Abstract
Several resting-state neuroimaging studies have indicated abnormal regional homogeneity (ReHo) in chronic schizophrenia; however, little work has been conducted to investigate naïve patients with first-episode schizophrenia (FES). Even less investigated is the association between ReHo measures and clinical symptom severity in naïve patients with FES. The current study evaluated ReHo alterations in whole brain, and assessed the correlations between ReHo measures and clinical variables in naïve patients with FES. Forty-four naïve patients with FES and 26 healthy controls (HC) underwent resting-state functional magnetic resonance imaging (rs-fMRI). Group-level analysis was utilized to analyze the ReHo differences between FES and HC in a voxel-by-voxel manner. Severity of symptoms was evaluated using a five-factor model of the Positive and Negative Syndrome Scale (PANSS). The correlation between the severity of symptoms and ReHo map was examined in patients using voxel-wise correlation analyses within brain areas that showed a significant ReHo alteration in patients compared with controls. Compared with the healthy control group, the FES group showed a significant decrease in ReHo values in the left medial frontal gyrus (MFG), right precentral gyrus, left superior temporal gyrus (STG), left left middle temporal gyrus (MTG), left thalamus, and significant increase in ReHo values in the left MFG, left inferior parietal lobule (IPL), left precuneus, and right lentiform nucleus (LN). In addition, the correlation analysis showed the PANSS total score negatively correlated with ReHo in the right precentral gyrus and positively correlated with ReHo in the left thalamus, the positive factor positively correlated with ReHo in the right thalamus, the disorganized/concrete factor positively correlated with ReHo in left posterior cingulate gyrus (PCG), the excited factor positively correlated with ReHo in the left precuneus, and the depressed factor negatively correlated with ReHo in the right postcentral gyrus and positively correlated with ReHo in the right thalamus. Our results indicate that widespread ReHo abnormalities occurred in an early stage of schizophrenic onset, suggesting a potential neural basis for the pathogenesis and symptomatology of schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is a chronic, severe, and disabling mental disease, which affects about 1% of the entire population and imposes a considerable burden on patients, their families, and society (Tandon et al. 2010). The etiology and pathogenesis of schizophrenia are not yet clear. Given that the brain consumes a considerable amount of energy even in the absence of tasks (when a subject is awake and in the resting state), studies of the resting-state brain function are likely to provide important information (Raichle and Gusnard 2005). Numerous studies of electroencephalography (EEG) (Pascual-Marqui et al. 1999), positron-emission tomography (PET) (Levy et al. 1992) and single photon emission computed tomography (SPECT) (Catafau et al. 1994) have provided evidence that abnormal spontaneous neuronal activity is potentially the underlying pathophysiologic mechanism of schizophrenia. Thus, studying the resting-state electric activity is crucial to understanding the neurobiological substrate of schizophrenia because the abnormal brain activity in the resting state may play a crucial role in the neurobehavioral manifestations of schizophrenia (Xiao et al. 2017). Abnormal spontaneous neuronal activity in schizophrenia has been widely reported in the literature; however, the results are inconsistent (Ebmeier et al. 1993; Kaplan et al. 1993; Boutros et al. 2008; Parellada et al. 1994; Knott et al. 2001; Lee et al. 2006).
Resting-state functional magnetic resonance imaging (rs-fMRI), a novel magnetic resonance imaging technique, has been successfully implemented to reveal regional activity and functional connectivity in schizophrenia patients (Whitfield-Gabrieli et al. 2009; Liu et al. 2006). In recent rs-fMRI studies, a regional homogeneity (ReHo) approach has been adopted to analyze the blood oxygen level-dependent (BOLD) signal of the brain. Based on the previous findings that functional activity of fMRI data is more likely to occur in clusters of several spatially contiguous voxels than in a single voxel (Katanoda et al. 2002; Tononi et al. 1998), ReHo assumes that a given voxel is temporally similar to those of its neighbors, and could be used to detect the localized functional connectivity or synchronization of information processing with little interference from external stimuli, so it is better than conventional MRI. Moreover, increasing evidences show that local functional homogeneity has neurobiological relevance to anatomical, developmental and neurocognitive factors, leading to its contribution to serving as a neuroimaging marker to investigate the human brain function, behaviors and neuropsychiatric disorders (Jiang and Zuo 2016; Jiang et al. 2015a). Futhermore, researchers have demonstrated ReHo is an rs-fMRI metric with high test-retest reliability because it has high robustness against tempo-spatial noise and outliers (Zuo et al. 2013; Zuo and Xing 2014), which is important due to its direct implication on its clinical applications. A recent meta-analysis (Xiao et al. 2017) of ReHo data, pooling data of 316 schizophrenic patients from 7 studies, concluded that several regions showed abnormal synchronization compared with healthy controls. In these studies, schizophrenia patients showed increased ReHo in right superior frontal gyrus and right superior temporal gyrus (STG), and decreased ReHo in left fusiform gyrus, left STG, left postcentral gyrus, and right precentral gyrus, which suggest that altered spontaneous brain activity in the resting state may be related to the pathophysiology of schizophrenia (Malaspina et al. 2004). However, the results can be challenged due to lack of control for confounding factors such as illness duration, long-term exposure to antipsychotic medication, and possible progressive brain atrophy (Lui et al. 2010; Pardo et al. 2011; Hu et al. 2016).
The study of naïve patients with first-episode schizophrenia (FES) is particularly instrumental in understanding the neurobiology of schizophrenia, to some extent due to the opportunity to circumvent the impact of potential confounders including illness chronicity, medication effects, and the medical and psychiatric comorbidities that accompanied by chronicity of illness (Buckley and Evans 2006). Compared to many studies with chronic patients, relatively few studies have been undertaken to explore patients with naïve patients with FES (Xiao et al. 2017; Jiang et al. 2015b; Yang et al. 2014; Li et al. 2015). The results have also been inconsistent. For example, both decreased and increased ReHo have been reported in patients with FES (Chen et al. 2013; Fang 2013). However, some authors only found increased ReHo (Xiong 2016) or only found decreased ReHo (Jiang et al. 2010). Thus, there is a need to explore ReHo alterations in patients with FES.
As far as we know, studies on the relationship between ReHo and clinical symptoms in FES are really sparse. Though several studies have examined the relationship in chronic schizophrenia, results are mixed. For instance, the ReHo in the dorso-medial prefrontal cortex showed a negative correlation with delusion severity in one study (Gao et al. 2015). However, no associations between ReHo map and the clinical symptoms were identified in several other studies (Liu et al. 2016; Yu et al. 2013; Cui et al. 2016). One study showed (Yu et al. 2013) that abnormal ReHo was found to be associated with impaired ability of specific information processing and integration in chronic schizophrenia. The inconsistent conclusion from these studies likely attributes to the small sample size, different patient characteristics (naïve vs medicated, acute vs chronic, in active phase vs in remission, etc), limited brain regions investigated, and other confounding factorsassociated with the illness chronicity and medication. In the current study, which has a relatively larger sample than those used in previous studies of naïve patients, we also examined correlations between ReHo alteration and the psychotic symptoms in patients with FES, using the five-factor model of the Positive and Negative Syndrome Scale (PANSS), better capturing the PANSS structure in patients with schizophrenia (Jerrell and Hrisko 2013a; Jerrell and Hrisko 2013b; Lancon et al. 2000; Wallwork et al. 2012).
Materials and methods
Subjects
Seventy-two naïve Chinese inpatients (48 males) with a first episode were recruited at the Nanjing Brain Hospital from February, 2014 to March, 2015. All patients were diagnosed using Structured Clinical Interview for DSM-IV Patient Edition (SCID-I/P), and met the criteria for schizophrenia. The patients had a mean ± SD age of 24.2 ± 6.0 years (range, 17–44), a mean ± SD duration of illness of 10.6 ± 9.1 months (range, 1–24), and a mean ± SD education of 12.2 ± 3.1 years (range, 2–20).
Thirty-one healthy controls (18 males), matched for age, gender, and education, were recruited from Nanjing by advertisements posted in the local community. Current mental status and personal or family history of any mental disorder were assessed by a trained and experienced psychiatrist. None of the controls presented a personal or family history of psychiatric disorder. The controls had a mean ± SD age of 22.3 ± 3.6 years (range, 18–31) and a mean ± SD education of 13.7 ± 2.9 years (range, 9–20).
General inclusion criteria for all the groups included (1) aged 15–45 years, (2) right-handed, (3) an ability to understand the survey instructions and contents. General exclusion criteria included a history of significant head injury, seizures, cerebrovascular disease, other neurological disease, impaired thyroid function, learning difficulties, and DSM-IV criteria for alcohol or substance abuse or dependence in the past year.
Psychopathological assessment in patients
Two specialists who were unaware of the study objectives rated the patient’s psychopathology using the PANSS (Kay et al. 1987, 1988). PANSS is a widely used multidimensional instrument for evaluating psychotic symptoms and a five-factor model is regarded to better capture the PANSS structure in patients with schizophrenia (Jerrell and Hrisko 2013a, b; Lancon et al. 2000; Wallwork et al. 2012). The PANSS provided the total score and five factor scores including positive (delusions, hallucinations, grandiosity, and unusual thought content), negative (blunted affect, emotional withdrawal, poor rapport, passive/apathetic social withdrawal, lack of spontaneity, and motor retardation), disorganized/concrete (conceptual disorganization, difficulty in abstract thinking, and poor attention), excited (excitement, hostility, uncooperativeness, and poor impulse control), and depressed factor (anxiety, guilt feelings, and depression).
Imaging acquisition
MRI data were acquired using a 3.0-T Siemens Verio scanner with a standard head coil padded with foam to reduce head motion and scanner noise. Sagittal three-dimensional T1-weighted images were acquired using a brain volume sequence with the following parameters: repetition time (TR) = 2530 ms; echo time (TE) = 2.3 ms; inversion time = 900 ms; flip angle (FA) = 7°; field of view (FOV) = 256 mm × 256 mm; matrix = 256 × 256; slice thickness = 1 mm, gap = 0.5 mm; 192 sagittal slices; and acquisition time = 353 s. RS functional BOLD images were acquired using a gradient-echo single-shot echo planar imaging sequence with the following parameters: TR/TE = 2000/30 ms, FOV = 240 mm × 240 mm, matrix = 64 × 64, FA = 90°, slice thickness = 4 mm, no gap, 30 interleaved transverse slices, 171 volumes, and acquisition time = 346 s. All subjects were instructed to lie still with their eyes closed, relax, move as little as possible, think of nothing, and not fall asleep in particular during RS-fMRI data acquisition (Biswal et al. 1995; Zang et al. 2004; Greicius et al. 2003). All MRI were visually inspected to ensure that only images without visible artifacts were included in subsequent analyses.
Data preprocessing and processing
The fMRI data preprocessing was performed based on Matlab 2012a platform and Data Processing Assistant for Resting-State fMRI (DPARSF, Advance edition) (Chao-Gan and Yu-Feng 2010; Yan et al. 2016). The data were calculated in original space warped by diffeomorphic anatomical registration via an exponentiated Lie algebra (DARTEL). The first ten volumes for each participant were discarded to allow the signal to reach equilibrium and the participants to adapt to the scanning noise. The remaining volumes were corrected for the acquisition time delay between slices. All participants’ resting-state data were within the defined motion thresholds (i.e., translational or rotational motion parameters <2.5 mm or 2.5°). Then, images were spatially realigned to the first image of each dataset and movement parameters were assessed for each subject and corrected using the Friston 24 approach (Friston et al. 1996). The data were scrubbed to further correct for movement artefacts using Frame-wise Displacement (FD, 0.5 mm) using a linear interpolation approach (Power et al. 2012). Several nuisance covariates (six motion parameters, their 1st time derivatives, the global brain signal, the white matter signal, and the cerebrospinal fluid signal) were regressed out as nuisance covariates. The datasets were then band-pass filtered in a frequency range of 0.01–0.08 Hz. ReHo was performed on a voxel-by-voxel basis by calculating Kendall’s coefficient of concordance of time series within a cluster of neighboring voxels in original space, and the details of the method were described elsewhere (Zang et al. 2004). Cubic clusters of 27 voxels were used and the ReHo value of every cubic cluster was assigned to the central voxel (Zang et al. 2004). To minimize the whole-brain effect, voxel ReHo values were scaled by dividing each subject’s value by the mean value of their whole-brain ReHo. In the normalization step, DARTEL (Ashburner 2007) was used to normalize the data using each subject’s structural image before removing linear drift. Then, each ReHo map was spatially smoothed with a Gaussian filter of 4 mm full width at half maximum (FWHM). Finally, we obtained the swmReHo for statistical analysis.
Statistical analysis
Group-level analyses were carried out to examine brain regions with significant detectable ReHo abnormalities in schizophrenia. The ReHo maps were compared between FES and HC using permutation-based statistical analysis with 5000 permutations, with age, gender, education, and the probability of gray matter as covariates. The statistical threshold was set at p < 0.01, correcting for multiple comparisons by threshold-free cluster enhancement (TFCE) (Smith and Nichols 2009). The resultant significant ReHo decrease/increase areas in patients were used as inclusion masks in the following voxel-wise correlation analysis.
Voxelwise correlation analyses were carried out to explore the correlation between the ReHo map and the total score and each component of PANSS (positive, negative, disorganized–concrete, excited, and depressed factor score) in schizophrenia patients, respectively. Age, gender, education, and illness duration were entered into the model as covariates of no interest. The above-mentioned masks were used to include only those voxels with significant group differences. The permutation-based nonparametric inference was undertaken with 5000 permutations. The statistical significance level was thresholded for correction of multiple comparisons using TFCE of 0.05.
Statistical analysis of demographic and clinical data was performed using SPSS software (PASW Statistics for Windows, Version 18.0. Chicago: SPSS Inc). To compare the differences between the two groups, two-sample t-test was used for continuous variables and Chi-square test for categorical variables. The height threshold of statistical significance was set at p < 0.05.
Results
Demographic and clinical data
Data from 27 subjects (23 FES, 4 HC) were excluded due to excessive head movement (more than 2.5 mm maximum displacement in x, y, z or 2.5°of angular motion) during the fMRI acquisition; and additional 6 subjects (5 FES, 1 HC) were excluded due to poor quality of normalized images of ReHo. Therefore, the data from 70 subjects (44 FES, 26 HC) were available and used for all analyses. The subject characteristics of FES and HC groups are shown in Table 1. There were no significant differences between FES and HC groups in age (two-sample t-test, t = 0.98, p = 0.33), gender (Chi-square test,χ2 = 0.20, p = 0.66), and education (two-sample t-test, t = −1.50, p = 0.12).
Group differences in ReHo
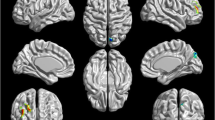
In the current study, decreased ReHo values were observed in the FES group compared with HC in the left medial frontal gyrus (MFG), right precentral gyrus, left STG, left middle temporal gyrus (MTG), and left thalamus (p < 0.01 for all, corrected by TFCE; Fig. 1 and Table 2). In contrast, increased ReHo values were found mainly in the left MFG, left inferior parietal lobule (IPL), left precuneus, and right lentiform nucleus (LN) in the FES group relative to HC (p < 0.01 for all, corrected by TFCE; Fig. 1 and Table 3). The comparison between the two groups was adjusted for age, gender, illness duration and the probability of gray matter as covariates. Then, the subsequent correlation analysis in patients was performed within the ReHo decrease mask and the ReHo increase mask, respectively.
Brain regions with significantly altered ReHo in schizophrenic patients compared to control subjects. Statistically significant differences in gray matter volume were defined as p < 0.01, TFCE corrected after correcting for age, gender, education, illness duration and the probability of gray matter. Warm color indicates that ReHo is higher in the schizophrenic patient group than in the healthy control group, and vice versa
Correlations between ReHo values and PANSS scores
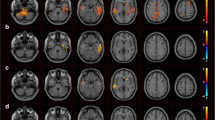
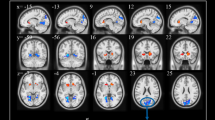
To investigate the relationship between the ReHo values and PANSS scores in FES, we performed the voxel-wise correlation analyses between the ReHo map and PANSS scores, using age, gender, education, and illness duration as covariates. There was a significant negative correlation between the PANSS total score (r = −0.40, p < 0.05, corrected by TFCE; Fig. 2a) and the ReHo value in the right precentral gyrus, and a significant positive correlation between the PANSS total score (r = 0.49, p < 0.05, corrected by TFCE; Fig. 2b) and the ReHo value in the left thalamus. Significantly positive correlation between the positive factor (r = 0.45, p < 0.05, corrected by TFCE; Fig. 2c) and ReHo value was observed in the right Thalamus. Moreover, we also found the disorganized/concrete factor (r = 0.38, p < 0.05, corrected by TFCE; Fig. 2d) was significantly positively correlated with ReHo value in the left posterior cingulate gyrus (PCG). There was a significant positive correlation between the excited factor (r = 0.44, p < 0.05, corrected by TFCE; Fig. 2e) and the ReHo value in the left precuneus. In addition, the depressed factor was significantly negative with ReHo value in the right postcentral gyrus (r = −0.38, p < 0.05, corrected by TFCE; Fig. 2f), and was significantly positive with ReHo value in the right thalamus (r = 0.50, p < 0.05, corrected by TFCE; Fig. 2g). The results of these voxel-wise correlation analyses are shown in Table 4.
The correlation analysis showed the PANSS total score negatively correlated with ReHo in the right precentral gyrus (a) and positively correlated with ReHo in the left thalamus (b), the positive factor positively correlated with ReHo in the right thalamus (c), the disorganized/concrete factor positively correlated with ReHo in left PCG (d), the excited factor positively correlated with ReHo in the left precuneus (e), and the depressed factor negatively correlated with ReHo in the right postcentral gyrus (f) and positively correlated with ReHo in the right thalamus (g). Significant correlations between PANSS and ReHo were defined as p < 0.05, TFCE corrected after correcting for age, gender, education, and illness duration. Warm and cool color indicate positive and negative correlation, respectively
Discussion
In the current study, a fully automated voxel-based method was used to measure the whole-brain local synchronization of the spontaneous activity in a voxel-wise way in naïve patients with FES, as well as its association with clinical symptoms. We found that ReHo in left MFG, right precentral gyrus, left STG, left MTG, and left thalamus was lower in schizophrenic patients compared to controls, while ReHo in left MFG, left IPL, left precuneus, and left LN was higher. Furthermore, the correlation analysis revealed that the psychopathology measures significantly correlated with abnormal ReHo in various brain regions, including the right precentral gyrus, left thalamus, right thalamus, left PCG, left precuneus, and right pestilential gyrus. These findings indicate that the ReHo abnormalities are present even at the early stage of FES. The ReHo abnormalities could be a potential neural basis for schizophrenia, suggesting that abnormal regional spontaneous neuronal activity at rest might contribute to the clinical symptoms of the illness.
In this study, we found that widespread ReHo abnormalities in several brain regions in naïve patients with FES compared with that of control subjects, which was partly consistent with the results of previous ReHo studies in chronic schizophrenic (Liu et al. 2006, 2016; Gao et al. 2015; Yu et al. 2013; Liao et al. 2012), as well as in FES (Chen et al. 2013; Cui et al. 2016). All these findings suggest that widespread disruption of local synchronization of the spontaneous activity may be involved in the psychopathology of schizophrenia. However, the results from these studies are not the exactly same. For example, decreased ReHo in the frontal lobe has been reported in patients with schizophrenia (Liu et al. 2006, 2016). However, some authors failed to replicate the finding and even found increased ReHo in the frontal lobe (Chen et al. 2013; Gao et al. 2015; Cui et al. 2016). Several factors may account for the discrepancy, such as differences in ReHo analysis, including head motion regression model, spacial normalize, and nuisance covariates regression; different illness courses (acute vs chronic or active phase vs remission); the subtypes of schizophrenic patients recruited; or the ethnic heterogeneity.
Most brain regions that showed abnormal ReHo values in this study belonged to the default mode network (DMN) (Whitfield-Gabrieli et al. 2009), such as MFG, MTG, IPL, and precuneus, which was consistent with the findings reported by other studies (Liu et al. 2006, 2016). These findings indicate that DMN abnormality are present even at the early stage of FES. DMN is deactivated during a wide range of attention-demanding tasks but is active during rest with a high degree of connectivity across the brain regions (i.e., temporal correlations between brain regions) (Whitfield-Gabrieli and Ford 2012; Hu et al. 2017). Recently, increasing numbers of reports have explored the role of the DMN in schizophrenia, and indicate that abnormalities in the DMN are strongly correlated with psychopathology and cognitive deficits of schizophrenia (Hu et al. 2017). Previous imaging studies implied that frontal lobe dysfunction possibly contributed to the emergence of negative symptoms (Roth et al. 2004; Semkovska et al. 2001), and the right middle frontal cortex was particularly vulnerable to long-term effects of schizophrenia (Premkumar et al. 2008). Recently, one study using 3 T imaging and reliable manual parcellation showed that reduced gray matter volume in the prefrontal cortex was specifically related to negative symptoms, such as affective flattening, with deficits in cognitive switching (Ohtani et al. 2014). Several neuroimaging studies have indicated that temporal lobe abnormalities possibly play a prominent role in the emergence of psychotic symptoms of auditory hallucinations, thought disorder, and positive psychotic symptoms in schizophrenia (Levitt et al. 2010; Shenton et al. 1992). Additionally, delusions of passivity in schizophrenic patients are associated with hyperactivity together with reductions in gray matter volume of the IPL (Danckert et al. 2004; Maruff et al. 2005). The precuneus is the key component of the DMN (Jardri et al. 2013; Mondino et al. 2016; Fair et al. 2008), and one study has indicated a crucial role of precuneus in visual-spatial imagery, episodic memory retrieval, self-processing, and consciousness (Cavanna and Trimble 2006). The present study also showed decreased ReHo in the precentral gyrus in schizophrenia, which was consistent with the previous study (Yu et al. 2013). The precentral gyrus is the key part of the mirror neuron system, which is believed to play an important role in social cognition, including perception of facial expressions, emotions, and individual desires (Jani and Kasparek 2017; Watanuki et al. 2016). A meta-analysis of neuroimaging studies (Jani and Kasparek 2017) reported that the precentral gyrus was more engaged in the schizophrenia patients who performed better in facial emotion perception and less engaged in the schizophrenia patients who performed worse. Consistent with findings of previous study (Chen et al. 2013), our results indicated that patients with schizophrenia had lower ReHo in the STG than control subjects. Decreased gray matter volumes of the STG in patients with schizophrenia also demonstrated in a voxel-based morphometry study (Kim et al. 2017), and reduced gray matter volume in STG was specifically associated with the psychopathology of schizophrenia. Our findings also showed that ReHo abnormalities in brain regions, including LN and thalamus, which are involved in the basal ganglia-thalamocortical circuitry. The basal ganglia-thalamocortical circuitry plays a strong role in multiple aspects of motor, executive/associative function, and emotion/motivation (Obeso et al. 2014). In conclusion, these findings indicate that the ReHo abnormalities are present even at the early stage of FES, suggesting that abnormal regional spontaneous neuronal activity at rest may contribute to the pathophysiology of schizophrenia.
The current study has shown that the PANSS scores significantly correlated with abnormal ReHo in various brain regions, including the right precentral gyrus, left thalamus, right thalamus, left PCG, left precuneus, and postcentral gyrus, suggesting that ReHo in distinct brain regions may underlie different clinical symptoms of schizophrenia. The correlation between the PANSS total score and the clusters including the precentral gyrus is not shown in the literature before but appears understandable based on early research work. Previous studies have suggested that the precentral gyrus plays a strong role in social cognition, and the overactivity of precentral gyrus can cause emotional processing dysfunction in schizophrenia (Jani and Kasparek 2017; Watanuki et al. 2016; Taylor et al. 2012). Furthermore, a recent study showed that facial emotion perception was significantly correlated with PANSS total score (Tseng et al. 2013). Our study provides anatomical evidence supporting the involvement of disturbed localized functional connectivity of precentral gyrus in clinical symptom severity in patients with schizophrenia. As the principal relay station for sensory information destined for the cerebral cortex, the thalamus is involved in many functions, including coordinating, planning (Andreasen et al. 1998) and complex cognition (Simpson et al. 2010). Interestingly, our current results show that ReHo in the thalamus is significantly correlated with the PANSS total score, positive factor, and depressed factor, respectively. One hypothesis advanced to explain the variety of symptoms of schizophrenia is that the midline neural circuits which mediate attention and information processing are dysfunctional; indeed, the ventrolateral and lateral areas of the thalamus, which project to the cingulate gyrus, temporal cortex, and parietal cortex, are seen to be most involved (Andreasen et al. 1994). A recent study (Meng et al. 2017) in schizophrenia has shown that the fractional amplitude of low frequency fluctuations of thalamus is likely responsible for positive symptoms of schizophrenia. Besides, Kumari et al. (2016) reported depression scores associated positively with activation of the thalamus during presentation of facial expressions of fear. Several studies (Garrity et al. 2007; Harrison et al. 2007; Zhou et al. 2007) have revealed that overactivity of the PCG in patients with schizophrenia at rest, and positive symptoms concluding hallucinations, delusions, and confused thoughts, were found to correlate with increased activity in the PCG. Our results regarding a correlation between the PANSS disorganized/concrete factor and ReHo in the left PCG partly support the above findings. Our results demonstrated that the PANSS excited factor showed a significant correlation with the ReHo in the precuneus in schizophrenia. The excited factor, a simple and intuitive scale to assess agitated patients, consists of 5 items: excitement, tension, hostility, uncooperativeness, and poor impulse control (Lindenmayer et al. 2008). The schizophrenic patients show significantly reduced effective connectivity from the precuneus to the amygdala when looking at fearful compared to neutral faces, and the disconnection may be a factor contributing toward fear related social behavior, especially agitation and aggressive behaviour (Mukherjee et al. 2012). On some level, the current study demonstrates that the precuneus plays an important role in the excitement in schizophrenia. A recent study showed that a significant association between gray matter volume in the postcentral gyrus with scale values for depressive symptoms in healthy subjects. Similarly, in our patient group, significantly negative correlation between the PANSS depressed factor and ReHo was observed in the right postcentral gyrus. Thus, postcentral gyrus likely contributes to the depression in schizophrenicpatients. Taken together, these findings suggest that disruption in the brain function in different brain regions may account for different core symptoms of schizophrenia early in the course of illness. However, given that the data on relationship between ReHo values and clinical variables in FES are sparse,the picture emerging is that these findings deserve further validation in patients with FES.
Several limitations necessitate caution when interpreting the results of the current study. Our sample size of participants is still relatively small although it is comparable to most fMRI studies in FES to date. Future studies with larger samples and multiple centers are desirable to confirm our findings. Also, owing to the cross-sectional design, we can not conclude the exact progressive features of these brain alterations in patients. Future longitudinal studies may help provide further insight into whether ReHo deficits are a cause or a consequence of schizophrenia. Another note is that, although a voxel-based approach has an advantage in terms of objectivity and no intentional measurements, it could come at the price of potential registration or normalization errors. Lastly, the patients who were not able to keep the head still were excluded from the study, raising the possibility that our results are not representative of all patients with schizophrenia.
References
Andreasen, N. C., et al. (1994). Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science, 266(5183), 294–298.
Andreasen, N. C., Paradiso, S., & O'Leary, D. S. (1998). "Cognitive dysmetria" as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophrenia Bulletin, 24(2), 203–218.
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113.
Biswal, B., Zerrin Yetkin, F., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541.
Boutros, N. N., Arfken, C., Galderisi, S., Warrick, J., Pratt, G., & Iacono, W. (2008). The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Research, 99(1–3), 225–237.
Buckley, P. E., Evans, D.. (2006). First-episode schizophrenia. A window of opportunity for optimizing care and outcomes. Postgrad Med Spec No: p. 5–19.
Catafau, A. M., Parellada, E., Lomeña, F. J., Bernardo, M., Pavía, J., Ros, D., Setoain, J., & Gonzalez-Monclús, E. (1994). Prefrontal and temporal blood flow in schizophrenia: resting and activation technetium-99m-HMPAO SPECT patterns in young neuroleptic-naive patients with acute disease. Journal of Nuclear Medicine, 35(6), 935–941.
Cavanna, A. E., & Trimble, M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain, 129(Pt 3), 564–583.
Chao-Gan, Y., & Yu-Feng, Z. (2010). DPARSF: A MATLAB toolbox for "pipeline" data analysis of resting-state fMRI. Frontiers in Systems Neuroscience, 4, 13.
Chen, J., et al. (2013). Comparative study of regional homogeneity in schizophrenia and major depressive disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics, 162B(1), 36–43.
Cui, L. B., Liu, K., Li, C., Wang, L. X., Guo, F., Tian, P., Wu, Y. J., Guo, L., Liu, W. M., Xi, Y. B., Wang, H. N., & Yin, H. (2016). Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophrenia Research, 173(1–2), 13–22.
Danckert, J., Saoud, M., & Maruff, P. (2004). Attention, motor control and motor imagery in schizophrenia: implications for the role of the parietal cortex. Schizophrenia Research, 70(2–3), 241–261.
Ebmeier, K., et al. (1993). Single-photon emission computed tomography with 99mTc-exametazime in unmediated schizophrenic patients. Biological Psychiatry, 33(7), 487–495.
Fair, D. A., Cohen, A. L., Dosenbach, N. U. F., Church, J. A., Miezin, F. M., Barch, D. M., Raichle, M. E., Petersen, S. E., & Schlaggar, B. L. (2008). The maturing architecture of the brain's default network. Proceedings of the National Academy of Sciences of the United States of America, 105(10), 4028–4032.
Fang, L. (2013). Resting-state functional magnetic resonance imaging study of brain function in the first-episode paranoid-type schizophrenia patients. Nanjing: The Fourth School of Clinical Medicine, Nanjing Medical University.
Friston, K. J., Williams, S., Howard, R., Frackowiak, R. S. J., & Turner, R. (1996). Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine, 35(3), 346–355.
Gao, B., Wang, Y., Liu, W., Chen, Z., Zhou, H., Yang, J., Cohen, Z., Zhu, Y., & Zang, Y. (2015). Spontaneous activity associated with delusions of schizophrenia in the left medial superior frontal gyrus: A resting-state fMRI study. PLoS One, 10(7), e0133766.
Garrity, A. G., Pearlson, G. D., McKiernan, K., Lloyd, D., Kiehl, K. A., & Calhoun, V. D. (2007). Aberrant "default mode" functional connectivity in schizophrenia. The American Journal of Psychiatry, 164(3), 450–457.
Greicius, M. D., Krasnow, B., Reiss, A. L., & Menon, V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–258.
Harrison, B. J., Yücel, M., Pujol, J., & Pantelis, C. (2007). Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophrenia Research, 91(1–3), 82–86.
Hu, M. L., et al. (2016). Short-term effects of risperidone monotherapy on spontaneous brain activity in first-episode treatment-naive schizophrenia patients: a longitudinal fMRI study. Scientific Reports, 6, 34287.
Hu, M. L., Zong, X. F., Mann, J. J., Zheng, J. J., Liao, Y. H., Li, Z. C., He, Y., Chen, X. G., & Tang, J. S. (2017). A review of the functional and anatomical default mode network in schizophrenia. Neuroscience Bulletin, 33(1), 73–84.
Jani, M., & Kasparek, T. (2017). Emotion recognition and theory of mind in schizophrenia: a meta-analysis of neuroimaging studies. The World Journal of Biological Psychiatry, 1–31.
Jardri, R., Thomas, P., Delmaire, C., Delion, P., & Pins, D. (2013). The neurodynamic organization of modality-dependent hallucinations. Cerebral Cortex, 23(5), 1108–1117.
Jerrell, J. M., & Hrisko, S. (2013a). A comparison of the PANSS pentagonal and van Der Gaag 5-factor models for assessing change over time. Psychiatry Research, 207(1–2), 134–139.
Jerrell, J. M., & Hrisko, S. (2013b). Utility of two PANSS 5-factor models for assessing psychosocial outcomes in clinical programs for persons with schizophrenia. Schizophr Res Treatment, 2013, 705631.
Jiang, L., & Zuo, X. N. (2016). Regional homogeneity: a multimodal, multiscale neuroimaging marker of the human connectome. The Neuroscientist, 22(5), 486–505.
Jiang, S., Zhou, B., & Liao, Y. (2010). Primary study of resting state functional magnetic resonance imaging in early onset schizophrenia using ReHo. Zhong Nan Da Xue Xue Bao. Yi Xue Ban, 35(9), 947–951.
Jiang, L., Xu, T., He, Y., Hou, X. H., Wang, J., Cao, X. Y., Wei, G. X., Yang, Z., He, Y., & Zuo, X. N. (2015a). Toward neurobiological characterization of functional homogeneity in the human cortex: regional variation, morphological association and functional covariance network organization. Brain Structure & Function, 220(5), 2485–2507.
Jiang, L., Xu, Y., Zhu, X. T., Yang, Z., Li, H. J., & Zuo, X. N. (2015b). Local-to-remote cortical connectivity in early- and adulthood-onset schizophrenia. Translational Psychiatry, 5, e566.
Kaplan, R. D., Szechtman, H., Franco, S., Szechtman, B., Nahmias, C., Garnett, E. S., List, S., & Cleghorn, J. M. (1993). Three clinical syndromes of schizophrenia in untreated subjects relation to brain glucose activity measured by position emission tomography (PET). Schizophrenia Research, 11(1), 47–54.
Katanoda, K., Matsuda, Y., & Sugishita, M. (2002). A spatio-temporal regression model for the analysis of functional MRI data. NeuroImage, 17(3), 1415–1428.
Kay, S. R., Fiszbein, A., & Opler, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261–276.
Kay, S. R., Opler, L. A., & Lindenmayer, J. P. (1988). Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Research, 23(1), 99–110.
Kim, G. W., Kim, Y. H., & Jeong, G. W. (2017). Whole brain volume changes and its correlation with clinical symptom severity in patients with schizophrenia: A DARTEL-based VBM study. PLoS One, 12(5), e0177251.
Knott, V., Labelle, A., Jones, B., & Mahoney, C. (2001). Quantitative EEG in schizophrenia and in response to acute and chronic clozapine treatment. Schizophrenia Research, 50(1–2), 41–53.
Kumari, V., Peters, E., Guinn, A., Fannon, D., Russell, T., Sumich, A., Kuipers, E., Williams, S. C. R., & ffytche, D. H. (2016). Mapping depression in schizophrenia: a functional magnetic resonance imaging study. Schizophrenia Bulletin, 42(3), 802–813.
Lancon, C., et al. (2000). Stability of the five-factor structure of the positive and negative syndrome scale (PANSS). Schizophrenia Research, 42(3), 231–239.
Lee, S. H., Wynn, J. K., Green, M. F., Kim, H., Lee, K. J., Nam, M., Park, J. K., & Chung, Y. C. (2006). Quantitative EEG and low resolution electromagnetic tomography (LORETA) imaging of patients with persistent auditory hallucinations. Schizophrenia Research, 83(2–3), 111–119.
Levitt, J. J., et al. (2010). A selective review of volumetric and morphometric imaging in schizophrenia. Current Topics in Behavioral Neurosciences, 4, 243–281.
Levy, A. V., Gomez-Mont, F., Volkow, N. D., Corona, J. F., Brodie, J. D., & Cancro, R. (1992). Spatial low frequency pattern analysis in positron emission tomography: a study between normals and schizophrenics. Journal of Nuclear Medicine, 33(2), 287–295.
Li, H. J., Xu, Y., Zhang, K. R., Hoptman, M. J., & Zuo, X. N. (2015). Homotopic connectivity in drug-naive, first-episode, early-onset schizophrenia. Journal of Child Psychology and Psychiatry, 56(4), 432–443.
Liao, H., Wang, L., Zhou, B., Tang, J., Tan, L., Zhu, X., Yi, J., Chen, X., & Tan, C. (2012). A resting-state functional magnetic resonance imaging study on the first-degree relatives of persons with schizophrenia. Brain Imaging and Behavior, 6(3), 397–403.
Lindenmayer, J. P., Bossie, C. A., Kujawa, M., Zhu, Y., & Canuso, C. M. (2008). Dimensions of psychosis in patients with bipolar mania as measured by the positive and negative syndrome scale. Psychopathology, 41(4), 264–270.
Liu, H., Liu, Z., Liang, M., Hao, Y., Tan, L., Kuang, F., Yi, Y., Xu, L., & Jiang, T. (2006). Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. Neuroreport, 17(1), 19–22.
Liu, C., Xue, Z., Palaniyappan, L., Zhou, L., Liu, H., Qi, C., Wu, G., Mwansisya, T. E., Tao, H., Chen, X., Huang, X., Liu, Z., & Pu, W. (2016). Abnormally increased and incoherent resting-state activity is shared between patients with schizophrenia and their unaffected siblings. Schizophrenia Research, 171(1–3), 158–165.
Lui, S., Li, T., Deng, W., Jiang, L., Wu, Q., Tang, H., Yue, Q., Huang, X., Chan, R. C., Collier, D. A., Meda, S. A., Pearlson, G., Mechelli, A., Sweeney, J. A., & Gong, Q. (2010). Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by "resting state" functional magnetic resonance imaging. Archives of General Psychiatry, 67(8), 783–792.
Malaspina, D., Harkavy-Friedman, J., Corcoran, C., Mujica-Parodi, L., Printz, D., Gorman, J. M., & van Heertum, R. (2004). Resting neural activity distinguishes subgroups of schizophrenia patients. Biological Psychiatry, 56(12), 931–937.
Maruff, P., et al. (2005). Reduced volume of parietal and frontal association areas in patients with schizophrenia characterized by passivity delusions. Psychological Medicine, 35(6), 783–789.
Meng, X., et al. (2017). Predicting individualized clinical measures by a generalized prediction framework and multimodal fusion of MRI data. NeuroImage, 145, 218–229.
Mondino, M., Jardri, R., Suaud-Chagny, M. F., Saoud, M., Poulet, E., & Brunelin, J. (2016). Effects of Fronto-temporal transcranial direct current stimulation on auditory verbal hallucinations and resting-state functional connectivity of the left Temporo-parietal junction in patients with schizophrenia. Schizophrenia Bulletin, 42(2), 318–326.
Mukherjee, P., Whalley, H. C., McKirdy, J. W., McIntosh, A. M., Johnstone, E. C., Lawrie, S. M., & Hall, J. (2012). Lower effective connectivity between amygdala and parietal regions in response to fearful faces in schizophrenia. Schizophrenia Research, 134(2–3), 118–124.
Obeso, J. A., Rodriguez-Oroz, M. C., Stamelou, M., Bhatia, K. P., & Burn, D. J. (2014). The expanding universe of disorders of the basal ganglia. The Lancet, 384(9942), 523–531.
Ohtani, T., Levitt, J. J., Nestor, P. G., Kawashima, T., Asami, T., Shenton, M. E., Niznikiewicz, M., & McCarley, R. W. (2014). Prefrontal cortex volume deficit in schizophrenia: a new look using 3T MRI with manual parcellation. Schizophrenia Research, 152(1), 184–190.
Pardo, B. M., Garolera, M., Ariza, M., Pareto, D., Salamero, M., Valles, V., Delgado, L., & Alberni, J. (2011). Improvement of cognitive flexibility and cingulate blood flow correlates after atypical antipsychotic treatment in drug-naive patients with first-episode schizophrenia. Psychiatry Research, 194(3), 205–211.
Parellada, E., Catafau, A. M., Bernardo, M., Lomeña, F., González-Monclús, E., & Setoain, J. (1994). Prefrontal dysfunction in young acute neuroleptic-naive schizophrenic patients: A resting and activation SPECT study. Psychiatry Research: Neuroimaging, 55(3), 131–139.
Pascual-Marqui, R. D., Lehmann, D., Koenig, T., Kochi, K., Merlo, M. C. G., Hell, D., & Koukkou, M. (1999). Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Research, 90(3), 169–179.
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154.
Premkumar, P., Fannon, D., Kuipers, E., Cooke, M. A., Simmons, A., & Kumari, V. (2008). Association between a longer duration of illness, age and lower frontal lobe grey matter volume in schizophrenia. Behavioural Brain Research, 193(1), 132–139.
Raichle, M. E., & Gusnard, D. A. (2005). Intrinsic brain activity sets the stage for expression of motivated behavior. The Journal of Comparative Neurology, 493(1), 167–176.
Roth, R. M., Flashman, L. A., Saykin, A. J., McAllister, T. W., & Vidaver, R. (2004). Apathy in schizophrenia: reduced frontal lobe volume and neuropsychological deficits. The American Journal of Psychiatry, 161(1), 157–159.
Semkovska, M., Bedard, M. A., & Stip, E. (2001). Hypofrontality and negative symptoms in schizophrenia: synthesis of anatomic and neuropsychological knowledge and ecological perspectives. Encephale, 27(5), 405–415.
Shenton, M. E., Kikinis, R., Jolesz, F. A., Pollak, S. D., LeMay, M., Wible, C. G., Hokama, H., Martin, J., Metcalf, D., Coleman, M., & McCarley, R. W. (1992). Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. The New England Journal of Medicine, 327(9), 604–612.
Simpson, E. H., Kellendonk, C., & Kandel, E. (2010). A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron, 65(5), 585–596.
Smith, S. M., & Nichols, T. E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98.
Tandon, R., Nasrallah, H. A., & Keshavan, M. S. (2010). Schizophrenia, "just the facts" 5. Treatment and prevention. Past, present, and future. Schizophrenia Research, 122(1–3), 1–23.
Taylor, S. F., Kang, J., Brege, I. S., Tso, I. F., Hosanagar, A., & Johnson, T. D. (2012). Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biological Psychiatry, 71(2), 136–145.
Tononi, G., McIntosh, A. R., Russell, D. P., & Edelman, G. M. (1998). Functional clustering: identifying strongly interactive brain regions in neuroimaging data. NeuroImage, 7(2), 133–149.
Tseng, H. H., Chen, S. H., Liu, C. M., Howes, O., Huang, Y. L., Hsieh, M. H., Liu, C. C., Shan, J. C., Lin, Y. T., & Hwu, H. G. (2013). Facial and prosodic emotion recognition deficits associate with specific clusters of psychotic symptoms in schizophrenia. PLoS One, 8(6), e66571.
Wallwork, R. S., Fortgang, R., Hashimoto, R., Weinberger, D. R., & Dickinson, D. (2012). Searching for a consensus five-factor model of the positive and negative syndrome scale for schizophrenia. Schizophrenia Research, 137(1–3), 246–250.
Watanuki, T., Matsuo, K., Egashira, K., Nakashima, M., Harada, K., Nakano, M., Matsubara, T., Takahashi, K., & Watanabe, Y. (2016). Precentral and inferior prefrontal hypoactivation during facial emotion recognition in patients with schizophrenia: A functional near-infrared spectroscopy study. Schizophrenia Research, 170(1), 109–114.
Whitfield-Gabrieli, S., & Ford, J. M. (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology, 8, 49–76.
Whitfield-Gabrieli, S., Thermenos, H. W., Milanovic, S., Tsuang, M. T., Faraone, S. V., McCarley, R. W., Shenton, M. E., Green, A. I., Nieto-Castanon, A., LaViolette, P., Wojcik, J., Gabrieli, J. D. E., & Seidman, L. J. (2009). Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 106(4), 1279–1284.
Xiao, B., Wang, S., Liu, J., Meng, T., He, Y., & Luo, X. (2017). Abnormalities of localized connectivity in schizophrenia patients and their unaffected relatives: a meta-analysis of resting-state functional magnetic resonance imaging studies. Neuropsychiatric Disease and Treatment, 13, 467–475.
Xiong, Y. (2016). Resting state fMRI study of amplitude of low-frequency fluctuation and regional homogeneity in early onset schizophrenia. Taiyuan: First Hospital, Sanxi Medical University.
Yan, C. G., Wang, X. D., Zuo, X. N., & Zang, Y. F. (2016). DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics, 14(3), 339–351.
Yang, Z., et al. (2014). Brain network informed subject community detection in early-onset schizophrenia. Scientific Reports, 4, 5549.
Yu, R., Hsieh, M. H., Wang, H. L. S., Liu, C. M., Liu, C. C., Hwang, T. J., Chien, Y. L., Hwu, H. G., & Tseng, W. Y. I. (2013). Frequency dependent alterations in regional homogeneity of baseline brain activity in schizophrenia. PLoS One, 8(3), e57516.
Zang, Y., Jiang, T., Lu, Y., He, Y., & Tian, L. (2004). Regional homogeneity approach to fMRI data analysis. NeuroImage, 22(1), 394–400.
Zhou, Y., Liang, M., Tian, L., Wang, K., Hao, Y., Liu, H., Liu, Z., & Jiang, T. (2007). Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophrenia Research, 97(1–3), 194–205.
Zuo, X. N., & Xing, X. X. (2014). Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neuroscience and Biobehavioral Reviews, 45, 100–118.
Zuo, X. N., Xu, T., Jiang, L., Yang, Z., Cao, X. Y., He, Y., Zang, Y. F., Castellanos, F. X., & Milham, M. P. (2013). Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. NeuroImage, 65, 374–386.
Acknowledgements
This work is partly supported by the Natural Science Foundation of Jiangsu Province (Grants No BK20151076) and the project of Jiangsu Provincial Health Department (General Program No: H201442,Y2013004), the Six talent peaks project in Jiangsu Province (NO2014-WSN-055).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All research procedures were approved by the Medical Research Ethics Committee of Nanjing Brain Hospital, and were conducted in accordance with the 1964 Helsinki declaration and its later amendments.
Informed consent
Written informed consent of the schizophrenic patient was obtained from his/her legally authorized representative and the control provided written informed consent himself/herself after totally understanding the purpose of our study.
Conflicts of interest
Xiaoxin Zhao, Jingjing Yao, Yiding Lv, Xinyue Zhang, Chongyang Han, Lijun Chen, Fangfang Ren, Zhuma Jin, Yuan Li, and Yuxiu Sui declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zhao, X., Yao, J., Lv, Y. et al. Abnormalities of regional homogeneity and its correlation with clinical symptoms in Naïve patients with first-episode schizophrenia. Brain Imaging and Behavior 13, 503–513 (2019). https://doi.org/10.1007/s11682-018-9882-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-018-9882-4