed, and percent signal change (incongruent minus congruent) in both clusters was positively correlated with AUD severity and self-reported negative affect. Neuropsychological task performance and self-reported impulsivity were not highly correlated with AUD severity. Hierarchical regression analyses indicated that percent signal change (incongruent minus congruent) in cerebellum was independently associated with negative affect after controlling for recent and chronic drinking. In a subset of individuals (n = 23) reduction in cerebellar percent signal change (incongruent minus congruent) was correlated with increases in percent days abstinent over 3 weeks. BOLD activation during this Stroop task may therefore be an important objective marker of AUD severity and negative affect. The potential importance of the cerebellum in emotion regulation and AUD severity is highlighted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Loss of control of alcohol use is a primary symptom of an alcohol use disorder (AUD) (American Psychiatric Association 2013) and may be partially mobilized by global impairments in cognitive control (Charney et al. 2010; Houben et al. 2011; Kwako et al. 2015; Mayer et al. 2013; Wilcox et al. 2014; Wilcox et al. 2015). Abberations in neural systems involved in regulating behavior and inhibiting prepotent responses can render someone more vulnerable to impulses to use alcohol, even when there is a desire to cut down or stop (Camchong et al. 2013; Houben et al. 2011; Kwako et al. 2015; Petit et al. 2014; Rupp et al. 2016; Schmidt et al. 2017; Wilcox et al. 2014). However, the measure of the integrity of these systems most strongly related to AUD severity is not definitively established.
Performance on tasks of response inhibition, selective attention/distractor interference control (i.e. Stroop) and working memory can be used as a probe for this vulnerability, being associated with AUD severity and diagnosis, but results are mixed regarding the relationships between AUD and many of these measures of cognitive control or with self-reported impulsivity (Joos et al. 2013a; Joos et al. 2013b; Wilcox et al. 2014). Inconsistency across studies may exist, in part, because self-report may be sensitive to daily mood fluctuations, and because such a wide variety of tasks are used within the cognitive control domains (Wilcox et al. 2014).
Because it measures brain function directly, and is objective, there is reason to believe that BOLD signal [obtained through functional magnetic resonance imaging (fMRI)] during tasks of cognitive control may be a more suitable measure than indirect measures, such as task performance and self-report, of this underlying vulnerability. FMRI during tasks of cognitive control have demonstrated varying results in AUD; during response inhibition tasks AUD demonstrate lower activation in the cognitive control network [prefrontal cortex, insula, striatum] during inhibition in AUD compared to controls, or in AUD with greater severity (Claus et al. 2013; Karch et al. 2008; Li et al. 2009; Schmaal et al. 2013), whereas Stroop-like tasks are associated with greater activation in AUD and other substance use disorders in a number of regions in the cognitive control network when all trials are combined (Mayer et al. 2013; Wilcox et al. 2015), and in a more limited network (PCC, precuneus, cerebellum) when group differences in incongruent versus congruent contrast maps are examined (Hatchard et al. 2015; Schulte et al. 2012); the directionality of associations are mixed during tasks of working memory (Desmond et al. 2003; Park et al. 2011; Wilcox et al. 2014).
Impaired emotion regulation can cause higher levels of negative affect, is associated with AUD severity, and likely contributes to loss of control of alcohol use through negative reinforcement (Wilcox et al. 2016). Impairments in cognitive control may contribute to dysregulated emotion: these constructs are associated (Hendricks and Buchanan 2015), and studies show that enhancement of cognitive control through training reduces anxiety (Sari et al. 2015). Third, the insula and fronto-parietal circuit, including lateral and dorsomedial prefrontal cortex, are believed play important roles in both cognitive control and emotion regulation in individuals with substance use disorder and without substance use disorder (Ochsner et al. 2012; Viviani 2014; Wilcox et al. 2014; Wilcox et al. 2016).
To our knowledge, only one study has explored relationships between negative affect and brain activation during a pure task of cognitive control (without emotional stimuli) in AUD (Karch et al. 2008), showing that anxious individuals with AUD have increased brain activation during a cognitive control task than non-anxious AUD. More work in this area is important to disambiguate the circuitry involved in processing emotional cues versus those involved in top-down control during tasks of emotion regulation (Crane et al. 2016). In addition, no study has examined the degree to which the relationships between impairment in cognitive control and AUD severity is driven by its association with negative affect.
In this study, which was a secondary analysis of a group of participants who were also undergoing a clinical trial of prazosin for the treatment of AUD (Wilcox et al. 2018b), our first aim was to examine the relationships between brain activation in the cognitive control network during a Stroop task (Mayer et al. 2013; Wilcox et al. 2015) and AUD severity at baseline. We hypothesized that greater activation during incongruent compared to congruent trials in the cognitive control network would be associated with greater AUD severity (Aim 1a). We also hypothesized that greater activation during incongruent compared to congruent trials would be more strongly correlated with (and a more sensitive predictor of) AUD severity than self-reported impulsivity and performance on neuropsychological tasks of cognitive control (Aim 1b). We also aimed to examine the relationships between brain activation and negative affect, and hypothesized that greater negative affect would be associated with greater activation in the cognitive control network during incongruent compared to congruent trials (Karch et al. 2008) (Aim 2). Our third aim was to explore whether prazosin, belonging to a class of medications (alpha-1 antagonists) showing efficacy for reducing drinking in this and other samples (Kenna et al. 2016; Simpson et al. 2015; Simpson et al. 2009; Wilcox et al. 2018b), affected brain activation in a subset of participants (n = 23) who received a second scan 3 weeks later, and whether changes in activation was related to changes in drinking (Aim 3). We hypothesized that prazosin would reduce activation during incongruent relative to congruent trials, and that larger reductions in activation would be associated with larger reductions in drinking over time.
Material and methods
Participants
Participants were 33 (63.6% male) individuals with AUD interested in cutting back or quitting drinking. Participants ranged in age from18 to 65 (39.7 ± 11.3) and were recruited through newspapers, online postings like craigslist, and flyers. 36 individuals were initially recruited and scanned, but one was excluded for unusable scan data, one for an anatomical abnormality and one for excessive motion. All participants were subsequently enrolled in a 6-week double-blind, placebo-controlled trial of prazosin for the treatment of AUD [https://clinicaltrials.gov/ct2/show/NCT01916941, (Wilcox et al. 2018b)], of whom 23 received a second brain scan. For inclusion, participants were required to have at least four drinking days during the past month, and to meet criteria for alcohol dependence (First et al. 2002) in the past three months. Participants were excluded if they were currently receiving alcohol treatment, taking daily medications that could influence BOLD signal or treatment outcome, suffering from significant medical problems or particular psychiatric diagnoses (PTSD, bipolar I, current dependence on another drug other than nicotine or cannabis). See supplementary information for more details on inclusion/exclusion and trial procedures.
Clinical measures
General measures
All participants completed the Fagerstrom Test for Nicotine Dependence (FTND) (Heatherton et al. 1991), and underwent a Structured Clinical Interview for DSM Disorders (SCID) (First et al. 2002) administered by a research assistant, a urine toxicology screen, and a Clinical Institute Withdrawal Assessment of Alcohol Scale (CIWA; excluded for score > 8) (Sullivan et al. 1989) prior to the imaging session.
Measures of cognitive control and function
All participants performed a Stroop color and word test (Golden 1978) from which the Interference T score (standardized score with mean of 50 and standard deviation of 10) was calculated. In addition, all participants completed a Connors Continuous Performance Test (CPTII) (Conners 2000) in which participants are asked to click the space bar when they are presented with any letter other than “X”; they are to refrain from clicking if they see the letter “X”. Primary measures from the CPT were T scores for the errors of commission and hit reaction time scores (measures of inattentiveness), given their previously observed relationship with AUD or AUD severity (Wilcox et al. 2014). Secondary measures were errors of omission, CPTD’ (ability to discriminate between targets and non-targets), and hit reaction time block change (change in reaction time over the task) given their association with related pathologies like attention deficit hyperactivity disorder (Epstein et al. 2003; Miranda et al. 2012). All participants also completed a measure that estimates intelligence (Weschsler Test of Adult Reading; WTAR) (Whitney et al. 2010).
Measures of alcohol consumption, AUD severity and related measures
The Timeline Follow-back calendar (Sobell et al. 1992) was used to determine alcohol and other drug use during the previous 90 days using percent days abstinent and drinks per week. Measures of drinking in the 90 days prior to the scan visit and the 7 days prior to the scan (recent drinks per week and recent percent days abstinent) were calculated. In addition, all participants completed measures of AUD severity including the Alcohol Use Disorders Identification Test (AUDIT) (Babor et al. 1992), a standard measure of AUD severity, and the Drinkers Inventory of Consequences (DRINC) (Forcehimes et al. 2007), which addresses consequences of heavy drinking over the past 3 months. In addition, all participants reported the number of years since onset of problem drinking during administration of the SCID.
Measures of impulsivity
The Barratt Impulsiveness Scale total score (Patton et al. 1995) was our primary measure of impulsivity given its known relationship with AUD severity in previous work (Wilcox et al. 2014).
Measures of negative affect
We administered two measures of negative affect, the PROMIS Anxiety and PROMIS Depression Scales (Schalet et al. 2016). In addition, a measure of emotion regulation (Wilcox et al. 2016), the Affective Lability Scale (Look et al. 2010) was obtained.
Imaging task
A multisensory numeric Stroop task was presented to all participants during fMRI scanning and was similar to previous published work (Mayer et al. 2011; Mayer et al. 2013; Wilcox et al. 2015). Each 10s block consisted of simultaneously presented multisensory (visual and auditory) congruent or incongruent numeric stimuli (targets) occurring at a low (0.33 Hz) frequency. Each block started with the cue word (exemplary visual angle = 7.69°) “LOOK” or “HEAR” followed by a stream of target numbers presented visually (one, two, or three; exemplary visual angle = 9.73°). If the cue word was “LOOK,” participants were instructed to press a button corresponding to the number for the visual stimuli and ignore the number that was simultaneously presented aurally. If the cue word was “HEAR,” subjects attended to the aural number stream while ignoring visual targets. There was a 1325 ms delay between the presentation of the cue (175 ms duration) and the presentation of the first target number (200 ms duration) to maximize attentional focus. The inter-block interval varied between 8, 10, and 12 s to decrease temporal expectations and permit modeling of the baseline (visual fixation plus baseline gradient noise). There were 3 trials per block. A total of 24 trials per trial type (96 total trials), and 8 blocks of each type (32 total blocks), were presented across two separate imaging runs. Before being placed in the scanner, participants practiced the behavioral task until demonstrating competency.
Multisensory Stroop task performance
Two measures of task performance during the multisensory Stroop task were obtained during each of four trial types (auditory incongruent, auditory congruent, visual incongruent, visual congruent): an accuracy measure, and reaction time on the task.
MR imaging and analyses
A high-resolution MPRAGE T1 sequence and whole-brain echo-planar image were collected on a 3 Tesla Siemens Trio scanner (acquisition details in supplementary information). All participants were inspected for significant anatomic abnormalities (one subject was dropped for large ventricles) and then analyzed for excessive frame-wise head motion (greater than three times the interquartile range on two or more of six parameters) compared to the rest of their cohort (one was determined to have excessive head motion using these criteria and was excluded). No subjects were found to be behavioral outliers (accuracy or reaction time).
The Analysis of Functional NeuroImages (AFNI) software package (Version 16.0.15) (Cox 1996) was used to generate functional images using standard pre-processing techniques (time-slice correction, motion correction, 6 mm Gaussian full-width half-maximum spatial filter, and spatial normalization to Talairach space resampled to 3x3x3 mm). A voxel-wise deconvolution analysis was then performed to generate a single hemodynamic response function (HRF) that spanned the first 22 s post-stimulus onset (to account for the fact that the HRF generally peaks at 4–6 s and lasts 10–15 s) for each trial-type with error trials modeled separately (Mayer et al. 2011; Mayer et al. 2013; Wilcox et al. 2015). Paired t-tests were performed (Incongruent vs. Congruent trials) and corrected for multiple comparisons using 3dClustsim. Percent signal change estimates were calculated based on the beta coefficients for the images occurring 6 to 14 s post-cue onset for each relevant trial type, and then divided by the average model intercept.
For all evoked task-associated findings, false positives were corrected at p < 0.001 (input volume voxel-wise threshold) and alpha <0.05 (p threshold for clusters, minimum cluster size of 896 μl based on based on 10,000 Monte-Carlo simulations and using spherical autocorrelation) within a white matter exclusion mask (mask details in supplementary information). In a subset of participants (n = 23) a second scan (Scan2) was performed approximately 3 weeks later, for which preprocessing techniques were identical. Mean percent signal change (incongruent minus congruent) estimates were calculated from the Scan2 images in the significant clusters identified from the whole brain analysis during the baseline scan.
Correlations
For most but not all measures, variables were normally distributed. In the cases where they were not normally distributed [i.e. if either the Kolmogorov-Smirnov or Sharpiro Wilk test p value was less than 0.05], variables were transformed to create variables with normal distributions [drinks per week in the previous 90 days, and recent drinks per week in the 7 days prior to the scan, CPT errors of omission, WTAR, CPTD’, CPT hit reaction time, Affective Lability Scale score]. When transformations were performed but we were unable to create normally distributed variables [CIWA, multisensory Stroop task behavioral measures, percent days abstinent in the previous 90 days], non-parametric statistics were used.
Corrections for multiple comparisons
Given the high number of correlation analyses performed, we corrected for multiple comparisons using false discovery rate within each aim (Aim 1a, Aim 1b, Aim 2) for our primary measures, results from which are reported in the corresponding tables (Tables 2, 3, 4, 5, 6).
Results
Sample characteristics
All subjects met criteria for alcohol dependence (American Psychiatric Association 2013) within the last month except for one subject who met 2/7 criteria for alcohol dependence in the last month, but had 2/7 additional criteria for dependence and one abuse criteria in the past, prior to 1 month ago. Fourty-2 % (42.4%) were Hispanic/Latino and 51.5% were Caucasian, 18.2% were Native American, 18.1% were mixed/other, 15.1% were unemployed, and mean years of education for the sample was 14.2, standard deviation (SD) = 1.92. Thirteen participants were smokers and 3 participants had current (past month) marijuana dependence. Table 1, supplementary information and the clinical trial manuscript provide further sample details (Wilcox et al. 2018b).
At the time of the scan, all individuals had a breath alcohol level of <0.01 and a CIWA of less than 4 (mean = 0.82, SD = 1.11). Six individuals were positive for THC, and 3 for benzodiazepines but no participants reported using illicit drugs in the 24 h before the scan. Six individuals reported drinking alcohol within 24 h of the scan (1, 2, 3, 3.2, 5, and 12.7 standard drinks, respectively).
Behavioral results during the fMRI task
Behavioral results during the task were as expected (Mayer et al. 2017; Mayer et al. 2013; Wilcox et al. 2015), demonstrating greater accuracy and higher reaction times for congruent compared to incongruent trials, as well as a main effect of modality, with participants having significantly slower reaction times during auditory trials [mean = 760.23, standard error (SE) = 23.79] than visual trials (mean = 717.29, SE = 22.62) (details in supplementary information).
Imaging results
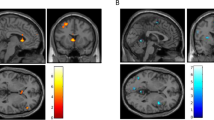
Greater activation was observed within 2 clusters: bilateral cerebellum (vermis; 3963 μl) (mean percent signal change incongruent = 0.247, SD = 0.213; congruent = 0.130 = SD 0.193) and left superior/middle temporal gyrus (BAs 21,22) (2941 μl) (mean percent signal change incongruent = 0.33, SD = 0.221; mean percent signal change congruent = 0.243, SD = 0.224) for incongruent compared to congruent trials (Fig. 1). Two variables for use in the analyses that follow were subsequently created by extracting percent signal change for incongruent minus congruent stimuli from these 2 clusters: cerebellum (Cerebellum) and temporal gyrus (Temp).
This figure depicts regions showing differences in activation during incongruent compared to congruent trials during a multisensory Stroop task (bilateral cerebellum, left, talairach x = −4; left temporal gyrus, right, talairach z = 2). Activation maps are color-coded according to the magnitude of the voxelwise p threshold for a paired T test comparing incongruent to congruent stimuli
Relationships between imaging results and possible confounding variables
None of the two imaging variables were related to smoking status, CIWA, age or gender [ps > 0.1; rho (CIWA)/pearsons (age) < .2; T (gender, smoking) < 0.2]. Brain activation during incongruent minus congruent stimuli was negatively correlated with recent drinks per week (Cerebellum r = −0.335, p = 0.057; Temp r = 0.38, p = 0.03), so we control for this (to control for effects of recent drinking) in the relevant analyses that follow. Comparing those who drank 24 h before the scan and those who did not, there were no significant differences between groups (ps > 0.15).
Correlations between imaging results and AUD severity and drinking (aim 1a)
Our primary AUD severity measures were AUDIT and DRINC, and drinking measures (percent days abstinent and drinks per week) in the previous 90 days. Both clusters (Cerebellum>Temp) were positively correlated with AUD severity (AUDIT, DRINC) (Fig. 2 for DRINC). These effects remained significant or were trending towards significance after adding recent (previous 7 days) drinks per week as a covariate (Tables 2 and 3).
Correlations between cognitive control task performance/impulsivity and AUD severity/drinking (aim 1b)
Primary measures of cognitive control were the CPT errors of comission, CPT hit reaction time, and Stroop Interference T scores. For impulsivity, the Barratt Impulsivity Scale score was our primary measure. WTAR, CPTD’, CPT hit reaction time block change and CPT errors of omission T scores were secondary measures. Barratt Impulsivity Scale score was significantly positively correlated with DRINC but this did not meet criteria for significance after correcting for multiple comparisons. Cerebellum and Temp were therefore more robustly associated with AUD severity (Table 2, 3) compared to the primary and secondary cognitive task performance measures (Table 4). Task performance on the multisensory Stroop task was not significantly associated with AUD severity measures (supplementary information).
Correlations between imaging results and negative affect measures (aim 2)
Negative affect measures were obtained with the Affective Lability Scale, and the PROMIS Anxiety and Depression Scales. Both clusters (Cerebellum>Temp) were positively correlated with the negative affect measures (0.001 < ps < 0.039) (Tables 5 and 6, Fig. 3 for PROMIS Anxiety). This association persisted upon correction for recent (previous 7 days) drinks per week (Tables 5 and 6).
Since Cerebellum and Temp were correlated with both AUD severity and negative affect, we performed a series of hierarchical regression analyses to clarify the nature of their relationships. In the first step, the negative affect measures (Affective Lability Scale, PROMIS Anxiety, PROMIS Depression) were entered as dependent variables and AUD severity (AUDIT or DRINC), number of years with a drinking problem, and recent drinks per week were entered as independent variables (total of 6 models run). In the second step the imaging variable (either Cerebellum or Temp) was added as a predictor (total of 12 models run). These analyses indicated that adding Cerebellum to the model significantly increased model fit in 5/6 of these models, whereas adding Temp to the model only significantly increased fit for one. By contrast, if analogous analyses were performed with AUD severity as a dependent variable, and negative affect measures, number of years with a drinking problem and recent drinks per week as predictors, neither Cerebellum nor Temp increased model fit.
Aim 3. Longitudinal analyses (aim 3)
A repeated measures ANOVA with Time (baseline vs. Scan 2) as a within subjects factor and Condition (prazosin vs. placebo) as a between subjects factor indicated that prazosin treatment was not associated with significant changes in percent signal change (incongruent minus congruent) compared to placebo in Cerebellum or Temp [Condition*Time effect for Cerebellum: F = 0.088, p = 0.770; Condition*Time effect for Temp: F = 0.190, p = 0.667). However, change in Cerebellum from baseline to Scan2 (Scan2-baseline) was correlated with increases in percent days abstinent [recent percent days abstinent during the 7 days prior to the second scan minus percent days abstinent over the 90 days prior to the second scan] (rho = −0.554, p = 0.006) and reductions in drinks per week (recent drinks per week in the 7 days prior to the second scan minus drinks per week over the 90 days prior to the second scan) (rho = 0.369, p = 0.084), indicating that greater reductions in percent signal change (incongruent minus congruent) were associated with greater reductions in drinking. Change in Temp was not correlated with changes in drinking, nor were changes in scores from the Barratt Impulsivity Scale, the Affective Lability Scale, or the PROMIS Anxiety or Depression Scales (rho<0.4, ps > 0.1).
Discussion
In summary, greater activation during incongruent relative to congruent trials in cerebellum and temporal lobe during a Stroop task was positively correlated with AUD severity even when controlling for recent (previous 7 days) drinking. Moreover, reductions in drinking from baseline to a second scan at 3 weeks were correlated with reductions in cerebellar activation. Furthermore, greater activation in both regions to incongruent relative to congruent stimuli was positively correlated with self-reported negative affect. Finally, in cerebellum, this relationship was independent of the effects of current drinking, AUD severity and length of time with a drinking problem.
The finding that AUD severity was positively correlated with brain activation during a Stroop task in the cerebellum is in-line with previous work showing greater activation in fronto-cerebellar circuits during working memory (Desmond et al. 2003) in AUD relative to controls and with work showing greater activation to incongruent versus congruent stimuli in young alcohol-using adults (Hatchard et al. 2015). It also supports a growing literature on the importance of the cerebellum in drug craving (Froeliger et al. 2015; Moreno-Rius and Miquel 2017), although there was not a relationship with craving in our sample, per se (data not reported). That AUD severity was correlated with greater activation in temporal lobe has not, to our knowledge, been highlighted in previous work, although temporal grey matter loss is noted in AUD for example (Chanraud et al. 2007) and greater activation in temporal lobe during a conflict task in individuals with a positive family history of AUD (Acheson et al. 2014) has been observed. Moreover, as stated, increases in percent days abstinent and reductions in drinks per week were associated with reductions in percent signal change (incongruent minus congruent) in cerebellum, which could either indicate that reductions in percent signal change were a mechanism of drinking reduction, and could be a treatment target, or that reductions were simply a consequence of drinking reduction.
That BOLD signal in cerebellum was robustly correlated with measures of negative affect was especially interesting. Furthermore, according to supplemental regression analyses, the cerebellar signal appeared to be independently associated with negative affect, correcting for AUD severity, number of years of alcohol drinking, and recent alcohol exposure. By contrast, BOLD signal in cerebellum did not add any predictive power above and beyond levels of negative affect when AUD severity was the dependent variable in a regression. This indicates that the relationship between AUD severity and BOLD signal during incongruent compared to congruent stimuli was probably being effected primarily through negative affect, rather than through the effects of chronic alcohol use (which we felt was best measured in our dataset with AUD severity). This is important because negative affect is believed to be a motivator for alcohol consumption in AUD (Koob and Le Moal 2008; Wilcox et al. 2016; Wilcox and Tonigan 2016) and provides support for the possibility that cerebellar activation during this task may be a treatment target.
Unfotunately, prazosin, which was associated with modest reductions in drinks per week in the clinical trial (Wilcox et al. 2018b), did not affect percent signal change in the cerebellum or temporal lobe relative to placebo. Prazosin is an alpha-1 antagonist, and this class of medications has shown some efficacy for AUD treatment in the sample examined in this manuscript (Wilcox et al. 2018b) and in other samples (Kenna et al. 2016; Simpson et al. 2015; Simpson et al. 2009). There is reason to believe that the noradrenergic system may also play an important role in both cognitive control, negative affect and emotion regulation (Arnsten et al. 1999; Arnsten et al. 2015; Fox et al. 2012; Le et al. 2011; Raskind et al. 2003). Therefore, although prazosin modestly reduced drinking in this sample (Wilcox et al. 2018b), our findings indicate that this treatment was likely not acting via effects on cerebellar or temporal lobe function.
In the absence of an intervention that affects cerebellar signal, it is impossible to parse whether the negative affect is causing the higher BOLD signal in cerebellum, perhaps by way of heightened adrenergic tone, as stress is known to affect cognitive function (Arnsten et al. 2015) or if the higher signal is a marker of poor cerebellar function which is driving the negative affect. Supporting the former, increased anxiety can trigger deterioration in cognitive control, as evidenced by studies showing that negative affective stimuli can impair performance on tasks requiring inhibition and cognitive control (Hobson et al. 2014; Roelofs et al. 2009; Wingenfeld et al. 2009), or that rumination is associated with difficulty inhibiting negative information when switching from negative to positive affective blocks on a Go No-Go task (Hilt et al. 2014). There is also support for the latter possibility, as evidenced by a growing appreciation in the literature of the role that the cerebellum, and especially the vermis (the region activated by our task, dubbed the limbic cerebellum), plays in emotion regulation, emotional processing (Adamaszek et al. 2017; Anderson et al. 2006; Frodl et al. 2011; Lupo et al. 2015; Schmahmann 2010) and impulsivity (Park et al. 2014). From a circuitry perspective, it is known that the cerebellar vermis projects to the medial prefrontal cortex and the limbic system (Adamaszek et al. 2017; Caligiore et al. 2017; De Vidovich et al. 2016; Roostaei et al. 2014). The principal output of the cerebellum to the prefrontal cortex is believed to be inhibitory (Casula et al. 2016; Daskalakis et al. 2005), and so hyperactivation in this area could lead to excessive inhibitory drive to the prefrontal cortex, taking important emotional-regulatory functions “off line”. That cerebellar dysfunction could contribute to dysregulated emotion deserves further exploration.
Although the relationships between activation in the temporal lobe and negative affect were no longer significant upon correcting for AUD severity, this still deserves further exploration as a potential biomarker of negative affect and emotion regulation, as it was not possible for us to isolate the effects of AUD severity from those of negative affect levels. Temporal lobe (middle and superior) is activated during similar versions of this Stroop task in cocaine use disorder (Mayer et al. 2013) and this area may also be related to emotion regulation during reappraisal (Morawetz et al. 2016) and may link prefrontal with subcortical regions involved in affect regulation (Morawetz et al. 2016).
There have been mixed results in previous work regarding the relationship between performance on tasks of cognitive control and AUD severity (Wilcox et al. 2014), but few studies have compared the degree to which fMRI signal versus self-report scales or task performance on widely-utilized neuropsychiatric tests is related to AUD severity, important in light of the fact that fMRI is more expensive. That the BOLD signal was more strongly correlated with AUD severity than performance on the cognitive neuropsychiatric tests is notable. The relative importance of fMRI in this sample was supported by the fact that changes in percent days abstinent and drinks per week over time were correlated with changes in cerebellar BOLD signal, but not with changes in self-reported impulsivity or negative affect, when measured with questionnaires that correlated with drinking at baseline.
Although these results indicate that fMRI has the potential outperform behavioral measures, and be a more robust biomarker of disease severity, there are several things that would need to be demonstrated prior to deployment of fMRI to obtain this signal as, say, a treatment-outcome predictor, into the clinical arena. Some of the more obvious barriers to the use of fMRI in the clinical setting, as opposed to neuropsychiatric testing, are higher cost, cross-scanner inconsistency, and difficulty of widespread implementation, particularly when cognitive tasks are utilized to evoke brain activity (resting state or anatomical data are generally more simple to acquire) (Milham et al. 2017; Wilcox et al. 2018a). Furthermore, one of the major limitations of these kinds of results and others like it, is the challenge of replication (Milham et al. 2017; Wilcox et al. 2018a); this finding has not, yet, been replicated in an independent sample. Before we will be able to utilize fMRI in clinical populations to identify individuals with more severe illness who might need increased support, or to identify subtypes more likely to respond to particular treatments (treatment matching), we will need more replication studies, and we will need to collect more large longitudinal datasets to permit utilization of more state of the art prediction analysis methods (i.e. machine learning with cross-validation) (Milham et al. 2017; Wilcox et al. 2018a). That said, these kinds of findings can still inform research and treatment development. A study is currently underway to investigate whether inhibitory repetitive transcranial magnetic stimulation to the cerebellum affects alcohol use and negative affect in treatment-seeking individuals with AUD (R21 AA026573). The idea for this study came from these results and other work demonstrating that targeting the cerebellum with neurostimulation may alter one’s capacity for emotion regulation and levels of negative affect (Adamaszek et al. 2017; Anderson et al. 2006; Frodl et al. 2011; Froeliger et al. 2015; Park et al. 2014).
There are a number of additional limitations to this work. As previously alluded to, it cannot be determined whether neural activation is causing negative affect and is a treatment target, or if negative affect is causing greater activation. Future work utilizing interventions in a prospective placebo-controlled design targeting brain activation in these regions and/or negative affect can better answer some of these questions. Second, we did not have a control group; however our sample had a range of AUD severity. Finally, we did not have other potentially important measures of impulsivity such as delay discounting and risky decision making which have both been demonstrated to predict alcohol use (Courtney et al. 2012), and which may be more robust than some of the measures of impulsivity and cognitive control employed in this sample which were not highly correlated with AUD severity.
In conclusion, our results indicate that imaging may provide a sensitive measure of AUD severity, and that cerebellar activation during a cognitive control task is independently related to negative affect, controlling for AUD severity. The potential importance of the cerebellum, a previously under-appreciated structure in the pathophysiology of psychiatric disorders, as a biomarker of negative affect and impaired emotion regulation in AUD is highlighted.
References
Acheson, A., Franklin, C., Cohoon, A. J., Glahn, D. C., Fox, P. T., & Lovallo, W. R. (2014). Anomalous temporoparietal activity in individuals with a family history of alcoholism: Studies from the Oklahoma family health patterns project. Alcoholism, Clinical and Experimental Research, 38, 1639–1645. https://doi.org/10.1111/acer.12420.
Adamaszek, M., D’Agata, F., Ferrucci, R., Habas, C., Keulen, S., Kirkby, K. C., Leggio, M., Mariën, P., Molinari, M., Moulton, E., Orsi, L., van Overwalle, F., Papadelis, C., Priori, A., Sacchetti, B., Schutter, D. J., Styliadis, C., & Verhoeven, J. (2017). Consensus paper: Cerebellum and emotion. Cerebellum, 16, 552–576. https://doi.org/10.1007/s12311-016-0815-8.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders. In Fifth edition: DSM-V-TR®. Author. Washington, DC.
Anderson, C. M., Lowen, S. B., & Renshaw, P. F. (2006). Emotional task-dependent low-frequency fluctuations and methylphenidate: Wavelet scaling analysis of 1/f-type fluctuations in fMRI of the cerebellar vermis. Journal of Neuroscience Methods, 151, 52–61. https://doi.org/10.1016/j.jneumeth.2005.09.020.
Arnsten A, Mathew R, Ubriani R, Taylor J, Li B-M (1999) Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function vol 45.
Arnsten, A. F., Raskind, M. A., Taylor, F. B., & Connor, D. F. (2015). The effects of stress exposure on prefrontal cortex: Translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress, 1, 89–99. https://doi.org/10.1016/j.ynstr.2014.10.002.
Babor T, de la Fuente J, Saunders J, Grant M (1992) The alcohol use disorders identification test. Guidelines for use in primary health care.
Caligiore, D., Pezzulo, G., Baldassarre, G., Bostan, A. C., Strick, P. L., Doya, K., Helmich, R. C., Dirkx, M., Houk, J., Jörntell, H., Lago-Rodriguez, A., Galea, J. M., Miall, R. C., Popa, T., Kishore, A., Verschure, P. F. M. J., Zucca, R., & Herreros, I. (2017). Consensus paper: Towards a systems-level view of cerebellar function: The interplay between cerebellum, basal ganglia, and cortex. Cerebellum, 16, 203–229. https://doi.org/10.1007/s12311-016-0763-3.
Camchong, J., Stenger, A., & Fein, G. (2013). Resting-state synchrony in long-term abstinent alcoholics. Alcoholism, Clinical and Experimental Research, 37, 75–85. https://doi.org/10.1111/j.1530-0277.2012.01859.x.
Casula, E. P., Pellicciari, M. C., Ponzo, V., Stampanoni Bassi, M., Veniero, D., Caltagirone, C., & Koch, G. (2016). Cerebellar theta burst stimulation modulates the neural activity of interconnected parietal and motor areas. Scientific Reports, 6, 36191. https://doi.org/10.1038/srep36191.
Chanraud, S., Martelli, C., Delain, F., Kostogianni, N., Douaud, G., Aubin, H. J., Reynaud, M., & Martinot, J. L. (2007). Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology, 32, 429–438. https://doi.org/10.1038/sj.npp.1301219.
Charney, D. A., Zikos, E., & Gill, K. J. (2010). Early recovery from alcohol dependence: Factors that promote or impede abstinence. Journal of Substance Abuse Treatment, 38, 42–50. https://doi.org/10.1016/j.jsat.2009.06.002.
Claus, E. D., Feldstein Ewing, S. W., Filbey, F. M., & Hutchison, K. E. (2013). Behavioral control in alcohol use disorders: Relationships with severity. Journal of Studies on Alcohol and Drugs, 74, 141–151.
Conners CK (2000) Conners' Continuous Performance Test II: Computer Program for Windows Technical Guide and Software Manual. Multi-Health Systems, North Tonawanda, NY.
Courtney, K. E., Arellano, R., Barkley-Levenson, E., Gálvan, A., Poldrack, R. A., MacKillop, J., David Jentsch, J., & Ray, L. A. (2012). The relationship between measures of impulsivity and alcohol misuse: An integrative structural equation modeling approach. Alcoholism, Clinical and Experimental Research, 36, 923–931. https://doi.org/10.1111/j.1530-0277.2011.01635.x.
Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173.
Crane, N. A., Jenkins, L. M., Dion, C., Meyers, K. K., Weldon, A. L., Gabriel, L. B., Walker, S. J., Hsu, D. T., Noll, D. C., Klumpp, H., Phan, K. L., Zubieta, J. K., & Langenecker, S. A. (2016). Comorbid anxiety increases cognitive control activation in major depressive disorder. Depression and Anxiety, 33, 967–977. https://doi.org/10.1002/da.22541.
Daskalakis, Z. J., Christensen, B. K., Fitzgerald, P. B., Fountain, S. I., & Chen, R. (2005). Reduced cerebellar inhibition in schizophrenia: A preliminary study. The American Journal of Psychiatry, 162, 1203–1205. https://doi.org/10.1176/appi.ajp.162.6.1203.
De Vidovich, G. Z., et al. (2016). Repetitive TMS on left cerebellum affects impulsivity in borderline personality disorder: A pilot study. Frontiers in Human Neuroscience, 10, 582. https://doi.org/10.3389/fnhum.2016.00582.
Desmond, J. E., Chen, S. H., DeRosa, E., Pryor, M. R., Pfefferbaum, A., & Sullivan, E. V. (2003). Increased frontocerebellar activation in alcoholics during verbal working memory: An fMRI study. Neuroimage, 19, 1510–1520.
Epstein, J. N., Erkanli, A., Conners, C. K., Klaric, J., Costello, J. E., & Angold, A. (2003). Relations between continuous performance test performance measures and ADHD behaviors. Journal of Abnormal Child Psychology, 31, 543–554.
First MB, Spitzer RL, Gibbon M, Williams JBW (2002) Structured clinical interview for DSM-IV-TR Axis I disorders, research version, patient edition with psychotic screen (SCID-I/P W/ PSY SCREEN).
Forcehimes, A. A., Tonigan, J. S., Miller, W. R., Kenna, G. A., & Baer, J. S. (2007). Psychometrics of the drinker inventory of consequences (DrInC). Addictive Behaviors, 32, 1699–1704. https://doi.org/10.1016/j.addbeh.2006.11.009.
Fox, H. C., Anderson, G. M., Tuit, K., Hansen, J., Kimmerling, A., Siedlarz, K. M., Morgan, P. T., & Sinha, R. (2012). Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: Preliminary findings. Alcoholism, Clinical and Experimental Research, 36, 351–360. https://doi.org/10.1111/j.1530-0277.2011.01628.x.
Frodl, T., Scheuerecker, J., Schoepf, V., Linn, J., Koutsouleris, N., Bokde, A. L. W., Hampel, H., Möller, H. J., Brückmann, H., Wiesmann, M., & Meisenzahl, E. (2011). Different effects of mirtazapine and venlafaxine on brain activation: An open randomized controlled fMRI study. The Journal of Clinical Psychiatry, 72, 448–457. https://doi.org/10.4088/JCP.09m05393blu.
Froeliger, B., McConnell, P. A., Stankeviciute, N., McClure, E. A., Kalivas, P. W., & Gray, K. M. (2015). The effects of N-acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: A double-blind, placebo-controlled fMRI pilot study. Drug and Alcohol Dependence, 156, 234–242. https://doi.org/10.1016/j.drugalcdep.2015.09.021.
Golden, C. J. (1978). Stroop color and word test: A manual for clinical and experimental uses. In Skoelting. Illinois: Chicago.
Hatchard, T., Smith, A. M., Halchuk, R. E., Longo, C. A., Fried, P. A., Hogan, M. J., & Cameron, I. (2015). Effects of low-level alcohol use on cognitive interference: An fMRI study in young adults. Alcohol, 49, 7–13. https://doi.org/10.1016/j.alcohol.2014.07.020.
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., & Fagerstrom, K. O. (1991). The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. British Journal of Addictions, 86, 1119–1127.
Hendricks, M. A., & Buchanan, T. W. (2015). Individual differences in cognitive control processes and their relationship to emotion regulation. Cognition & Emotion, 30, 1–13. https://doi.org/10.1080/02699931.2015.1032893.
Hilt, L. M., Leitzke, B. T., & Pollak, S. D. (2014). Cognitive control and rumination in youth: The importance of emotion. Journal of Experimental Psychopathology, 5, 302–313. https://doi.org/10.5127/jep.038113.
Hobson, N. M., Saunders, B., Al-Khindi, T., & Inzlicht, M. (2014). Emotion down-regulation diminishes cognitive control: A neurophysiological investigation. Emotion, 14, 1014–1026. https://doi.org/10.1037/a0038028.
Houben, K., Nederkoorn, C., Wiers, R. W., & Jansen, A. (2011). Resisting temptation: Decreasing alcohol-related affect and drinking behavior by training response inhibition. Drug and Alcohol Dependence, 1–5.
Joos, L., Goudriaan, A. E., Schmaal, L., Fransen, E., van den Brink, W., Sabbe, B. G., & Dom, G. (2013a). Effect of modafinil on impulsivity and relapse in alcohol dependent patients: A randomized, placebo-controlled trial. European Neuropsychopharmacology, 23, 948–955. https://doi.org/10.1016/j.euroneuro.2012.10.004.
Joos, L., Goudriaan, A. E., Schmaal, L., van den Brink, W., Sabbe, B. G., & Dom, G. (2013b). Effect of modafinil on cognitive functions in alcohol dependent patients: A randomized, placebo-controlled trial. Journal of Psychopharmacology, 27, 998–1006. https://doi.org/10.1177/0269881113503505.
Karch, S., Jäger, L., Karamatskos, E., Graz, C., Stammel, A., Flatz, W., Lutz, J., Holtschmidt-Täschner, B., Genius, J., Leicht, G., Pogarell, O., Born, C., Möller, H. J., Hegerl, U., Reiser, M., Soyka, M., & Mulert, C. (2008). Influence of trait anxiety on inhibitory control in alcohol-dependent patients: Simultaneous acquisition of ERPs and BOLD responses. Journal of Psychiatric Research, 42, 734–745. https://doi.org/10.1016/j.jpsychires.2007.07.016.
Kenna, G. A., Haass-Koffler, C. L., Zywiak, W. H., Edwards, S. M., Brickley, M. B., Swift, R. M., & Leggio, L. (2016). Role of the alpha1 blocker doxazosin in alcoholism: A proof-of-concept randomized controlled trial. Addiction Biology, 21, 904–914. https://doi.org/10.1111/adb.12275.
Koob, G. F., & Le Moal, M. (2008). Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363, 3113–3123. https://doi.org/10.1098/rstb.2008.0094.
Kwako, L. E., Momenan, R., Litten, R. Z., Koob, G. F., & Goldman, D. (2015). Addictions Neuroclinical assessment: A neuroscience-based framework for addictive disorders. Biological Psychiatry, 80, 179–189. https://doi.org/10.1016/j.biopsych.2015.10.024.
Le, A. D., Funk, D., Juzytsch, W., Coen, K., Navarre, B. M., Cifani, C., & Shaham, Y. (2011). Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology, 218, 89–99. https://doi.org/10.1007/s00213-011-2178-7.
Li, C. S., Luo, X., Yan, P., Bergquist, K., & Sinha, R. (2009). Altered impulse control in alcohol dependence: Neural measures of stop signal performance. Alcoholism, Clinical and Experimental Research, 33, 740–750. https://doi.org/10.1111/j.1530-0277.2008.00891.x.
Look, A. E., Flory, J. D., Harvey, P. D., & Siever, L. J. (2010). Psychometric properties of a short form of the affective lability scale (ALS-18). Pers Individ Dif, 49, 187–191. https://doi.org/10.1016/j.paid.2010.03.030.
Lupo, M., Troisi, E., Chiricozzi, F. R., Clausi, S., Molinari, M., & Leggio, M. (2015). Inability to process negative emotions in cerebellar damage: A functional transcranial Doppler sonographic study. Cerebellum, 14, 663–669. https://doi.org/10.1007/s12311-015-0662-z.
Mayer, A. R., Teshiba, T. M., Franco, A. R., Ling, J., Shane, M., Stephen, J. M., & Jung, R. E. (2011). Modelling conflict and error in the medial frontal cortex. Human Brain Mapping, 33, 2843–2855.
Mayer, A. R., Wilcox, C. E., Teshiba, T. M., Ling, J. M., & Yang, Z. (2013). Hyperactivation of the cognitive control network in cocaine use disorders during a multisensory Stroop task. Drug and Alcohol Dependence, 133, 235–241. https://doi.org/10.1016/j.drugalcdep.2013.04.029.
Mayer, A. R., Ryman, S. G., Hanlon, F. M., Dodd, A. B., & Ling, J. M. (2017). Look Hear! The prefrontal cortex is stratified by modality of sensory input during multisensory cognitive control. Cerebral Cortex, 27, 2831–2840. https://doi.org/10.1093/cercor/bhw131.
Milham, M. P., Craddock, R. C., & Klein, A. (2017). Clinically useful brain imaging for neuropsychiatry: How can we get there? Depression and Anxiety, 34, 578–587. https://doi.org/10.1002/da.22627.
Miranda, M. C., Barbosa, T., Muszkat, M., Rodrigues, C. C., Sinnes, E. G., Coelho, L. F. S., Rizzuti, S., Palma, S. M. M., & Bueno, O. F. A. (2012). Performance patterns in Conners' CPT among children with attention deficit hyperactivity disorder and dyslexia. Arquivos de Neuro-Psiquiatria, 70, 91–96.
Morawetz, C., Bode, S., Baudewig, J., Jacobs, A. M., & Heekeren, H. R. (2016). Neural representation of emotion regulation goals. Human Brain Mapping, 37, 600–620. https://doi.org/10.1002/hbm.23053.
Moreno-Rius, J., & Miquel, M. (2017). The cerebellum in drug craving. Drug and Alcohol Dependence., 173, 151–158. https://doi.org/10.1016/j.drugalcdep.2016.12.028.
Ochsner, K. N., Silvers, J. A., & Buhle, J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–E24. https://doi.org/10.1111/j.1749-6632.2012.06751.x.
Park, M. S., Sohn, S., Park, J. E., Kim, S. H., Yu, I. K., & Sohn, J. H. (2011). Brain functions associated with verbal working memory tasks among young males with alcohol use disorders. Scandinavian Journal of Psychology, 52, 1–7. https://doi.org/10.1111/j.1467-9450.2010.00848.x.
Park, C. A., Kang, C. K., Son, Y. D., Choi, E. J., Kim, S. H., Oh, S. T., Kim, Y. B., Park, C. W., & Cho, Z. H. (2014). The effects of caffeine ingestion on cortical areas: Functional imaging study. Magnetic Resonance Imaging, 32, 366–371. https://doi.org/10.1016/j.mri.2013.12.018.
Patton, J. H., Stanford, M. S., & Barratt, E. S. (1995). Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology, 51, 768–774.
Petit, G., Cimochowska, A., Kornreich, C., Hanak, C., Verbanck, P., & Campanella, S. (2014). Neurophysiological correlates of response inhibition predict relapse in detoxified alcoholic patients: Some preliminary evidence from event-related potentials. Neuropsychiatric Disease and Treatment, 10, 1025–1037. https://doi.org/10.2147/NDT.S61475.
Raskind, M. A., Peskind, E. R., Kanter, E. D., Petrie, E. C., Radant, A., Thompson, C. E., Dobie, D. J., Hoff, D., Rein, R. J., Straits-Tröster, K., Thomas, R. G., & McFall, M. M. (2003). Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: A placebo-controlled study. The American Journal of Psychiatry, 160, 371–373.
Roelofs, K., Minelli, A., Mars, R. B., van Peer, J., & Toni, I. (2009). On the neural control of social emotional behavior. Social Cognitive and Affective Neuroscience, 4, 50–58. https://doi.org/10.1093/scan/nsn036.
Roostaei, T., Nazeri, A., Sahraian, M. A., & Minagar, A. (2014). The human cerebellum. a review of physiologic neuroanatomy. Neurol Clin, 32, 859–869. https://doi.org/10.1016/j.ncl.2014.07.013.
Rupp, C. I., Beck, J. K., Heinz, A., Kemmler, G., Manz, S., Tempel, K., & Fleischhacker, W. W. (2016). Impulsivity and alcohol dependence treatment completion: Is there a neurocognitive risk factor at treatment entry? Alcoholism, Clinical and Experimental Research, 40, 152–160. https://doi.org/10.1111/acer.12924.
Sari, B. A., Koster, E. H., Pourtois, G., & Derakshan, N. (2015). Training working memory to improve attentional control in anxiety: A proof-of-principle study using behavioral and electrophysiological measures. Biological Psychology, 121, 203–212. https://doi.org/10.1016/j.biopsycho.2015.09.008.
Schalet, B. D., Pilkonis, P. A., Yu, L., Dodds, N., Johnston, K. L., Yount, S., Riley, W., & Cella, D. (2016). Clinical validity of PROMIS depression, anxiety, and anger across diverse clinical samples. Journal of Clinical Epidemiology, 73, 119–127. https://doi.org/10.1016/j.jclinepi.2015.08.036.
Schmaal, L., Joos, L., Koeleman, M., Veltman, D. J., van den Brink, W., & Goudriaan, A. E. (2013). Effects of modafinil on neural correlates of response inhibition in alcohol-dependent patients. Biological Psychiatry, 73, 211–218. https://doi.org/10.1016/j.biopsych.2012.06.032.
Schmahmann, J. D. (2010). The role of the cerebellum in cognition and emotion: Personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychology Review, 20, 236–260. https://doi.org/10.1007/s11065-010-9142-x.
Schmidt, T. P., Pennington, D. L., Cardoos, S. L., Durazzo, T. C., & Meyerhoff, D. J. (2017). Neurocognition and inhibitory control in polysubstance use disorders: Comparison with alcohol use disorders and changes with abstinence. Journal of Clinical and Experimental Neuropsychology, 39, 22–34. https://doi.org/10.1080/13803395.2016.1196165.
Schulte, T., Muller-Oehring, E. M., Sullivan, E. V., & Pfefferbaum, A. (2012). Synchrony of corticostriatal-midbrain activation enables normal inhibitory control and conflict processing in recovering alcoholic men. Biological Psychiatry, 71, 269–278. https://doi.org/10.1016/j.biopsych.2011.10.022.
Simpson, T. L., et al. (2009). A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcoholism, Clinical and Experimental Research, 33, 255–263. https://doi.org/10.1111/j.1530-0277.2008.00807.x.
Simpson, T. L., Malte, C. A., Dietel, B., Tell, D., Pocock, I., Lyons, R., Varon, D., Raskind, M., & Saxon, A. J. (2015). A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcoholism, Clinical and Experimental Research, 39, 808–817. https://doi.org/10.1111/acer.12703.
Sobell LC, Sobell MB, Litten RZ, Allen JP (1992) Timeline follow-back: A technique for assessing self-reported alcohol consumption, Timeline Follow-Back.
Sullivan, J., Sykora, K., Schneiderman, J., Naranjo, C., & Sellers, E. (1989). Assessment of alcohol withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). British Journal of Addiction, 84, 1353–1357.
Viviani, R. (2014). Neural correlates of emotion regulation in the ventral prefrontal cortex and the encoding of subjective value and economic utility. Frontiers in Psychiatry, 5, 123. https://doi.org/10.3389/fpsyt.2014.00123.
Whitney, K. A., Shepard, P. H., Mariner, J., Mossbarger, B., & Herman, S. M. (2010). Validity of the Wechsler test of adult Reading (WTAR): Effort considered in a clinical sample of U.S. military veterans. Applied Neuropsychology, 17, 196–204. https://doi.org/10.1080/09084282.2010.499787.
Wilcox, C. E., & Tonigan, J. S. (2016). Changes in depression mediate the effects of AA attendance on alcohol use outcomes. American Journal of Drug and Alcohol Abuse, 44, 103–112.
Wilcox, C. E., Dekonenko, C. J., Mayer, A. R., Bogenschutz, M. P., & Turner, J. A. (2014). Cognitive control in alcohol use disorder: Deficits and clinical relevance. Reviews in the Neurosciences, 25, 1–24. https://doi.org/10.1515/revneuro-2013-0054.
Wilcox, C. E., Mayer, A. R., Bogenschutz, M. P., Ling, J., Dekonenko, C., & Cumbo, H. (2015). Cognitive control network function in alcohol use disorder before and during treatment with lorazepam. Substance Use & Misuse, 50, 40–52. https://doi.org/10.3109/10826084.2014.957771.
Wilcox, C. E., Pommy, J. M., & Adinoff, B. (2016). Neural circuitry of impaired emotion regulation in substance use disorders. American Journal of Psychiatry, 173, 344–361.
Wilcox, C. E., Abbott, C. C., & Calhoun, V. D. (2018a). Alterations in resting-state functional connectivity in substance use disorders and treatment implications. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 91, 79–93. https://doi.org/10.1016/j.pnpbp.2018.06.011.
Wilcox, C. E., Tonigan, J. S., Bogenschutz, M. P., Clifford, J., Bigelow, R. C., & Simpson, T. L. (2018b). A randomized, placebo-controlled, clinical trial of prazosin for the treatment of alcohol use disorder. Journal of Addiction Medicine, 12, 339–345.
Wingenfeld, K., Rullkoetter, N., Mensebach, C., Beblo, T., Mertens, M., Kreisel, S., Toepper, M., Driessen, M., & Woermann, F. G. (2009). Neural correlates of the individual emotional Stroop in borderline personality disorder. Psychoneuroendocrinology, 34, 571–586. https://doi.org/10.1016/j.psyneuen.2008.10.024.
Funding
This work was supported by the National Institutes of Health grant number K23-AA021156 awarded to Claire Wilcox.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Claire Wilcox, Mr. Joshua Clifford, Mr. Josef Ling, Dr. Andrew R. Mayer, Ms. Rose Bigelow, Dr. Michael Bogenschutz and Dr. J. Scott Tonigan have no conflicts of interest to report.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Wilcox, C.E., Clifford, J., Ling, J. et al. Stroop-related cerebellar and temporal activation is correlated with negative affect and alcohol use disorder severity. Brain Imaging and Behavior 14, 586–598 (2020). https://doi.org/10.1007/s11682-019-00126-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-019-00126-3