Abstract
Cerebral microbleeds (CMB), suspected markers of hemorrhage-prone microangiopathy, are common in patients with cerebrovascular disease and in those with cognitive impairment. Their longitudinal relationship with cognitive decline and incident dementia in non-demented community-dwelling older individuals has been insufficiently examined. 302 adults aged 70–90 participating in the population-based Sydney Memory and Ageing Study underwent a susceptibility-weighted imaging (SWI) MRI sequence. The relationship of CMB with performance on neuropsychological tests was examined both cross-sectionally and longitudinally, over a mean of 4 years. The association with cases of incident dementia during this period was also examined. The prevalence of CMB was 20%. In cross-sectional analysis, after adjusting for demographics and vascular risk factors, there was a significant association between the presence of CMB and poorer executive function. CMB were not associated with global cognition or other cognitive domains. On longitudinal analysis, after adjusting for demographics and vascular risk factors, there was a greater decline in visuospatial ability in those with CMB compared to those without. The presence of CMB was not associated with increased progression to dementia. CMB are associated with impairments in specific cognitive domains: executive function and decline in visuospatial ability, independent of other markers of CVD including white matter hyperintensities. This suggests a direct contribution of CMB to cognitive impairment although no significant difference in incident dementia rates was observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Vascular pathology is associated with cognitive impairment and dementia, and several markers of cerebral small vessel disease (SVD) can be visualized using magnetic resonance imaging (MRI). In addition to white matter hyperintensities (WMH) and lacunes, cerebral microbleeds (CMB) have been recognized as a marker of SVD. CMB are visualized as punctate hypointense lesions on paramagnetic-sensitive MRI sequences, in particular susceptibility weighted imaging (SWI), and can be found throughout the cerebral lobes, basal ganglia, cerebellum and brainstem (Greenberg et al. 2009). They correspond to small perivascular haemosiderin deposits and represent breakdown products from prior microscopic hemorrhages (Fazekas et al. 1999; Shoamanesh and Benavente 2011).
CMB are found in both cognitively normal and impaired individuals, and their prevalence increases with age from 7% at age 45–50 years to 36% at 80 years and older (Poels et al. 2010). While their prevalence is highest in vascular dementia (65–85%), CMB are also frequently found in Alzheimer’s disease (AD) (18–32%) and mild cognitive impairment (MCI) (20–43%) (Yates et al. 2014). The distribution of CMB in the brain differs according to underlying pathology. CMB in the subcortical and deep regions are thought to be associated with atherosclerotic/hypertensive small vessel disease and in contrast, CMB in lobar regions are more likely associated with cerebral amyloid angiopathy (CAA) (Greenberg et al. 2009; Vernooij et al. 2008).
Once thought to be clinically silent, the majority of cross-sectional studies have reported an association of CMB with impaired executive function, attention and processing speed and, in some studies, global cognition (Poels et al. 2012; Qiu et al. 2008; Wu et al. 2014). The location and number of CMB within the brain are also pertinent, with several large studies (Ding et al. 2017; Vernooij et al. 2008) and a recent meta-analysis (Wu et al. 2014) suggesting that cognitive impairment is seen in the presence of multiple CMB. Moreover, CMB located in the lobar and deep regions, but not infratentorial lesions (Wu et al. 2014; Yakushiji et al. 2015), have an association with cognitive impairment. The studies examining the longitudinal impact of CMB on cognitive decline and incident dementia have reported inconsistent results (Chiang et al. 2015; Ding et al. 2017; Chung et al. 2016; Ayaz et al. 2010; Haller et al. 2010; Kirsch et al. 2009; Akoudad et al. 2016; Miwa et al. 2014; Romero et al. 2017). There are multiple reasons for the inconsistent findings: lack of common consensus criteria for CMB; diverse populations; different imaging parameters; and diverse use of cognitive measurement. In the context of this inconsistency, and the scarcity of longitudinal studies, the aims of this study were to determine whether the presence of CMB, measured both globally and in specific cerebral regions, was associated with cognitive impairment cross-sectionally, and with cognitive decline and incident dementia over four years of follow-up.
METHODS
Subjects
Participants were drawn from the population based longitudinal Sydney Memory and Ageing study (MAS) (Sachdev et al. 2010), an ongoing study which began in 2005 and focuses on cognitive decline in the community-dwelling elderly. Subjects were aged 70–90 years, living in the community and able to complete their assessments in English. Exclusion criteria were diagnosis of dementia or other psychiatric or central nervous system disorder at wave 2. There have been four waves of this study, two years apart. At each wave, participants underwent an MRI scan, comprehensive neuropsychological assessment, medical examination, blood collection, and APOE genotyping (wave 1). For this study, we used data from wave 2, when SWI was introduced to the MRI protocol. Written consent was obtained from all participants. The methodological details and ethics approval of the study have been previously published (Sachdev et al. 2010).
Radiological examination
MRI was performed on a Philips 3 T Achieva Quasar Dual scanner (Philips Medical Systems, Best, Netherlands). The parameters for the SWI sequence were: repetition time (TR) = 25.33 ms, time to echo (TE) = 40.33 ms; slice thickness = 1.1, of field of view (FOV) = 240 × 132 × 215 with overlap of 0.55 mm (over-contiguous) with no gap between slices producing a spatial resolution of 0.536 × 0.536 × 0.50 mm3/voxel. A 3D T1-weighted sequence (1 × 1 × 1 mm3, TR/TE = 6.39/2.9 ms) and a T2-weighted fluid attenuation inversion recovery (FLAIR) sequence (TR/TE/TI = 10,000/110/2800 ms; thickness 3.5 mm; 0.898 × 0.898 mm2) were also performed. Total WMH volume was assessed with automated methods using FLAIR and T1-weighted images, and adjusted for total intra-cranial volume (Wen and Sachdev 2004).
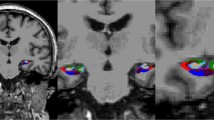
All images were co-registered using Statistical Parametric Mapping version 5 (SPM5) (Wellcome Department of Cognitive Neurology, London, UK, 1999) using T1 images as reference and SWI images as source. CMB were defined as round hypointense foci <10 mm in diameter seen on SWI, at least half surrounded by brain parenchyma and distinct from potential mimics such as iron and calcium deposits, vessel flow voids and bone (Greenberg et al. 2009). Symmetrical hypointensities in the globus pallidus likely representing calcium or non-hemorrhagic iron deposition were excluded. Hypointensities within the subarachnoid space were deemed to be pial blood vessels. The co-registered T1 images were used to determine precise anatomical localization and help exclude sulcal flow voids.
All images were analyzed using MRIcron version 15 (www.nitrc.org/projects/mricron/). For each image, the location, number and size (based on maximum diameter) of any identified CMB were recorded. The counted anatomical locations included the frontal, temporal, parietal and occipital lobes, the basal ganglia including the caudate, putamen and globus pallidus, the thalamus, brainstem and cerebellum. Any uncertain lesions were reviewed with an experienced neuroradiologist (JC). Twenty scans were excluded due to excessive motion or susceptibility artefacts. Participants whose scans were excluded did not significantly differ from the included participants in age, sex, blood pressure, education or cognitive impairment. All images were analyzed blind to clinical data.
Inter-observer reliability on 10 randomly selected scans (CMB absent vs. present) between the primary rater (AS) with an experienced neuroradiologist (JC), was Cohen’s κ = 0.615, corresponding to ‘good’ agreement. Intra-observer reliability on 20 randomly selected scans rated 2 months apart was Cohen’s κ = 1.0, corresponding to ‘very good’ agreement.
Neuropsychological assessment
Neuropsychological assessment, administered by trained research psychologists, consisted of a battery of tests grouped into cognitive domains: attention and processing speed (Digit Symbol-Coding (Wechsler 1997a), and Trail Making Test (TMT) A (Strauss et al. 2006)), memory (Logical Memory (Wechsler 1997b), Rey Auditory Verbal Learning Test (RAVLT), (Strauss et al. 2006) and Benton Visual Retention Test (Benton et al. 1996)), language (Boston Naming Test – 30 items (Kaplan 2001), Semantic Fluency (Animals) (Strauss et al. 2006)), visuospatial (Block Design (Wechsler 1981)), and executive function (Controlled Oral Word Association Test (FAS) (Strauss et al. 2006) and Trail Making Test (TMT) B (Strauss et al. 2006)). Raw test scores were transformed to z-scores using the baseline mean and SD values of a healthy reference subsample (n = 723 MAS participants). Domain scores were calculated by averaging the z-scores of component tests (except for visuo-spatial which was represented by a single test) and a global cognition score was calculated by averaging the domain scores. These composite scores were standardized so that the healthy reference group had means and SDs of 0 and 1 respectively.
Diagnostic classification was based on a multidisciplinary consensus panel consisting of old age psychiatrists, neuropsychiatrists and neuropsychologists using established criteria for MCI (Winblad et al. 2004) and DSM-IV (American Psychiatric Association 2000)/DSM-5 (American Psychiatric Association 2013) criteria for dementia conducted at each wave (Sachdev et al. 2010).
Assessment of covariates
Data on potential risk factors for CMB in the MAS study were collected through face-to-face assessments by trained research psychologists. Participants gave a blood sample for cholesterol, lipoprotein levels and genetic analysis including apolipoprotein gene (APOE) status. Hypertension was defined as a blood pressure of ≥140/90 mm/Hg taken as the mean of two seated readings or if the participant was ever diagnosed as having hypertension by their doctor. Diabetic status was determined by a prior medical diagnosis or a fasting blood glucose value >7 mmol/L.
Cardiovascular risk was computed based on the research of the Framingham Stroke Study (D’Agostino et al. 2008). Specifically, our variable was based on the 10-year risk prediction of general cardiovascular disease (http://www.framinghamheartstudy.org/risk-functions/cardiovascular-disease/index.php), using the following: sex; current smoking status (self-reported); diabetic status; systolic blood pressure; total cholesterol; high-density lipoprotein (HDL); currently on antihypertensive medication. When blood analyses were unavailable, Body Mass Index (BMI) was used instead of cholesterol and HDL data (as per Framingham protocol). See D’Agostino et al. (2008) for the weighting of the variables in the index. We excluded age from the score calculator as the range of ages of 70 years plus in our sample would have produced a ceiling effect (or at least very little variation) in the calculated risk score. Age was therefore included as a separate covariate in the analysis.
Statistical analysis
First, the numbers of CMB in the lobar and deep regions of the brain, as well as the whole brain (lobar, deep or infratentorial regions), were calculated. The lobar brain region was defined as comprising the frontal, temporal, parietal and occipital lobes, and the deep region comprising the basal ganglia and thalamus. A subgroup was created with CMB only in the lobar regions (strictly lobar distribution). Individuals with CMB in more than one regions were considered to have a mixed distribution. A strictly deep group was not created as there were insufficient numbers for meaningful analysis. An infratentorial group was not created as several studies reported that CMB in this location were not associated with cognitive impairment (Wu et al. 2014; Yakushiji et al. 2014). Two variables were formed to record CMB numbers: a binary variable of CMB present versus no CMB and a categorical three-level variable of 0 CMB, 1 CMB and multiple (≥ 2) CMB. The effects of multiple (≥ 2) CMB were explored as multiple microbleeds have been more consistently associated with cognitive deficits than a single CMB (Banerjee et al. 2016) and the rating of one CMB has been reported to be less reliable (Cordonnier et al. 2009; Gregoire et al. 2009).
Descriptive statistics for sample characteristics at baseline were presented for subsamples with and without CMB, and also for the subsample with multiple microbleeds. To examine differences in demographic and medical characteristics between those with no CMB and the other two subsamples, independent samples t-tests were used for continuous variables, Mann-Whitney U tests were used for non-normal continuous variables (absolute value of skewness >1) and Pearson’s chi-square tests or Fisher’s exact test for categorical variables.
Distributions of the dependent variables were inspected for normality and extreme values. Where necessary, distributions were transformed to more closely approximate the normal distribution (absolute value of skewness less than one). Also, to minimize the influence of extreme values on statistical outcomes, scores were Winsorized where necessary so that upper and lower values were reduced to three standard deviations above or below the mean.
A series of ANCOVAs (Analysis of Covariance) were used to examine differences in domain and global cognition measures at wave 2 between first, the no CMB group and the CMB present group and second, between the no CMB and the multiple CMB group. This was done for the CMB in the whole brain and separately for those with a strictly lobar distribution. Analyses were performed with covariates age, sex and education (Model 1), repeated with the additional covariates of cardiovascular risk score and APOE-ε4 (Model 2) and finally with the addition of markers of cerebral SVD; WMH volume and presence of lacunes (Model 3).
For the longitudinal analysis, linear mixed-models (LMMs) were used to examine the association of the two CMB variables with changes in global and domain scores across the three- time-points; waves 2, 3 and 4. In these analyses, wave was treated as a repeated measures factor, the CMB variables, between-subject factors, and interactions between the CMB and wave were included in the eqs. A random intercept term was also included. Estimated means were obtained for each of the time points and charted. Custom contrasts were then used to examine differences in cognitive changes (linear trend) between the CMB groups across the three waves. As for the cross-sectional analyses, the longitudinal analyses were performed with covariates age, sex and education (Model 1), repeated with the additional covariates cardiovascular risk score, APOE-ε4 (Model 2) and with the additional markers of cerebral SVD; WMH volume and presence of lacunes (Model 3). Analyses were performed both for CMB in the in the whole brain and separately for those with a strictly lobar distribution.
Logistic regression models were used to estimate the odds ratio (95% Confidence Interval) of developing dementia between waves 2 and 4 on the basis of the two CMB factors described above; the binary categorical variable and the 3-level factor (≥2 CMB vs none), across the whole brain and then for those with a strictly lobar distribution of CMB. These analyses were performed with the same covariates as the LMM analysis above (Models 1 to 3). Due to potential attrition bias, a conservative sensitivity analysis was performed, assuming that the proportion of non-completers at wave 4 was the same as the proportion of completers have dementia. This was done by re-running the logistic regression, randomly re-assigning non-completers to diagnostic categories (dementia vs. no dementia) to make up the correct assumed proportions.
All analyses were performed using the SPSS statistical package (IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp).
RESULTS
Of the 302 participants, 60 (20%) had at least one CMB. Of these 41 (68%) had CMB only in a lobar distribution; 4 (7%) had CMB only in a deep distribution and 15 (25%) had CMB in a mixed distribution and/or the infratentorial area. Half had only one CMB (n = 30, 50%) and half had more than one CMB (n = 30, 50%).
Baseline characteristics of the study population are reported in Table 1. Total WMH volume significantly differed between CMB present and CMB absent groups (U = 8667.5, z = 2.91, p = 0.004) and between the multiple CMB and CMB absent groups (U = 4571.5, z = 2.60, p = 0.009). No other demographic or medical characteristics significantly differed between the groups.
Cross-sectional results
When adjusted for age, sex and education, there was no association between either the presence of CMB or multiple CMB (vs no CMB) anywhere in the brain (whole brain) and global cognition or the domains attention and processing speed, language, visuospatial ability and memory (Table 2). There were significant associations between the presence of CMB and executive dysfunction, for both the presence of CMB (cf. no CMB; mean difference in z-score − 0.35; 95%CI -0.63 to −0.07, p = 0.015) and when examining participants with multiple (≥2) microbleeds (cf. no CMB; mean difference in z-score − 0.53; 95%CI -0.90 to −0.17, p = 0.004).
These associations remained significant when further adjusted for the presence of cardiovascular risk score and APOE-ε4 status (Model 2) (Table 3). Moreover, when additionally adjusted for the markers of SVD; WMH volume and the presence of lacunes (Model 3), those individuals with multiple microbleeds retained a significant association with executive function. (cf. no CMB; mean difference in z-score − 0.41; 95%CI -0.78 to −0.03, p = 0.035).
Longitudinal results
Results for Model 1 (adjusted for age, sex and education) are presented in Table 4 and displayed in Fig. 1, for CMB across the whole brain. The mean duration between wave 2 and wave 4 was 47.6 months (SD 1.9; range 40–57). Thirty-four individuals (11%) did not attend for wave 4 cognitive testing. Compared to those who attended wave 4, at baseline (wave 2) these 34 participants were significantly older, were more likely to have received a diagnosis of MCI and had worse cognition (MMSE and significantly reduced impairments in global cognition, executive function and memory and a trend towards worse attention and processing speed). They did not differ significantly in their gender, cardiovascular risk score, education, WMH volume or proportion with lacunes.
There was a strong association between CMB and longitudinal change in visuospatial ability. Participants with any CMB (whole brain) had a significantly faster decline (linear trend) in visuospatial function score compared to those with no CMB (Contrast estimate 0.26; SE 0.11; t(531.92) = 2.27, p = 0.024), adjusted for age, gender and education. This association remained significant in Model 3, after further adjusting for presence of cardiovascular risk, APOE-ε4 status, WMH and lacunes (Contrast estimate 0.28; SE 0.12; t(496.61) = 2.41, p = 0.016). These associations remained significant when examining only those participants with a strictly lobar distribution of microbleeds. These associations, although similar in magnitude, were no longer statistically significant when those with multiple whole brain CMB were analyzed, with only a non-significant trend toward significance (Contrast estimate 0.28; SE 0.15; t(532.63) = 1.82, p = 0.069). Please see Supplemental Table 2 for full results.
There was an association between CMB and longitudinal change in executive function. Participants with multiple CMB (whole brain) had a significantly slower decline (linear trend) in executive function score compared to those with no CMB (Contrast estimate −0.26; SE 0.13; t(511.22) = −1.99, p = 0.047, adjusted for age, gender and education. This association no longer remained significant after further adjusting for the presence of cardiovascular risk, WMH and lacunes (Model 3). There was not an association between executive function and participants with any CMB across the whole brain or those with CMB in a strictly lobar distribution. Please see Supplemental Table 2 for full results.
Whole brain CMB were not associated with decline in global cognition, attention and processing speed, language or memory.
Dementia diagnosis
Table 5 reports the association of CMB with incident dementia. Nine percent of those without CMB had dementia at wave 4, compared to 15% of those with a CMB. This was not a significant result however; (presence of any CMB cf. CMB OR 1.77; 95%CI 0.70–4.48, p = 0.229), when adjusted for age, sex and education. These results did not change when only the groups with multiple microbleeds or those with a strictly lobar distribution of CMB were examined. Nor did the results significantly change with the sensitivity analysis (presence of any CMB cf. no CMB OR 1.54; 95%CI 0.65–3.65, p = 0.322).
DISCUSSION
In this population based study of non-demented older adults, we found a CMB prevalence of 20%. On cross-sectional analysis, after adjusting for demographics and cardiovascular risk factors, participants with CMB were more likely to have impairments in executive function compared to those without CMB. When examined longitudinally over four years this association was not sustained. In contrast to the cross-sectional results, on longitudinal analysis, those with CMB were more likely to have a greater decline in visuospatial function that those without CMB. Those with CMB at baseline were not more likely to have or develop dementia during the follow up period.
The literature on the association of CMB with cognitive impairment is complex and at times contradictory. Consistent with our cross sectional results however, the majority of studies that found an association between CMB and cognitive impairment (and a recent meta-analysis (Li et al. 2017)) reported deficits in executive function (Akoudad et al. 2016; Meier et al. 2014; Qiu et al. 2010; Werring 2004; Yates et al. 2014), with several finding that executive function was the only cognitive domain associated with CMB (Meier et al. 2014; Patel et al. 2013). The association between CMB and greater decline in visuospatial function over time is less frequently reported and most studies did not investigate this cognitive domain specifically. Several Asian studies however, did find an association of CMB with visuospatial decline in cohorts including subcortical vascular dementia (Won Seo et al. 2007), cognitively impaired elders (Hilal et al. 2014) and community dwelling aged (Chung et al. 2016).
Executive function is one of the most commonly reported domains to be impacted by SVD generally, supporting the argument that CMB may be a proxy for generalized vascular disease and its subsequent sequelae. Increased numbers of CMB are often in regions thought to be the neuroanatomical substrate of these cognitive domains, i.e., the frontal and basal ganglia, with the hypothesis that lesions in these regions disrupt critical frontal-subcortical circuits (Werring 2004) producing specific cognitive impairments (Martinez-Ramirez et al. 2014). We found that individuals with a strictly lobar distribution of CMB did not have impaired executive function. This may suggest the association is driven by deep cerebral SVD, or represent the limitations of a smaller sub-sample size. Participants with CMB had worse executive function at wave 2, but not a steeper decline in executive function with time, which may be explained by the particularly poor executive function results of the CMB group in wave 2. This affected the slope of the linear trend (Fig. 1) and meant not only did this group not decline faster, but paradoxically that in some analyses (those with multiple microbleeds across the whole brain) participants with CMB had an improvement in executive function over time (compared to those without CMB). We assessed visuospatial ability through Block Design, but this test incorporates a range of other cognitive abilities, including aspects of executive function and processing speed which are a particularly important contributor to performance above age 75 (Brown et al. 2012; Lezak 2004). We categorized the tests into domains a priori according to the principal cognitive function that they represented according to convention and psychological theory. However, neuropsychological tests are multifactorial in structure and even though a test may be designed to focus on one aspect of cognition, test performance involves multiple cognitive processes (McFarland 2013). This overlap and lack of clear delineation between different cognitive domains is one of the reasons for the complex and often contradictory results in the published literature. Indeed, when a principal component analysis was previously performed on the MAS data (unpublished) the first principal component comprised attention/processing speed, executive function and visuospatial factors. Interestingly Chung et al. (2016) reported that strictly lobar CMB were associated with impairments in a visuospatial executive function, but not with a verbal executive function domain.
There was a strong association between CMB presence and increased WMH volume. WMH are a well-established marker of SVD and our finding that CMB is associated with cognitive impairment independent of WMH volume supports the thesis that CMB produce cognitive impairment through direct mechanisms as well as being a general marker of overall cerebral vascular pathology. Several other studies have now shown that CMB are associated with cognitive impairment independent of WMH volume (Akoudad et al. 2016; Qiu et al. 2010; van Norden et al. 2011). Supportive evidence for this includes histopathological studies showing there is gliosis, necrosis or infarction damaging white matter around areas of CMB-related atherosclerotic or amyloid deposition damage (Fazekas et al. 1999; Schrag et al. 2010). CMB have also been shown to be correlated with white matter ultrastructure damage on diffusion tensor imaging (DTI) (Patel et al. 2013).
We did not find an association between CMB and incident dementia. Ding et al. (2017) reported higher dementia risk in their participants with three or more microbleeds. In models adjusted for other SVD markers, two other large population studies, the Framingham (Romero et al. 2017) and Rotterdam (Akoudad et al. 2016) studies reported associations between incident dementia and deep, but not lobar microbleeds. This was explained in two ways; that those with strictly lobar CMB may have lower burden of hypertensive arteriopathy and so may take longer for dementia to develop (Romero et al. 2017) and, that participants with deep or infratentorial microbleeds often had a higher microbleed count and more mixed microbleed location, potentially representing more severe pathology (Akoudad et al. 2016).
The associations of regional CMB with impairments is a significant interest of current research (Akoudad et al. 2016; Gregoire et al. 2013; Qiu et al. 2010; van Norden et al. 2011). In a meta-analysis, Wu et al. (2014), reported associations of deep and lobar CMB with cognitive impairment, but not infratentorial lesions. There may also be a differential expression of cognitive impairment depending on region, potentially related to the difference in underlying pathology, with CMB in the subcortical and deep regions being associated with atherosclerotic small vessel disease and CMB in lobar regions more likely associated with CAA (Greenberg et al. 2009; Vernooij et al. 2008). Different geographical populations also have a different distribution patterns of CMB, with mainly lobar CMB in Western populations and mainly deep or infratentorial in Asian populations (Yakushiji et al. 2012). An additional mechanism for CMB-associated cognitive impairment is the contribution of amyloid pathology through CAA, a very common pathological finding in AD (Martinez-Ramirez et al. 2014). The fact that visuospatial decline was seen even after adjustment for WMH and other markers of CVD disease and this relationship was sustained in those with strictly lobar CMB suggests that AD pathology may be a contributory factor. With significant AD pathology, we may have expected to see an association with memory impairment however, which did not occur.
The main limitation of our study was its size and although sufficiently powered for the main analysis, it was underpowered to look at the relative contribution of those with multiple CMB, with some recent studies suggesting this group plays a disproportionate role in cognitive impairment and dementia (Akoudad et al. 2016; Ding et al. 2017; Hilal et al. 2014). It was also underpowered to detect differences in those with a strictly deep distribution of microbleeds, a relatively rare finding in our cohort, with only four participants in this group. Another limitation was the attrition of the sample, with those not followed up being more cognitively impaired at baseline and their absence from the longitudinal data being a probable source of bias. Survival bias is a common issue in longitudinal studies, with participants who drop out of observational cohort studies having an increased likelihood of progression to dementia (Jacova et al. 2006), suggesting that our results may be an underestimate of the true effect. Eleven percent attrition over four years is reasonable and the use of mixed modelling techniques best accounts for missing data. Finally, we were not able to include an AD biomarker, such as amyloid PET. This could help delineate the relative contributions of CVD and AD pathology to cognitive impairment (Koncz and Sachdev 2018), via the presence of CMB.
Strengths of this study were its ability to control for a number of moderating and confounding factors. Full neuropsychological evaluation allowed detailed assessment of different cognitive domains and the use of multiple tests within a cognitive domain gave a more robust assessment. The use of SWI also allows a much greater detection of potential CMB less sensitive sequences such as gradient recalled echo (GRE) (Nandigam et al. 2008).
Further longitudinal studies are required to elicit the nuanced contribution of CMB to cognitive impairment and dementia, particularly with respect to individual cognitive domains. Future studies should be large enough to stratify the number of CMB into meaningful categories and the use of multicenter collaborations is recommended. This should allow meta-analysis (Charidimou et al. 2016) and account for the role of geographical difference and variations in neuropsychological tests in CMB associated cognitive impairment. CMB are an important independent predictor of specific cognitive impairments in an ageing population cohort and should be included when considering the burden of cerebrovascular disease.
References
Akoudad, S., Wolters, F. J., Viswanathan, A., & et al. (2016). Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurology, https://doi.org/10.1001/jamaneurol.2016.1017.
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC, USA: American Psychiatric Publishing.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author.
Ayaz, M., Boikov, A. S., Haacke, E. M., Kido, D. K., & Kirsch, W. M. (2010). Imaging cerebral microbleeds using susceptibility weighted imaging: one step toward detecting vascular dementia. Journal of Magnetic Resonance Imaging, 31(1), 142–148. https://doi.org/10.1002/jmri.22001.
Banerjee, G., Wilson, D., Jäger, H. R., & Werring, D. J. (2016). Novel imaging techniques in cerebral small vessel diseases and vascular cognitive impairment. Biochimica et Biophysica Acta, 1862(5), 926–938. https://doi.org/10.1016/j.bbadis.2015.12.010.
Benton, A. L., Sivan, A. B., & Spreen, O. (1996). Der Benton Test (7th ed.). Bern: Huber.
Brown, L. A., Brockmole, J. R., Gow, A. J., & Deary, I. J. (2012). Processing speed and visuospatial executive function predict visual working memory ability in older adults. Experimental Aging Research, 38(1), 1–19. https://doi.org/10.1080/0361073x.2012.636722.
Charidimou, A., Soo, Y., Heo, J. H., & Srikanth, V. (2016). A call for researchers to join the META-MICROBLEEDS Consortium. The Lancet Neurology, 15(9), 900. https://doi.org/10.1016/s1474-4422(16)30124-7.
Chiang, G. C., Cruz Hernandez, J. C., Kantarci, K., Jack, C. R., Weiner, M. W., & Initiative, A. s. D. N. (2015). Cerebral Microbleeds, CSF p-Tau, and Cognitive Decline: Significance of Anatomic Distribution. American Journal of Neuroradiology, 36(9), 1635–1641. https://doi.org/10.3174/ajnr.A4351.
Chung, C. P., Chou, K. H., Chen, W. T., Liu, L. K., Lee, W. J., Chen, L. K., et al. (2016). Strictly Lobar Cerebral Microbleeds Are Associated With Cognitive Impairment. Stroke, 47(10), 2497–2502. https://doi.org/10.1161/strokeaha.116.014166.
Cordonnier, C., Potter, G. M., Jackson, C. A., Doubal, F., Keir, S., Sudlow, C. L., et al. (2009). Improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS). Stroke, 40(1), 94–99. https://doi.org/10.1161/STROKEAHA.108.526996.
D'Agostino Sr., R. B., Vasan, R. S., Pencina, M. J., Wolf, P. A., Cobain, M., Massaro, J. M., et al. (2008). General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation, 117(6), 743–753. https://doi.org/10.1161/CIRCULATIONAHA.107.699579.
Ding, J., Sigurethsson, S., Jonsson, P. V., Eiriksdottir, G., Meirelles, O., Kjartansson, O., et al. (2017). Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology, 88(22), 2089–2097. https://doi.org/10.1212/wnl.0000000000003983.
Fazekas, F., Kleinert, R., Roob, G., Kleinert, G., Kapeller, P., Schmidt, R., et al. (1999). Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. American Journal of Neuroradiology, 20(4), 637–642.
Greenberg, S. M., Vernooij, M. W., Cordonnier, C., Viswanathan, A., Al-Shahi Salman, R., Warach, S., et al. (2009). Cerebral microbleeds: a guide to detection and interpretation. The Lancet Neurology, 8(2), 165–174. https://doi.org/10.1016/S1474-4422(09)70013-4.
Gregoire, S. M., Chaudhary, U. J., Brown, M. M., Yousry, T. A., Kallis, C., Jager, H. R., et al. (2009). The Microbleed Anatomical Rating Scale (MARS): Reliability of a tool to map brain microbleeds. Neurology, 73(21), 1759–1766. https://doi.org/10.1212/wnl.0b013e3181c34a7d.
Gregoire, S. M., Scheffler, G., Jäger, H. R., Yousry, T. A., Brown, M. M., Kallis, C., et al. (2013). Strictly lobar microbleeds are associated with executive impairment in patients with ischemic stroke or transient ischemic attack. Stroke, 44(5), 1267–1272. https://doi.org/10.1161/STROKEAHA.111.000245.
Haller, S., Bartsch, A., Nguyen, D., Rodriguez, C., Emch, J., Gold, G., et al. (2010). Cerebral microhemorrhage and iron deposition in mild cognitive impairment: susceptibility-weighted MR imaging assessment. Radiology, 257(3), 764–773. https://doi.org/10.1148/radiol.10100612.
Hilal, S. M. M. P. H., Saini, M. M. D., Tan, C. S. P., Catindig, J. A. M. D., Koay, W. I. B., Niessen, W. J. P., et al. (2014). Cerebral Microbleeds and Cognition: The Epidemiology of Dementia in Singapore Study. Alzheimer Disease & Associated Disorders, 28(2), 106–112.
Jacova, C., Hsiung, G. Y., & Feldman, H. H. (2006). Dropouts and refusals in observational studies: lessons for prevention trials. Neurology, 67(9 Suppl 3), S17–S20.
Kaplan, E. (2001). The Boston Naming Test. Philadelphia: Lippincott Williams Wilkins.
Kirsch, W., McAuley, G., Holshouser, B., Petersen, F., Ayaz, M., Vinters, H. V., et al. (2009). Serial susceptibility weighted MRI measures brain iron and microbleeds in dementia. Journal of Alzheimer's Disease, 17(3), 599–609. https://doi.org/10.3233/JAD-2009-1073.
Koncz, R., & Sachdev, P. S. (2018). Are the brain's vascular and Alzheimer pathologies additive or interactive? Current Opinion in Psychiatry, 31(2), 147–152. https://doi.org/10.1097/yco.0000000000000395.
Lezak, M. D. (2004). Neuropsychological assessment. USA: Oxford University Press.
Li, X., Yuan, J., Yang, L., Qin, W., Yang, S., Li, Y., et al. (2017). The significant effects of cerebral microbleeds on cognitive dysfunction: An updated meta-analysis. PLoS One, 12(9), e0185145. https://doi.org/10.1371/journal.pone.0185145.
Martinez-Ramirez, S., Greenberg, S. M., & Viswanathan, A. (2014). Cerebral microbleeds: overview and implications in cognitive impairment. Alzheimer's Research & Therapy, 6(3), 33. https://doi.org/10.1186/alzrt263.
McFarland, D. J. (2013). Modeling Individual Subtests of the WAIS IV with Multiple Latent Factors. PLoS One, 8(9), e74980. https://doi.org/10.1371/journal.pone.0074980.
Meier, I. B., Gu, Y., Guzaman, V. A., Wiegman, A. F., Schupf, N., Manly, J. J., et al. (2014). Lobar microbleeds are associated with a decline in executive functioning in older adults. Cerebrovascular Diseases, 38(5), 377–383. https://doi.org/10.1159/000368998.
Miwa, K., Tanaka, M., Okazaki, S., Yagita, Y., Sakaguchi, M., Mochizuki, H., et al. (2014). Multiple or mixed cerebral microbleeds and dementia in patients with vascular risk factors. Neurology, 83(7), 646–653. https://doi.org/10.1212/wnl.0000000000000692.
Nandigam, R. N. K., Viswanathan, A., Delgado, P., Skehan, M. E., Smith, E. E., Rosand, J., et al. (2008). MR Imaging Detection of Cerebral Microbleeds: Effect of Susceptibility-Weighted Imaging, Section Thickness, and Field Strength. American Journal of Neuroradiology, 30(2), 338–343. https://doi.org/10.3174/ajnr.a1355.
Patel, B., Lawrence, A. J., Chung, A. W., Rich, P., Mackinnon, A. D., Morris, R. G., et al. (2013). Cerebral microbleeds and cognition in patients with symptomatic small vessel disease. Stroke, 44(2), 356–361. https://doi.org/10.1161/STROKEAHA.112.670216.
Poels, M. M., Ikram, M. A., van der Lugt, A., Hofman, A., Niessen, W. J., Krestin, G. P., et al. (2012). Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology, 78(5), 326–333. https://doi.org/10.1212/WNL.0b013e3182452928.
Poels, M. M., Vernooij, M. W., Ikram, M. A., Hofman, A., Krestin, G. P., van der Lugt, A., et al. (2010). Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke, 41(10 Suppl), S103–S106. https://doi.org/10.1161/STROKEAHA.110.595181.
Qiu, C., Cotch, M. F., Sigurdsson, S., Garcia, M., Klein, R., Jonasson, F., et al. (2008). Retinal and Cerebral Microvascular Signs and Diabetes: The Age, Gene/Environment Susceptibility-Reykjavik Study. Diabetes, 57(6), 1645–1650. https://doi.org/10.2337/db07-1455.
Qiu, C., Cotch, M. F., Sigurdsson, S., Jonsson, P. V., Jonsdottir, M. K., Sveinbjrnsdottir, S., et al. (2010). Cerebral microbleeds, retinopathy, and dementia: the AGES-Reykjavik Study. Neurology, 75(24), 2221–2228. https://doi.org/10.1212/WNL.0b013e3182020349.
Romero, J. R., Beiser, A., Himali, J. J., Shoamanesh, A., DeCarli, C., & Seshadri, S. (2017). Cerebral microbleeds and risk of incident dementia: the Framingham Heart Study. Neurobiology of Aging, 54, 94–99. https://doi.org/10.1016/j.neurobiolaging.2017.02.018.
Sachdev, P. S., Brodaty, H., Reppermund, S., Kochan, N. A., Trollor, J. N., Draper, B., et al. (2010). The Sydney Memory and Ageing Study (MAS): methodology and baseline medical and neuropsychiatric characteristics of an elderly epidemiological non-demented cohort of Australians aged 70–90 years. International Psychogeriatrics, 22(08), 1248–1264. https://doi.org/10.1017/s1041610210001067.
Schrag, M., McAuley, G., Pomakian, J., Jiffry, A., Tung, S., Mueller, C., et al. (2010). Correlation of hypointensities in susceptibility-weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy: a postmortem MRI study. Acta Neuropathologica, 119(3), 291–302. https://doi.org/10.1007/s00401-009-0615-z.
Shoamanesh, A., & Benavente, O. (2011). Cerebral Microbleeds: Histopathological Correlation of Neuroimaging. Neurology, 76(9), A308–A308.
Strauss, E., Sherman, E. M. S., & Spreen, O. (2006). A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary (3rd ed.). New York, NY, USA: Oxford University Press.
van Norden, A. G., van den Berg, H. A., de Laat, K. F., Gons, R. A., van Dijk, E. J., & de Leeuw, F. E. (2011). Frontal and temporal microbleeds are related to cognitive function: the Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) Study. Stroke, 42(12), 3382–3386. https://doi.org/10.1161/STROKEAHA.111.629634.
Vernooij, M. W., van der Lugt, A., Ikram, M. A., Wielopolski, P. A., Niessen, W. J., Hofman, A., et al. (2008). Prevalence and risk factors of cerebral microbleeds: The Rotterdam Scan Study. Neurology, 70(14), 1208–1214. https://doi.org/10.1212/01.wnl.0000307750.41970.d9.
Wechsler, D. (1981). WAIS-R manual. New York: The Psychological Corporation.
Wechsler, D. (1997a). Wechsler Adult Intelligence Scale-III (WAIS-III) (3rd ed.). San Antonio, TX, USA: The Psychological Corporation.
Wechsler, D. (1997b). Wechsler Memory Scale (3rd ed.). San Antonio, TX, USA: The Psychological Corporation.
Wen, W., & Sachdev, P. (2004). The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. NeuroImage, 22(1), 144–154. https://doi.org/10.1016/j.neuroimage.2003.12.027.
Werring, D. J. (2004). Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain, 127(10), 2265–2275. https://doi.org/10.1093/brain/awh253.
Winblad, B., Palmer, K., Kivipelto, M., Jelic, V., Fratiglioni, L., Wahlund, L. O., et al. (2004). Mild cognitive impairment - beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine, 256(3), 240–246. https://doi.org/10.1111/j.1365-2796.2004.01380.x.
Won Seo, S., Hwa Lee, B., Kim, E. J., Chin, J., Sun Cho, Y., Yoon, U., et al. (2007). Clinical Significance of Microbleeds in Subcortical Vascular Dementia. Stroke, 38(6), 1949–1951. https://doi.org/10.1161/strokeaha.106.477315.
Wu, R. H., Feng, C., Zhao, Y., Jin, A. P., Fang, M., & Liu, X. (2014). A Meta-Analysis of Association between Cerebral Microbleeds and Cognitive Impairment. Medical Science Monitor, 20, 2189–2198.
Yakushiji, Y., Charidimou, A., Hara, M., Eriguchi, M., Noguchi, T., Nishihara, M., et al. (2015). Small vessel disease the concept of "total small vessel disease score" in healthy adults: Validation in the Kashima Scan study. [Conference Abstract]. International Journal of Stroke, 10, 374.
Yakushiji, Y., Eriguchi, M., Nanri, Y., Hara, H., Charidimou, A., Werring, D. J., et al. (2014). Basal Ganglia Cerebral Microbleeds and Global Cognitive Function: The Kashima Scan Study. Journal of Stroke and Cerebrovascular Diseases, https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.09.015.
Yakushiji, Y., Noguchi, T., Hara, M., Nishihara, M., Eriguchi, M., Nanri, Y., et al. (2012). Distributional impact of brain microbleeds on global cognitive function in adults without neurological disorder. Stroke, 43(7), 1800–1805. https://doi.org/10.1161/strokeaha.111.647065.
Yates, P. A., Villemagne, V. L., Ellis, K. A., Desmond, P. M., Masters, C. L., & Rowe, C. C. (2014). Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations. Frontiers in Neurology, 4, 205. https://doi.org/10.3389/fneur.2013.00205.
Acknowledgements
The authors thank all participants and their supporters in the Sydney Memory and Ageing Study (MAS), and the MAS research team.
Funding
This study was supported by the National Health and Medical Research Council (NHMRC) of Australia Program Grant (no. 350833) and Capacity Building Grant (no. 568940). Dr. Paradise was funded by an Australian National University/NHMRC NNIDR – DCRC Early Diagnosis and Prevention Shared Grant. Dr. Crawford and Dr. Kochan were supported by NHMRC Program Grant (no. 568969).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Matt Paradise, author Adam Seruga, author John D. Crawford, author Joga Chaganti, author Anbupalam Thalamuthu, author Nicole A. Kochan, author Henry Brodaty, author Wei Wen and author Perminder S. Sachdev declare they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
Electronic supplementary material
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Paradise, M., Seruga, A., Crawford, J.D. et al. The relationship of cerebral microbleeds to cognition and incident dementia in non-demented older individuals. Brain Imaging and Behavior 13, 750–761 (2019). https://doi.org/10.1007/s11682-018-9883-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-018-9883-3