Abstract
Crohn’s disease (CD) has been reported to relate with the functional and structural alterations in several local brain regions. However, it remains unknown whether the possible abnormalities of information transmission and integration between brain regions are associated with CD. The purpose of this study was to investigate the topological alterations of brain functional networks and the potential relationships between the neuroimaging findings and CD clinical characteristics. 43 remissive CD patients and 37 matched healthy controls (HCs) were recruited to obtain their resting-state functional magnetic resonance imaging (fMRI) data. Independent component analysis was applied to decompose fMRI data for building brain functional networks. The local and global topological properties of networks and connectivity of brain regions were computed within each group. We then examined the relationships between the topological patterns and CD clinical characteristics. Compared to HCs, CD patients exhibited disrupted local and global topological patterns of brain functional networks including the decreased nodal graph metrics in the subcortical, sensorimotor, cognitive control and default-mode networks and dysfunctional interactions within and among these four networks. The connectivity strength of putamen negatively correlated with CD duration in patients. Moreover, CD patients with high level of anxiety and/or depression had altered local topological patterns associated with anterior cingulate cortex (ACC), medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC) compared to other patients. By revealing CD-related changes in topological patterns of brain functional networks, our findings provide further neuroimaging evidence on the pathophysiology of CD involved in pain, sensory, emotional and/or cognitive processing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) is one of the most common, chronic inflammatory bowel disease (IBD) with persistent, relapsing and remitting diarrhea even bloody diarrhea, abdominal pain, weight loss and fever (Kalla et al. 2014; Ng et al. 2013). Despite high prevalence rate in young adults worldwide, the pathophysiology of CD remains obscure, resulting in unsatisfactory treatment (Clark et al. 2007). CD could cause long-term suffering and heavy economic burden for individual and society because of repeated gastrointestinal symptoms (Casellas et al. 2005; Pizzi et al. 2006). Additionally, as for many other chronic diseases, CD patients often endure concomitant psychological distress such as anxiety and depression (Agostini et al. 2013b; Bao et al. 2015). Therefore, it is imperative to investigate the pathophysiological mechanisms underlying CD.
Using functional and structural brain magnetic resonance imaging (fMRI and sMRI) technologies, accumulating studies have provided evidence that the dysfunctions of central nervous system (CNS) and brain-gut axis (BGA) are major contributing factors to the development of CD (Stasi and Orlandelli 2008). For example, Agostini et al. performed a stress-evoking task on CD patients and controls during fMRI scanning, and found different neural activities between two groups in insula and putamen (Agostini et al. 2013b). Using resting-state fMRI, one of our previous studies indicated that CD patients had altered local synchronization of neuron activity related to the increased regional homogeneity (ReHo) in precuneus, anterior cingulate cortex (ACC) and superior frontal cortex and decreased ReHo in thalamus, middle cingulate cortex and insula (Bao et al. 2016a). In addition, CD patients were reported to have morphological changes of brain grey matter (GM), associated with aberrant GM volumes and cortical thickness in prefrontal cortex, somatosensory area, and cingulate cortex (Agostini et al. 2013a; Bao et al. 2015). However, aforementioned studies just have focused on regional alterations of either blood-oxygenation level dependent signal (BOLD) or GM structure. Little is known about the possible abnormalities of information transmission and integration between brain regions in CD patients and the relationships between possible abnormalities and CD progression.
Topological analysis provides us a chance to characterize the whole brain as a topological network to uncover the complexity of the regional interactions. To our knowledge, the human brain in the resting state is topologically organized into a set of large-scale intrinsic connectivity networks (ICNs) with spatially distributed and functionally specific, such as the sensorimotor, cognitive control and default-mode networks (Tomasi and Volkow 2011; Liang et al. 2016). Recently, a more refined and fine-grained parcellation of these large-scale networks into a multitude of smaller ICNs/constituents (Allen et al. 2014; Damaraju et al. 2014; Yu et al. 2015; He et al. 2016) has been shown by independent component analysis (ICA), a data-driven approach (Calhoun and Adali 2012; Calhoun et al. 2001), in fMRI data. These smaller constituents are responsible for primary processing and transmission of neuronal information to support the large-scale networks work efficiently. A brain network with optimal topological configuration should have high efficiency and low cost characteristics (Sporns 2011). Graph theory, a mathematical technique of topological analysis, may help to detect the connectivity patterns of ICNs, quantify the topological properties of whole brain networks and examine the underlying relationships between graph indicators and clinical symptoms of disease (Mikail Rubinov and Olaf Sporns 2010). Moreover, topological analysis of whole brain functional networks has been implemented in several psychiatric disorders such as Alzheimer’s disease, bipolar and major depressive disorders (Dai et al. 2015; He et al. 2016). However, the related topological patterns of brain functional networks still remain largely unexplored for IBD including CD, currently.
In the present study, we tried to investigate the topological alterations of whole brain functional networks in CD patients compared to healthy controls (HCs) and to explore the relationships between neuroimaging findings and CD clinical characteristics. In light of the brain functional and structural changes involved in processing of painful-related, sensory, emotional and cognitive information in CD patients, we here hypothesized: (i) CD patients would display altered local and global topological patterns of brain functional networks related to subcortical, sensorimotor and cognitive control regions; (ii) the topological alterations might correlate with CD clinical characteristics (e.g., duration and psychological level). In pursuit of the aims, we applied ICA to decompose fMRI data and built whole brain functional networks in HC and CD groups. Topological properties of networks and connectivity between ICNs were calculated and compared between two groups. The relationships were examined between topological patterns and CD clinical characteristics.
Materials and methods
Participants
43 CD patients were enrolled in this study. A portion of patients were recruited from the specialist outpatient clinic for IBD in Shanghai Research Insititute of Acupuncture and Meridian and the other patients were recruited from Endoscopy Center of the hospital at Fudan University, China.
All the patients received the medical examinations including the systemic and gastrointestinal screening. The colonoscopy and pathological biopsy were conducted one month before MRI by a skilled endoscopist from the hospital who was blind to the experimental conditions. Inclusion criteria for the CD patients were as follows: (i) 18 to 50 years old, right-handed and more than 6 years of education; (ii) remaining in remission period for 12 months or longer; (iii) no CD-related abdominal surgery, no taking drugs for relieving pain during the past 3 months and no history of psychiatric disorders; (iv) no pregnancy or lactation, no claustrophobia and no metal implantation.
37 gender-, age-, and education-matched HCs were recruited through advertisements. None of participants in HC group had occurred any gastrointestinal problem, or had positive results in colonoscopy in previous years, or had high scores (≥ 7) of Hospital Anxiety and Depression Scale (HADS) (Zigmond and Snaith 1983), or had received any medication prior to MRI scans.
The gastrointestinal functions of all participants were evaluated by an experienced gastroenterologist from gastroenterology department of the hospital. To remove anyone with neurological or psychiatric disorders, the mental examinations were conducted by a specialist from the Shanghai Mental Health Center according to the structured psychiatric interview from the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (Bao et al. 2015, 2016a, b).
All research procedures of the present study were conducted in accordance with the Declaration of Helsinki and were approved by the local hospital subcommittee on human studies. All the subjects signed informed consent before participation, and all methods were carried out in accordance with the approved guidelines.
CD clinical characteristics
The Crohn’s disease activity index (CDAI) (Best et al. 1979) and inflammatory bowel disease questionnaire (IBDQ) (Irvine et al. 1994) were used to assess the condition of CD patient and the quality of life. The psychological level was examined by the HADS.
Imaging data acquisition
All participants’ neuroimaging data were collected using a Siemens 3-Tesla MRI scanner (Siemens Medical, Erlangen, Germany). CD patients and HCs were instructed to keep their eyes closed and to stay relaxed without thinking of anything in particular and falling asleep during MRI scanning. Functional images were acquired using an echo planar imaging (EPI) sequence with repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle (FA) = 90°, slice thickness = 5 mm, field of view (FOV) = 240 mm × 240 mm, matrix size = 64 × 64, in-plane resolution = 3.75 mm × 3.75 mm, and 32 sagittal slices. High-resolution T1-weighted structural images were collected with TR = 2300 ms, TE = 2.98 ms, FA = 9°, slice thickness = 1.0 mm, FOV = 256 mm × 256 mm, matrix size = 256 × 256, in-plane resolution = 1 mm × 1 mm, and 176 slices.
Imaging data preprocessing
Data preprocessing was performed with an automated pipeline on the CONN functional connectivity toolbox (17.h) (http://www.nitrc.org/projects/conn), a Matlab-based cross-platform software. For each participant, preprocessing procedures of the functional images included: (i) the removal of the first five volumes to avoid nonequilibrium effects of initial signals and to allow participants to adapt to the scanning environment; (ii) slice-timing and motion correction to the first volume; (iii) normalization into Montreal Neurologic Institute (MNI) space using EPI template and reslicing to 3 mm × 3 mm × 3 mm isotropic voxels; (iv) smoothing with a Gaussian filter of full width at half-maximum of 6 mm. Participants with head motion out of range (larger than 1.5 mm translation or 1.5° rotation) were excluded for further analysis.
Group ICA and post-processing
Preprocessed fMRI data were subjected to a group-level spatial ICA using the GIFT toolbox (http://mialab.mrn.org/software/gift). First, individual data were decomposed into 120 principal components via a standard economy-size dimension reduction algorithm. Then group data were reduced using the expectation–maximization (EM) algorithm to retain 100 principal components. The number of components for ICA is a relatively high model order and has been demonstrated that could achieve a more refined anatomical and functional segmentation of whole-brain (Abou-Elseoud et al. 2010; Kiviniemi et al. 2009). The Infomax ICA algorithm (Bell and Sejnowski 1995) was repeated 10 times in ICASSO (http://www.cis.hut.fi/projects/ica/icasso) to estimate the reliability of the decomposition and to obtain 100 group level independent components (ICs). The subject-specific ICs and related time courses were back-reconstructed using the GICA1 method (Calhoun et al. 2001; Erhardt et al. 2011). According to the prior studies (Allen et al. 2011, 2014; Cordes et al. 2000), the ICs were evaluated based on two expectations: (i) spatial maps of ICNs should have low overlap with the vascular and ventricular artifacts, white matter and cerebrospinal fluid and should exhibit peak activations in grey matter; (ii) time courses of ICNs should dominatly fluctuate within a low-frequency stage (less 0.1 Hz). 48 ICs were characterized as ICNs and the related subject-specific time courses were extracted for further analysis. To remove residual noising (Power et al. 2012; Allen et al. 2011), time courses underwent additional post-processing including (i) detrending linear, quadratic, and cubic trends; (ii) regression of six head motion parameters and their temporal derivatives; (iii) removal of detected outliers; (iv) band-pass filtering using 5th order Butterworth filter with (0.01–0.1) Hz cutoff frequency.
Functional network connectivity construction and topological properties
To measure the brain functional network topological patterns, we calculated Pearson correlation coefficients and their significance levels (i.e., p-values) among the 48 ICNs in their mean time series. To avoid possible spurious connections and erroneous evaluations of network topology caused by weak and non-significant links (Rubinov and Sporns 2010; Bassett and Gazzaniga 2011; Tijms et al. 2014; Wang et al. 2015), a thresholding procedure based on significance level was performed. Specifically, a threshold of p < 0.05 (Bonferroni corrected) was used to ensure only elements that survived this statistical threshold were retained. Notably, similar to previous studies, the diagonal and negative links were set to zero, because it is questionable whether the self-connections or negative connections are biologically meaningful (Power et al. 2011, 2013; Rubinov and Sporns 2010). Note that including the negative connections (by using their absolute values to reflect the connectivity strength) also did not alter our main results. Finally, the remaining connectivity values were normalized using Fisher’s z transformation, and then a weighted network was obtained for each participant, where the node represented the ICN and the edge represented the correlation coefficient connecting two ICNs (Lynall et al. 2010). In the present study, three nodal graph metrics, the weighted connectivity strength, clustering coefficient and local efficiency, were selected to elucidate local-level message interaction of brain functional networks. Four global graph metrics were used to assess global-level information communication among the whole brain, including the averaged clustering coefficient, averaged local efficiency, characteristic path length and global efficiency. All topological properties within each graph were computed using the brain connectivity toolbox (BCT) (http://www.nitrc.org/projects/bct/).
Statistical analysis
The clinical and demographic characteristics of participants were analyzed by SPSS 20.0 (https://www.ibm.com/analytics/us/en/technology/spss/). Two-sample t-tests were performed for the age, height, weight and education level and part of clinical characteristics. Fisher’s exact test was used to compare the difference of gender between the two groups. All of significance threshold were set at p < 0.05 (two-tail).
We applied two-sample t-tests to explore group differences between CD patients and HCs, including the topological properties (nodal and global graph metrics) and connectivity between nodes (ICNs). In order to assess differences in nodal graph metrics, two-sample t-tests were conducted in each nodal graph metric resulting in 144 t-tests (three comparisons (HCs vs. CD): weighted connectivity strength, clustering coefficient and local efficiency; each comparison was conducted across the 48 nodes) using false discovery rate (FDR) correction. In order to assess differences in global graph metrics, four two-sample t-tests were performed between CD and HC groups. In order to assess differences in connectivity, 1128 two-sample t-tests were conducted among connectivity values for each node with the other nodes ((48 × 48–48) / 2 = 1128 comparisons (HCs vs. CD); the connectivity matrix is symmetric and diagonal values were zero) using FDR correction. In order to examine the relationships between the possible alterations of topological patterns (topological properties and related connectivity) and CD duration or symptom severity, Pearson correlation was calculated only in the nodes and/or connectivity with significant between-group differences.
In addition, based on the HADS-A and HADS-D scores, we classified CD patients into two subgroups: patients with high HADS-A (greater than 7) and/or HADS-D (greater than 7) and patients with low HADS-A and HADS-D. The same t-tests were used to test the topological patterns differences (including the nodal and global graph metrics as well as connectivity) between two CD subgroups.
All of the contrasts were thresholded at p < 0.05 (FDR corrected). To avoid the effects of age, sex and education level, these parameters were included in the inter-group comparison model as covariates of no interest.
Results
Clinical and demographic characteristics
The clinical and demographic characteristics of 37 HCs and 43 CD patients are displayed in Table 1. There was no significant between-group differences in the gender, age, height, weight and education level (gender: p = 1.000; age: p = 0.412; height: p = 0.783; weight: p = 0.274; education level: p = 0.182). However, the anxiety (HADS-A) and depression (HADS-D) scores in CD patients were significant higher than HCs (HADS-A: p = 0.001; HADS-D: p = 0.020). There were twenty-one patients with significantly higher HADS-A and HADS-D (female/male: 15/9; HADS-A: 8.67 ± 3.41; HADS-D: 7.38 ± 3.81).
ICNs identification and functional network construction
The spatial maps of 48 ICNs are shown in Fig. 1. Based on the anatomical and presumed functional properties of spatial maps identified with group ICA, 48 ICNs were arranged into seven large-scale networks including the subcortical (SC), auditory (AUD), sensorimotor (SM), visual (VIS), cognitive control (CC), default-mode (DM) and cerebellar (CB) networks.
The connectivity between ICNs, averaged over participants in HC and CD groups is shown in Fig. 2. CD patients had a weaker connectivity compared to HCs.
Altered local topological patterns
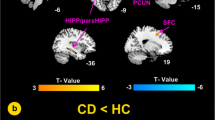
Compared to HCs, CD patients were only found to have significantly decreased nodal graph metrics in several ICNs. The detailed information of ICNs with significant between-group differences is summarized in Table 2 and Fig. 3 (a,b&c). As shown in Fig. 3a, CD patients had significantly decreased nodal connectivity strength in six ICNs, including the putamen, supplementary motor area (SMA), superior frontal gyrus (SFG), anterior midcingulate cortex (aMCC), precuneus and superior temporal gyrus (STG). CD patients also had decreased nodal clustering coefficient and nodal local efficiency, associated with the putamen, thalamus, postcentral gyrus, SMA, SFG, aMCC, supramarginal gyrus, precuneus and STG in CD patients (Fig. 3b, c).
Altered brain maps of CD-related nodal graph metrics and connectivity. Nodes in brain maps represent ICNs and are located at the peak activation points in the brain of corresponding ICNs. Colors of nodes correspond to the colors of ICNs’ anatomical and functional categories shown at the bottom of the figure. Edges connecting to nodes represent the connectivity between ICNs. Numbers in brain maps are the indexes of independent components identified as ICNs. Significant differences between HC and CD groups are shown in a) ~ d): a decreased connectivity strength in six ICNs; b decreased clustering coefficient in nine ICNs; c decreased local efficiency in nine ICNs and d decreased connectivity among ten ICNs (bilateral)
The significant between-group differences of connectivity are shown in Table 3 and Fig. 3d. Compared to HCs, CD patients had decreased connectivity of STG to precuneus, decreased connectivity of SMA to SFG, decreased connectivity of putamen to the insula, STG, precuneus, supramarginal gyrus and aMCC as well as decreased connectivity of aMCC to the insula, SMA, thalamus and precuneus.
Altered global topological patterns
The significant between-group differences of global graph metrics are displayed in Fig. 4. Compared to HCs, CD patients had decreased averaged clustering coefficient (p = 0.0029, Fig. 4a), averaged local efficiency (p = 0.0025, Fig. 4b) and global efficiency (p = 0.0024, Fig. 4c). While CD patients had increased characteristic path length (p = 0.00097, Fig. 4d).
The effects of CD clinical characteristics
The connectivity strength of putamen was decreased in CD patients compared to HCs, and this putamen-related connectivity strength significantly negatively correlated with CD duration (r = -0.46, p = 0.0015, Fig. 5a, b). However, no other significant correlation was found between CD clinical characteristics (e.g., CDAI, IBDQ and duration) and neuroimaging findings showing differences between CD and HC groups.
The effects of psychological factor
Compared to other patients, patients with significantly higher HADS-A and/or HADS-D had increased connectivity strength in medial prefrontal cortex (mPFC) and ACC, increased clustering coefficient in mPFC, ACC and posterior cingulate cortex (PCC), increased local efficiency in mPFC, ACC and PCC and increased connectivity between putamen and PCC, and caudate and mPFC (Fig. 6). However, no significant difference was found in global graph metrics between the two patient groups.
Altered brain maps of depression- and/or anxiety-related nodal graph metrics and connectivity. Nodes in brain maps represent ICNs and are located at the peak activation points in the brain of corresponding ICNs. Colors of nodes correspond to the colors of ICNs’ anatomical and functional categories shown at the bottom of the figure. Edges connecting to nodes represent the connectivity between ICNs. Significant differences in CD patients with high level of depression and/or anxiety compared to other patients are shown in a) ~ d): a increased connectivity strength in mPFC and ACC; b increased clustering coefficient in mPFC, ACC and PCC; c increased local efficiency in mPFC, ACC and PCC and d increased connectivity between mPFC and caudata, and PCC and putamen. mPFC: medial prefrontal cortex; ACC: anterior cingulate cortex; PCC: posterior cingulate cortex
Discussion
In the present study, we investigated the topological alterations of whole brain functional networks and the relationships between the possible alterations and clinical characteristics in CD patients. Compared to HCs, CD patients exhibited abnormal local and global topological patterns which could disturb the transmission of information. Specifically, altered local topological patterns were mainly located in the subcortical, sensorimotor, cognitive control and default-mode networks in CD patients. Moreover, the connectivity strength of putamen closely correlated with CD duration in the patient group. In addition, CD patients with significantly higher HADS-A and/or HADS-D had altered local topological patterns associated with ACC, mPFC and PCC compared to other patients.
In this study, brain functional connectivity networks were constructed based on connections between ICNs (nodes) and network nodes were defined by ICA, a natural and data-driven approach (Yu et al. 2015). In the recent studies, brain network nodes were also defined by parcellation approaches based on prior information, including predefined anatomical templates such as automated anatomical labeling (AAL) (Lynall et al. 2010), randomly generated templates (Fornito et al. 2010), and voxel-based divisions (Hedden et al. 2009). The efficacy of template-based or voxel-based parcellation approaches are limited, because the atlas-based regions of interest (ROIs) do not necessarily respect the functional boundaries of the human brain nor do they reflect individual subject differences (Craddock et al. 2012; Shirer et al. 2012; Smith et al. 2011). In contrast, ICA could maximally isolate spatially-independent component as functionally homogeneous nodes by analyzing the temporal coherence of BOLD signal (Calhoun and Allen 2013; Yu et al. 2011). Similar to those observed in previous studies using high model order ICA decompositions (Allen et al. 2014, 2011; Calhoun and Allen 2013), the brain functional connectivity network of this study presents modular organization within subcortical, auditory, sensorimotor, visual, cognitive control, default-mode and cerebellar networks, as well as anticorrelation between default-mode and task-positive ICNs (Fig. 2).
Statistical analysis results showed that CD patients had abnormal connectivity patterns (nodal graph metrics and connectivity between ICNs) related to the ICNs including the putamen, thalamus, postcentral gyrus, SMA, SFG, aMCC, insula, supramarginal gyrus, precuneus and STG. Most of the aforementioned ICNs have been reported in the previous neuroimaging studies of IBD. Compared to HCs, CD patients had decreased GM volumes in the aMCC (Agostini et al. 2013a). Zilou AK et al. observed that IBD patients had decreased GM volumes in the right SMA and left superior parietal lobe (Zikou et al. 2014). Jui-Yang Hong et al. found the patients with ulcerative colitis (UC), another kind of IBD, had the increased cortical thickness in the primary somatosensory cortex, and decreased cortical thickness in the insula (Hong et al. 2014). The results in this study overlapped the brain regions showing functional and structural abnormalities in our previous studies of CD (Bao et al. 2015, 2016, 2016). In addition, we also found that CD patients had declined functional coupling between the subcortical and cortical regions including the decreased connectivity of putamen to the aMCC, insula and precuneus. Thereby, the present findings may reveal that a host of ICNs with aberrant connectivity patterns are implicated in the pathophysiology of CD.
Three nodal graph metrics are involved in our study. Nodal connectivity strength is equal to the sum of the weighted edges (coefficients of connectivity) connecting to the node (e.g., ICN) (Yu et al. 2015) and can directly reflect the importance of the node in the corresponding brain functional network (Dai et al. 2015). Decreased nodal connectivity strength implies that there are weakened or broken edges connecting to the node, which result in disrupted connectivity patterns. Nodal clustering coefficient is equal to the possibility that the neighbors of a node are also connected with each other (Watts and Strogatz 1998). It represents the specialized processing capacity of a node within the densely interconnected graph (Mikail Rubinov and Olaf Sporns 2010). Decreased nodal clustering coefficients might represent the decreased local functional interconnections and subsequent information transfer efficiency. Nodal local efficiency is a measurement of the remaining processing capacity after the node is removed and reveals the fault tolerance of the nodal graph (Latora and Marchiori 2001). The decreased nodal local efficiency might induce the node being more vulnerable to being attacked. In the present study, decreased nodal graph metrics were mainly located in several ICNs including the putamen, thalamus, postcentral gyrus, SMA, SFG, aMCC, supramarginal gyrus, precuneus and STG in CD patients, compared to HCs. Functional and structural changes of these ICNs have been documented in previous studies of CD (Agostini et al. 2013a, b; Bao et al. 2015, 2016a, b). The present findings of altered connectivity patterns in these ICNs might provide further topological evidence to support the view that dysfunctions of CNS are implicated in the pathophysiology of CD.
Our study detected abnormalities in connectivity patterns of aMCC in CD patients, associated with decreased aMCC nodal graph metrics and decreased aMCC-related connectivities to the putamen, thalamus, SMA, insula and precuneus. To date, growing body of evidence indicates that aMCC and a range of regions connecting with aMCC might be involved in the chronic diseases, and play a crucial role in processing pain-related, cognitive or other negative feedback information. A review of aMCC claimed that it might activate for integrating information from pain, negative affect and cognition (Shackman et al. 2011). Combining of resting-state fMRI and electrical brain stimulation, Parvizi et al. indicated that electrical charge delivering in the aMCC might induce the changes of emotional and cognitive condition in epilepsy patients, and found the links of the aMCC to the frontoinsular as well as some subcortical regions (Parvizi et al. 2013). Thalamus is one of critical regions related to the interaction of BGA and regulation of homeostasis (Sherman 2016). SMA is involved in the sensory-discriminative of pain (Bao et al. 2016) as well as the processing of the affective (Cifre et al. 2012). Precuneus is usually activated in the self-reference process (Tao et al. 2015). Therefore, the present results show that altered aMCC connectivity patterns might bias CD patients toward unpleasant or painful perceptions, negative emotion and/or cognition.
We found abnormal connectivity patterns of the putamen in CD patients, which was associated with the duration of CD patients in this study. Putamen is activated during pain (Starr et al. 2011) and is reported to be related to sensory aspects of pain processing, and to regulate negative emotions and to be implicated in certain affective disorders, such as, depression and bipolar disorder (Park et al. 2017; Oquendo et al. 2007). In our study, CD patients had higher depression and anxiety levels than HCs. Putamen had altered connectivity to the aMCC and insula in CD patients, compared to HCs. Besides the aMCC, insula is also an important component in the circuit of emotional processing of grief, sadness and pain (Mee et al. 2006). Thereby, our findings could suggest that as a potential role, altered putamen connectivity patterns may be implicated in the abnormal CD psychological level or painful perceptions as well. In addition, putamen exhibited decreased connectivity strengths in patients following the increase of CD duration.
We also found significant changes of connectivity patterns in other ICNs related to the postcentral gyrus, SFG, STG and supramarginal gyrus in CD patients. Postcentral gyrus (BA 3) is situated in the primary somatosensory cortex and is responsible for integrating somatosensory information (Pijnenburg et al. 2015). SFG (BA 6) plays a role in the planning of complex and coordinated movements (Kakigi 2010). STG (BA 22) is involved in the peripheral selective attention (Seymour et al. 2017). And supramarginal gyrus (BA 40) is an important part in the processing of proprioceptive signals (Naito et al. 2016). Altered connectivity patterns in ICNs mentioned above might indicate the dysregulations of visceral and physical responses in CD patients.
Compared to HCs, global graph metrics in CD patients exhibited decreased averaged clustering coefficient, averaged local efficiency and global efficiency, and increased characteristic path length. Characteristic path length is an indicator of the overall routing efficiency in a graph (Achard and Bullmore 2007). Global efficiency is a measurement that examines the efficiency of nodes exchanging information concurrently (Achard and Bullmore 2007). The results of global graph metrics in this study may suggest reduced effective interactions across different ICNs in CD patients. However, no significant relationship was found between the global graph metrics and CD clinical characteristics, which might need further studies in the future.
In addition, CD patients with significantly high HADS-A and/or HADS-D had increased nodal graph metrics in ACC, mPFC and PCC, and related increased connectivity with putamen and caudate compared to other patients. Activation of the ACC contributes to various negative effects in chronic pain, including the unpleasantness of pain (Bliss et al. 2016). Moreover, activated ACC has been found in the study of patients with anxiety disorders and/or depression (Andreescu et al. 2017; Han et al. 2014). The function of mPFC is relevant to different aspects of social cognitive processing (Amodio and Frith 2006). Lane et al. found that mPFC was activated by painful stimuli induced by negative emotion and suggested that mPFC is involved in thinking about the unpleasantness of emotionally-arousing pictures (Amodio and Frith 2006; Lane et al. 1998). PCC is involved in episodic memory, emotional regulation and self-reflection, and has been found that had increased functional connectivity in major deprssion patients (Zhou et al. 2010). Caudate is known to be associated with emotional components of pain experience (Liu et al. 2017; Scott et al. 2006). As noted above, we thereby speculate that high level of depression and anxiety in CD patients could have a more adverse effect on regulating affects or cognition, which might further induce dysfunctions of pain modulatory system.
Some limitations of the present study should be addressed. Firstly, in order to investigate the effects of psychological factors, we classified CD patients into two subgroups in this study and found patients with significantly higher HADS-A and/or HADS-D had increased connectivity patterns in the mPFC, ACC and PCC. Actually, except to classification of CD patients, we also performed the statistical analysis between CD patients and HCs with anxiety and depression scores as additional covariates, and found the significant between-group differences were disappeared including nodal graph metrics of putamen (connectivity strength and local efficiency), thalamus (clustering coefficient and local efficiency), and connectivity of putamen to aMCC and insula. These distinguished findings might be due to different statistical analysis approaches and relevant subgroup of CD patients. Furthermore, more attentions should be paid to this issue in the future study. Secondly, in our study, we only focused on the changes of brain functional network topology in CD patients. Structural network topology also could provide crucial information about CD in brain-imaging studies. Future studies on investigating the structural network topology and a combination of the functional and structural network topology could help to better clarify the pathophysiology of CD.
Conclusion
The current study revealed significant topological alterations of brain functional networks in CD patients, associated with the key components of the pain, sensation, emotion and cognition systems. The altered connectivity patterns of putamen closely correlated with the CD duration, and the aberrant connectivity patterns of ACC, mPFC and PCC were related to higher level of depression and/or anxiety in CD patients. By revealing CD-related changes in topological patterns of brain functional networks, this study provides a fresh perspective and further evidence on the pathophysiology of CD.
References
Abou-Elseoud, A., Starck, T., Remes, J., Nikkinen, J., Tervonen, O., & Kiviniemi, V. (2010). The effect of model order selection in group PICA. Human Brain Mapping, 31(8), 1207–1216. https://doi.org/10.1002/hbm.20929.
Achard, S., & Bullmore, E. (2007). Efficiency and cost of economical brain functional networks. Plos Computational Biology, 3(2), e17. https://doi.org/10.1371/journal.pcbi.0030017.
Agostini, A., Benuzzi, F., Filippini, N., Bertani, A., Scarcelli, A., Farinelli, V., et al. (2013a). New insights into the brain involvement in patients with Crohn’s disease: a voxel-based morphometry study. Neurogastroenterology and Motility, 25(2), 147–182. https://doi.org/10.1111/nmo.12017.
Agostini, A., Filippini, N., Benuzzi, F., Bertani, A., Scarcelli, A., Leoni, C., et al. (2013b). Functional magnetic resonance imaging study reveals differences in the habituation to psychological stress in patients with Crohn’s disease versus healthy controls. Journal of the Mechanical Behavior of Biomedical Materials, 36(5), 477–487. https://doi.org/10.1007/s10865-012-9441-1.
Allen, E. A., Damaraju, E., Plis, S. M., Erhardt, E. B., Eichele, T., & Calhoun, V. D. (2014). Tracking whole-brain connectivity dynamics in the resting state. Cerebral Cortex, 24(3), 663–676. https://doi.org/10.1093/cercor/bhs352.
Allen, E. A., Erhardt, E. B., Damaraju, E., Gruner, W., Segall, J. M., Silva, R. F., et al. (2011). A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience, 5, 2, https://doi.org/10.3389/fnsys.2011.00002.
Amodio, D. M., & Frith, C. D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience, 7(4), 268–277. https://doi.org/10.1038/nrn1884.
Andreescu, C., Tudorascu, D., Sheu, L. K., Rangarajan, A., Butters, M. A., Walker, S., et al. (2017). Brain structural changes in late-life generalized anxiety disorder. Psychiatry Research, 268, 15–21. https://doi.org/10.1016/j.pscychresns.2017.08.004.
Bao, C., Liu, P., Liu, H., Jin, X., Calhoun, V. D., Wu, L., et al. (2016a). Different brain responses to electro-acupuncture and moxibustion treatment in patients with Crohn’s disease. Scientific Reports, 6, 36636. https://doi.org/10.1038/srep36636.
Bao, C. H., Liu, P., Liu, H. R., Wu, L. Y., Jin, X. M., Wang, S. Y., et al. (2016b). Differences in regional homogeneity between patients with Crohn’s disease with and without abdominal pain revealed by resting-state functional magnetic resonance imaging. Pain, 157(5), 1037–1044. https://doi.org/10.1097/j.pain.0000000000000479.
Bao, C. H., Liu, P., Liu, H. R., Wu, L. Y., Shi, Y., Chen, W. F., et al. (2015). Alterations in brain grey matter structures in patients with crohn’s disease and their correlation with psychological distress. Journal of Crohns & Colitis, 9(7), 532–540. https://doi.org/10.1093/ecco-jcc/jjv057.
Bassett, D. S., & Gazzaniga, M. S. (2011). Understanding complexity in the human brain. Trends in Cognitive Sciences, 15(5), 200–209. https://doi.org/10.1016/j.tics.2011.03.006.
Bell, A. J., & Sejnowski, T. J. (1995). An information-maximization approach to blind separation and blind deconvolution. Neural Computation, 7(6), 1129–1159.
Best, W. R., Becktel, J. M., & Singleton, J. W. (1979). Rederived values of the eight coefficients of the Crohn’s Disease Activity Index (CDAI). Gastroenterology, 77(4 Pt 2), 843–846.
Bliss, T. V., Collingridge, G. L., Kaang, B. K., & Zhuo, M. (2016). Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nature Reviews Neuroscience, 17(8), 485–496. https://doi.org/10.1038/nrn.2016.68.
Calhoun, V. D., & Adali, T. (2012). Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Review of Biomedical Engineering, 5, 60–73. https://doi.org/10.1109/rbme.2012.2211076.
Calhoun, V. D., Adali, T., Pearlson, G. D., & Pekar, J. J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14(3), 140–151.
Calhoun, V. D., & Allen, E. (2013). Extracting intrinsic functional networks with feature-based group independent component analysis. Psychometrika, 78(2), 243–259. https://doi.org/10.1007/s11336-012-9291-3.
Casellas, F., Vivancos, J. L., Sampedro, M., & Malagelada, J.-R. (2005). Relevance of the phenotypic characteristics of Crohn’s disease in patient perception of health-related quality of life. The American Journal of Gastroenterology, 100(12), 2737–2742.
Cifre, I., Sitges, C., Fraiman, D., Munoz, M. A., Balenzuela, P., Gonzalez-Roldan, A., et al. (2012). Disrupted functional connectivity of the pain network in fibromyalgia. Psychosomatic Medicine, 74(1), 55–62. https://doi.org/10.1097/PSY.0b013e3182408f04.
Clark, M., Colombel, J. F., Feagan, B. C., Fedorak, R. N., Hanauer, S. B., Kamm, M. A., et al. (2007). American gastroenterological association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21–23, 2006. Gastroenterology, 133(1), 312–339. https://doi.org/10.1053/j.gastro.2007.05.006.
Cordes, D., Haughton, V. M., Arfanakis, K., Wendt, G. J., Turski, P. A., Moritz, C. H., et al. (2000). Mapping functionally related regions of brain with functional connectivity MR imaging. American Journal of Neuroradiology, 21(9), 1636–1644.
Craddock, R. C., James, G. A., Holtzheimer, P. E. 3rd, Hu, X. P., & Mayberg, H. S. (2012). A whole brain fMRI atlas generated via spatially constrained spectral clustering. Human Brain Mapping, 33(8), 1914–1928. https://doi.org/10.1002/hbm.21333.
Dai, Z., Yan, C., Li, K., Wang, Z., Wang, J., Cao, M., et al. (2015). Identifying and Mapping Connectivity Patterns of Brain Network Hubs in Alzheimer’s Disease. Cerebral Cortex, 25(10), 3723–3742. https://doi.org/10.1093/cercor/bhu246.
Damaraju, E., Allen, E. A., Belger, A., Ford, J. M., McEwen, S., Mathalon, D. H., et al. (2014). Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clinical, 5, 298–308. https://doi.org/10.1016/j.nicl.2014.07.003.
Erhardt, E. B., Rachakonda, S., Bedrick, E. J., Allen, E. A., Adali, T., & Calhoun, V. D. (2011). Comparison of multi-subject ICA methods for analysis of fMRI data. Human Brain Mapping, 32(12), 2075–2095. https://doi.org/10.1002/hbm.21170.
Fornito, A., Zalesky, A., & Bullmore, E. T. (2010). Network scaling effects in graph analytic studies of human resting-state FMRI data. Frontiers in Systems Neuroscience, 4, 22. https://doi.org/10.3389/fnsys.2010.00022.
Han, K. M., Choi, S., Jung, J., Na, K. S., Yoon, H. K., Lee, M. S., et al. (2014). Cortical thickness, cortical and subcortical volume, and white matter integrity in patients with their first episode of major depression. Journal of Affective Disorders, 155, 42–48. https://doi.org/10.1016/j.jad.2013.10.021.
He, H., Yu, Q., Du, Y., Vergara, V., Victor, T. A., Drevets, W. C., et al. (2016). Resting-state functional network connectivity in prefrontal regions differs between unmedicated patients with bipolar and major depressive disorders. Journal of Affective Disorders, 190, 483–493. https://doi.org/10.1016/j.jad.2015.10.042.
Hedden, T., Van Dijk, K. R., Becker, J. A., Mehta, A., Sperling, R. A., Johnson, K. A., et al. (2009). Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. Journal of Neuroscience, 29(40), 12686–12694. https://doi.org/10.1523/jneurosci.3189-09.2009.
Hong, J. Y., Labus, J. S., Jiang, Z., Ashe-Mcnalley, C., Dinov, I., Gupta, A., et al. (2014). Regional neuroplastic brain changes in patients with chronic inflammatory and non-inflammatory visceral pain. PLoS One, 9(1), e84564. https://doi.org/10.1371/journal.pone.0084564.
Irvine, E. J., Feagan, B., Rochon, J., Archambault, A., Fedorak, R. N., Groll, A., et al. (1994). Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn’s Relapse Prevention Trial Study Group. Gastroenterology, 106(2), 287–296.
Kakigi, R. (2010). [Pain and itch perception in the human limbic system]. Rinsho Shinkeigaku, 50(11), 997–999.
Kalla, R., Ventham, N. T., Satsangi, J., & Arnott, I. (2014). Crohn’s disease. British Medical Journal, 349(7984), 27–31.
Kiviniemi, V., Starck, T., Remes, J., Long, X., Nikkinen, J., Haapea, M., et al. (2009). Functional segmentation of the brain cortex using high model order group PICA. Human Brain Mapping, 30(12), 3865–3886. https://doi.org/10.1002/hbm.20813.
Lane, R. D., Reiman, E. M., Axelrod, B., Yun, L. S., Holmes, A., & Schwartz, G. E. (1998). Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience, 10(4), 525–535.
Latora, V., & Marchiori, M. (2001). Efficient behavior of small-world networks. Physical Review Letters, 87(19), 198701.
Liang, X., Zou, Q., He, Y., & Yang, Y. (2016). Topologically Reorganized Connectivity Architecture of Default-Mode, Executive-Control, and Salience Networks across Working Memory Task Loads. Cerebral Cortex, 26(4), 1501–1511. https://doi.org/10.1093/cercor/bhu316.
Liu, P., Wang, G., Liu, Y., Zeng, F., Lin, D., Yang, X., et al. (2017). Disrupted intrinsic connectivity of the periaqueductal gray in patients with functional dyspepsia: a resting-state fMRI study. Neurogastroenterology and Motility, 29(8), https://doi.org/10.1111/nmo.13060.
Lynall, M. E., Bassett, D. S., Kerwin, R., McKenna, P. J., Kitzbichler, M., Muller, U., et al. (2010). Functional connectivity and brain networks in schizophrenia. Journal of Neuroscience, 30(28), 9477–9487. https://doi.org/10.1523/jneurosci.0333-10.2010.
Mee, S., Bunney, B. G., Reist, C., Potkin, S. G., & Bunney, W. E. (2006). Psychological pain: a review of evidence. Journal of Psychiatric Research, 40(8), 680–690.
Naito, E., Amemiya, K., & Morita, T. (2016). [Parietal Cortices and Body Information]. Brain and Nerve, 68(11), 1313–1320. https://doi.org/10.11477/mf.1416200595.
Ng, S. C., Tang, W., Ching, J. Y., Wong, M., Chow, C. M., Hui, A., et al. (2013). Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology, 145(1), 158–165. e152.
Oquendo, M. A., Hastings, R. S., Huang, Y. Y., Simpson, N., Ogden, R. T., Hu, X. Z., et al. (2007). Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Archives of General Psychiatry, 64(2), 201–208. https://doi.org/10.1001/archpsyc.64.2.201.
Park, I. H., Lee, B. C., Kim, J. J., Kim, J. I., & Koo, M. S. (2017). Effort-Based Reinforcement Processing and Functional Connectivity Underlying Amotivation in Medicated Patients with Depression and Schizophrenia. Journal of Neuroscience, 37(16), 4370–4380. https://doi.org/10.1523/jneurosci.2524-16.2017.
Parvizi, J., Rangarajan, V., Shirer, W. R., Desai, N., & Greicius, M. D. (2013). The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron, 80(6), 1359–1367. https://doi.org/10.1016/j.neuron.2013.10.057.
Pijnenburg, M., Brumagne, S., Caeyenberghs, K., Janssens, L., Goossens, N., Marinazzo, D., et al. (2015). Resting-State Functional Connectivity of the Sensorimotor Network in Individuals with Nonspecific Low Back Pain and the Association with the Sit-to-Stand-to-Sit Task. Brain Connect, 5(5), 303–311. https://doi.org/10.1089/brain.2014.0309.
Pizzi, L. T., Weston, C. M., Goldfarb, N. I., Moretti, D., Cobb, N., Howell, J. B., et al. (2006). Impact of chronic conditions on quality of life in patients with inflammatory bowel disease. Inflammatory Bowel Diseases, 12(1), 47–52.
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59(3), 2142–2154. https://doi.org/10.1016/j.neuroimage.2011.10.018.
Power, J. D., Cohen, A. L., Nelson, S. M., Wig, G. S., Barnes, K. A., Church, J. A., et al. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. https://doi.org/10.1016/j.neuron.2011.09.006.
Power, J. D., Schlaggar, B. L., Lessov-Schlaggar, C. N., & Petersen, S. E. (2013). Evidence for hubs in human functional brain networks. Neuron, 79(4), 798–813. https://doi.org/10.1016/j.neuron.2013.07.035.
Rubinov, M., & Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage, 52(3), 1059–1069.
Rubinov, M., & Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage, 52(3), 1059–1069. https://doi.org/10.1016/j.neuroimage.2009.10.003.
Scott, D. J., Heitzeg, M. M., Koeppe, R. A., Stohler, C. S., & Zubieta, J. K. (2006). Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. Journal of Neuroscience, 26(42), 10789–10795. https://doi.org/10.1523/jneurosci.2577-06.2006.
Seymour, J. L., Low, K. A., Maclin, E. L., Chiarelli, A. M., Mathewson, K. E., Fabiani, M., et al. (2017). Reorganization of neural systems mediating peripheral visual selective attention in the deaf: An optical imaging study. Hearing Research, 343, 162–175. https://doi.org/10.1016/j.heares.2016.09.007.
Shackman, A. J., Salomons, T. V., Slagter, H. A., Fox, A. S., Winter, J. J., & Davidson, R. J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12(3), 154–167. https://doi.org/10.1038/nrn2994.
Sherman, S. M. (2016). Thalamus plays a central role in ongoing cortical functioning. Nature Neuroscience, 19(4), 533–541. https://doi.org/10.1038/nn.4269.
Shirer, W. R., Ryali, S., Rykhlevskaia, E., Menon, V., & Greicius, M. D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex, 22(1), 158–165. https://doi.org/10.1093/cercor/bhr099.
Smith, S. M., Miller, K. L., Salimi-Khorshidi, G., Webster, M., Beckmann, C. F., Nichols, T. E., et al. (2011). Network modelling methods for FMRI. Neuroimage, 54(2), 875–891. https://doi.org/10.1016/j.neuroimage.2010.08.063.
Sporns, O. (2011). The human connectome: a complex network. Annals of the New York Academy of Sciences, 1224(1), 109–125.
Starr, C. J., Sawaki, L., Wittenberg, G. F., Burdette, J. H., Oshiro, Y., Quevedo, A. S., et al. (2011). The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain, 134(Pt 7), 1987–2004. https://doi.org/10.1093/brain/awr117.
Stasi, C., & Orlandelli, E. (2008). Role of the brain-gut axis in the pathophysiology of Crohn’s disease. Digestive Diseases, 26(2), 156–166. https://doi.org/10.1159/000116774.
Tao, Y., Liu, B., Zhang, X., Li, J., Qin, W., Yu, C., et al. (2015). The Structural Connectivity Pattern of the Default Mode Network and Its Association with Memory and Anxiety. Frontiers in Neuroanatomy, 9, 152. https://doi.org/10.3389/fnana.2015.00152.
Tijms, B. M., Yeung, H. M., Sikkes, S. A., Moller, C., Smits, L. L., Stam, C. J., et al. (2014). Single-subject gray matter graph properties and their relationship with cognitive impairment in early- and late-onset Alzheimer’s disease. Brain Connect, 4(5), 337–346. https://doi.org/10.1089/brain.2013.0209.
Tomasi, D., & Volkow, N. D. (2011). Association between functional connectivity hubs and brain networks. Cerebral Cortex, 21(9), 2003–2013. https://doi.org/10.1093/cercor/bhq268.
Wang, J., Wang, X., He, Y., Yu, X., Wang, H., & He, Y. (2015). Apolipoprotein E epsilon4 modulates functional brain connectome in Alzheimer’s disease. Human Brain Mapping, 36(5), 1828–1846. https://doi.org/10.1002/hbm.22740.
Watts, D. J., & Strogatz, S. H. (1998). Collective dynamics of ‘small-world’networks. Nature, 393(6684), 440–442.
Yu, Q., Erhardt, E. B., Sui, J., Du, Y., He, H., Hjelm, D., et al. (2015). Assessing dynamic brain graphs of time-varying connectivity in fMRI data: application to healthy controls and patients with schizophrenia. Neuroimage, 107, 345–355. https://doi.org/10.1016/j.neuroimage.2014.12.020.
Yu, Q., Plis, S. M., Erhardt, E. B., Allen, E. A., Sui, J., Kiehl, K. A., et al. (2011). Modular Organization of Functional Network Connectivity in Healthy Controls and Patients with Schizophrenia during the Resting State. Frontiers in Systems Neuroscience, 5, 103. https://doi.org/10.3389/fnsys.2011.00103.
Zhou, Y., Yu, C., Zheng, H., Liu, Y., Song, M., Qin, W., et al. (2010). Increased neural resources recruitment in the intrinsic organization in major depression. Journal of Affective Disorders, 121(3), 220–230. https://doi.org/10.1016/j.jad.2009.05.029.
Zigmond, A. S., & Snaith, R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67(6), 361–370.
Zikou, A. K., Kosmidou, M., Astrakas, L. G., Tzarouchi, L. C., Tsianos, E., & Argyropoulou, M. I. (2014). Brain involvement in patients with inflammatory bowel disease: a voxel-based morphometry and diffusion tensor imaging study. European Radiology, 24(10), 2499–2506. https://doi.org/10.1007/s00330-014-3242-6.
Funding
This study was supported by the National Natural Science Foundation of China under Grant Nos. 81,471,738, 81,771,918, 81,471,811, and the National Basic Research Program of China, Nos. 2009CB522900, 2015CB554501, 2014CB543203 and 2015CB856403, and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Ethical approval
All procedures performed in the present study were in accordance with the Declaration of Helsinki and were approved by the local hospital subcommittee on human studies. All participants signed informed consent forms prior to the investigation. The methods of this study were conducted in accordance with the approved guidelines.
Rights and permissions
About this article
Cite this article
Liu, P., Li, R., Bao, C. et al. Altered topological patterns of brain functional networks in Crohn’s disease. Brain Imaging and Behavior 12, 1466–1478 (2018). https://doi.org/10.1007/s11682-017-9814-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-017-9814-8