Abstract
Exercise may play a role in moderating eating behaviors. The purpose of this study was to examine the effect of an acute bout of exercise on neural responses to visual food stimuli in children ages 8–11 years. We hypothesized that acute exercise would result in reduced activity in reward areas of the brain. Using a randomized cross-over design, 26 healthy weight children completed two separate laboratory conditions (exercise; sedentary). During the exercise condition, each participant completed a 30-min bout of exercise at moderate-intensity (~ 67% HR maximum) on a motor-driven treadmill. During the sedentary session, participants sat continuously for 30 min. Neural responses to high- and low-calorie pictures of food were determined immediately following each condition using functional magnetic resonance imaging. There was a significant exercise condition*stimulus-type (high- vs. low-calorie pictures) interaction in the left hippocampus and right medial temporal lobe (p < 0.05). Main effects of exercise condition were observed in the left posterior central gyrus (reduced activation after exercise) (p < 0.05) and the right anterior insula (greater activation after exercise) (p < 0.05). The left hippocampus, right medial temporal lobe, left posterior central gyrus, and right anterior insula appear to be activated by visual food stimuli differently following an acute bout of exercise compared to a non-exercise sedentary session in 8–11 year-old children. Specifically, an acute bout of exercise results in greater activation to high-calorie and reduced activation to low-calorie pictures of food in both the left hippocampus and right medial temporal lobe. This study shows that response to external food cues can be altered by exercise and understanding this mechanism will inform the development of future interventions aimed at altering energy intake in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Physical activity promotion is important in children for their present health as well as to establish patterns of activity that will persist into adulthood (Kwon et al. 2015). The 2008 Physical Activity and Health Guidelines for Americans suggest that children obtain at least 60 min of physical activity (PA) per day, with 3 days including muscle strengthening and bone strengthening components (Physical Activity Guidelines Advisory Committee 2009). Unfortunately, evidence suggests that many children do not meet these guidelines (Ekelund et al. 2011).
The relationship between physical activity and weight management has long been an area of controversy and interest to researchers and practitioners (Kist et al. 2015). Physical activity increases energy expenditure (LeCheminant et al. 2009) and, thus, is thought to promote body weight maintenance (Donnelly et al. 2004). However, there has also been recent interest in whether or not physical activity and exercise influence energy intake or related correlates, such as appetite (Allsop et al. 2016; Chaput et al. 2016; Thivel et al. 2014).
Greater availability of neuroimaging techniques (e.g., functional MRI or fMRI) has allowed for a better understanding of the role of the brain in eating-related behaviors. Multiple studies have employed a protocol of passively showing pictures of food (high-calorie, low-calorie, neutral, etc.) while brain processes are examined (Yokum et al. 2011; Ng et al. 2011; Bruce et al. 2013). Initial studies of these picture-viewing tasks suggest that neural responses to visual food stimuli are associated with energy intake and predict weight management though very few have looked at actual energy intake (Murdaugh et al. 2012; Lawrence et al. 2012).
Accumulating evidence in adults suggests that acute bouts of exercise and exercise training may influence neural responses to pictures of food (Evero et al. 2012; Cornier et al. 2012; Crabtree et al. 2014; Hanlon et al. 2012; Killgore et al. 2013). For example, compared to a resting condition, adults showed increased dorsolateral prefrontal cortex (DLPFC) and decreased orbitofrontal cortex (OFC) and hippocampus activity after exercise when viewing high-calorie pictures (Killgore et al. 2013). Similarly, studies show decreased OFC, insula, and striatum (putamen specifically) activity to high-calorie foods following exercise rather than non-exercise conditions (Killgore et al. 2013; Evero et al. 2012). These findings fit nicely into the adult literature that suggests mesolimbic reward and emotion systems including the hippocampus, amygdala, basal ganglia (primarily the areas of the striatum), OFC, and insula are heavily involved in visual food processing and potentially feeding behaviors (Killgore et al. 2013; Spence et al. 2015; Berridge 2009; Crabtree et al. 2014; Lenard and Berthoud 2008).
The relationship between exercise and neural response to food pictures has also recently been investigated in adolescents using event-related potentials (ERPs) to demonstrate that food stimuli resulted in lower neural activation (i.e., decreased amplitude of the P3b ERP component) following exercise compared to non-food stimuli (Fearnbach et al. 2016). The difference in neural activity between food and non-food stimuli was not present following sedentary activity (Fearnbach et al. 2016). However, the extent to which the relationship between the brain response to food-related stimuli and exercise exists in children is not yet known. Related studies in children have indicated that exercise can increase cognitive control and improve self-regulatory processes (Hillman et al. 2009; Buck et al. 2008). Other exercise studies in children have noted structural brain differences, specifically in the hippocampus and dorsal striatum, of physically-fit children’s brains compared to unfit children (Chaddock et al. 2010a, b). Though the literature is still developing, evidence to date largely suggests the possibility of an exercise effect on neural processes and even neural structures in children.
With the growing concern over the lack of physical activity in children and the implications this may have on indicators of health, such as obesity, investigation of the interactions between exercise and neural responses to food is relevant. Therefore, the primary aim of this study was to examine the effect of an acute bout of exercise on neural responses (using fMRI) to food stimuli in children ages 8–11 years. The secondary aim of this study was to determine the influence of exercise on the neural response to different types of food pictures (low-calorie vs. high-calorie). We hypothesized that acute exercise would result in reduced activity in reward areas of the brain and specifically would result in reduced neural activity in response to high-calorie pictures of food.
Methods
Participants
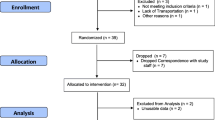
This study was approved by the University’s Institutional Review Board and all parents provided written informed consent and children provided assent prior to beginning. Participants were between the ages of 8–11 years. Each participating child was considered healthy, able to walk/jog continuously for 30 min, had normal and regular sleep patterns (as reported by a parent) (Hirshkowitz et al. 2015) and regularly consumed breakfast, lunch, and dinner over the previous 6 months. Children that participated in competitive year-round sports that practiced more than 3 times per week, were claustrophobic, actively dieting, had a metabolic disorder or disease, eating disorder, or had a characteristic (e.g., braces) not compatible with the MRI machine were excluded.
Study design and overview
Using a cross-over design, each participant completed two separate laboratory conditions (exercise and sedentary); determined randomly and counterbalanced. Exercise bouts and fMRI measurements were conducted at the MRI Research Facility located on the University campus. During the exercise condition, each participant completed a 30-min bout of exercise at moderate intensity (~ 67% HR maximum), on a motor-driven treadmill. Subsequently, each participant received the fMRI testing (within < 15 min), completing a task in which pictures of high- and low-calorie foods and blurred control pictures were shown. The sedentary condition was identical to the exercise condition except that participants sat at a table and played with games or read books available in the laboratory for a period of 30 min.
For both conditions, participants were scanned at the same time (between 8:00 and 10:00 am) to control for variations in brain response to food cues by time of day (Masterson et al. 2016), on the same day of the week, and with 1 week between conditions. Participants were instructed to follow the same schedule on the days of both conditions. Participants were required to have slept 8 + hours the night before, discontinued eating the previous night by 8 pm, and refrained from vigorous-intensity exercise or caffeine for 24 + hours prior to each condition. Participants were instructed to wear comfortable clothes and exercise shoes. All pre-study instructions where verified by parental report on the day of the study.
Procedures
After reporting to the MRI Facility, each research participant and his/her parent(s) was given an overview of the study; screened for safety, and provided informed consent/assent to participate. Next, demographic information, body weight and height, body composition, and pubertal development were assessed. Children completed the Freddy Fullness Scale, a validated child-appropriate script and picture of a person used to help each child understand and report their current level of fullness (Keller et al. 2006). Each participant then received instructions on the MRI session followed by a 30-min bout of exercise or 30 min of sedentary activity. Immediately following, each participant was positioned in the MRI scanner and MRI data were collected.
The second laboratory session was similar to the first session except: (1) only the Freddy Fullness scale was completed, and (2) the alternate exercise or sedentary condition was employed.
The exercise condition included a total of 30 min of moderate-intensity walking on a treadmill. This amount was similar to other studies (Hillman et al. 2011) and designed to prevent undue burden on the participants. Prior to the exercise session, each participant was fitted with a HR monitor (Polar Electro, Kempele, Finland). The protocol included: a 3-min warm-up, 3 min of walking at 3.0 mph, and 0.5% increase in grade every minute until each participant reached a steady state HR of ~ 135 bpm; thereafter, speed and grade were maintained. The target of 135 bpm was based on percentage of predicted maximum HR using the equation [208 – (0.7 × Age)] (Mahon et al. 2010). Based on the age of the participants (8 to 11 years of age), a HR target of ~ 135 bpm is approximately 67% of maximum HR or equivalent to the high end of moderate-intensity activity (Tompkins et al. 2015). Overall, HR was maintained within 3 bpm of the target. During the sedentary condition, each participant was able to play with toys, small games, or read books, but remained seated at a table.
Measurements
Height was measured using a Seca model 213 stadiometer and weight using a Seca model 813 digital scale (Seca, Chino, CA). Height and weight were used to determine body mass index (BMI; kg/m2). Children’s BMI was also reported by percentile according to age and sex. Body fat percentage was assessed by bioelectrical impedance assessment using an Omron HBF306C handheld body fat loss monitor (Omron Healthcare Inc., Lake Forest, IL). This model of BIA monitor has been shown to have reasonable accuracy in estimating body fat percentage in children (Jensky-Squires et al. 2008). BIA has previously been shown to be a reliable and practical method for estimating body fat percentage in children (Talma et al. 2013).
Parents completed a general demographic questionnaire and the Pubertal Development Scale. The Pubertal Development Scale was adapted from Carskadon and Acebo and assesses basic questions for the parents to answer regarding the child’s development toward puberty (Carskadon and Acebo 1993). The Freddy Fullness Scale was used to assess hunger levels as described above.
Functional MRI stimuli and procedure
The pictures of food and some procedures used for this study have been published previously (Masterson et al. 2016) and based on the work of Killgore et al. (Killgore et al. 2003). Pictures of food shown during the fMRI scan included: low-calorie foods (vegetables, fruit, and whole-grains) (n = 120); high-calorie foods (e.g., hamburgers, hotdogs, and ice-cream) (n = 120); and the same pictures but blurred (n = 240). Blurred pictures provided a baseline in order to control for visual stimulation. Images were presented to subjects using E-Prime via an MR-compatible monitor placed at the head of the MRI scanner and were viewed by means of a mirror mounted to the head coil. Each picture was presented for 2.5 s with a 0.5 s gap between pictures. Pictures were randomized to two blocks of 60 high-calorie foods and two blocks of 60 low-calorie foods. One block of each type of stimulus was shown during the exercise condition and the other block of each type was shown during the sedentary condition randomly assigned and counterbalanced. Corresponding blurred pictures were presented during the same functional run as their un-blurred counterparts as described below.
Within each block of 60 pictures, pictures were further subdivided into sub-blocks of pictures randomized for order. Within sub-blocks the order of pictures was also randomized. Blocks of stimuli were always followed by identical corresponding blocks of blurred images. In all, there were a total of 48 blocks consisting of 10 photos each. Each visit consisted of two runs each 6 min in length (120 pictures in each run) with a 1 min rest period to provide a short break in order to reduce participant movement during scans.
For each picture, participants were asked to respond to the question, “would you eat this in the morning or the evening,” by pressing a button on a fiber optic button box with his/her right hand indicating “morning” or “evening” to the question. For all blurred pictures, participants were asked to press a button when the photo changed. These tasks were used to increase level of engagement and prevent extensive mind wandering during the task.
MRI data acquisition
We have reported similar procedures for data acquisition and analysis previously (Masterson et al. 2016). All MRI data acquisition was completed at the MRI Facility using a Siemens TIM-Trio 3.0T MRI scanner (Siemens Trio, Erlangen, Germany). Scans were identical for both sessions. The following parameters were used to obtain a T1-weighted MPRAGE structural scan for each subject: TE = 2.26 ms; T = 1900 ms; flip angle = 9°; matrix size = 256 × 215 mm; field of view = 250 × 218 mm; 176 slices; slice thickness = 1 mm; voxel size = 0.977 × 0.977 × 1 mm; 1 total acquisition. T2*-weighted echo-planar images were obtained using the following parameters: TE = 28 ms; TR = 1800 ms; flip angle = 90°; matrix size = 64 × 64; field of view = 220 × 220 mm; 36 slices; slice thickness = 3 mm; voxel size = 3.4 × 3.4 × 3 mm; 270 total acquisitions.
Data analysis
The MRI data were preprocessed and analyzed using the Analysis of Functional NeuroImages (AFNI) suite of software. All functional runs were time shifted and corrected for participant motion both within and between scan runs. TRs with excessive motion (defined as > 0.6 mm translation or > 0.3° rotation) were excluded from analysis along with the TRs immediately preceding and following the motion event. Participants with an excessive number of motion events were excluded from further analysis. Structural scans were co-registered with the functional scans. Spatial normalization was accomplished by first aligning all scans to the AC-PC plane using a rigid body transformation. Further normalization was accomplished by aligning all structural scans to a study-specific template using Advanced Normalization Tools software (ANTs; Version 1.9; http://sourceforge.net/projects/advants/). A regression analysis was conducted on the functional data using AFNI program 3dDeconvolve. Six regressors coding for motion (three translations and three rotations) and regressors coding for scanner drift were included as conditions of no interest. Two additional regressors were created coding for high-calorie blocks and low-calorie blocks. Blocks were modeled by convolving the standard hemodynamic response function with a 30-s boxcar function. The blurred control image blocks were used as an implicit baseline for visual stimuli in the model. The results of the regression analysis were spatially filtered using a 5 mm FWHM Gaussian kernel. Data were then spatially normalized for group analysis using a study specific template. The conditions of interest for the group analysis were: sedentary low-calorie, sedentary high-calorie, exercise low-calorie, exercise high-calorie.
For the group-level analysis, we conducted an ANOVA on the whole-brain data with activity level (exercise, sedentary) and stimulus type (low-, high-calorie) as fixed factors and participants as a random factor. In order to correct for multiple comparisons, the results of these tests were thresholded with a voxel-wise p-value of p < 0.02 and 40 contiguous voxels for the spatial extent threshold. These parameters were determined by conducting 10,000 Monte Carlo simulations using AFNI’s 3dClustSim to yield an overall p-value of p < 0.05. Thresholded statistical maps were subjected to visual inspection to find meaningful effects. Average beta coefficients were extracted from within the areas of activation and were subjected to further analysis (ANOVA and t-tests) in SPSS. A paired samples t-test was used to determine differences in Freddy Fullness scores between conditions.
Results
Twenty-six participants completed the study. Of these, 10 were female, 16 were male, and all were Caucasian. The mean age was 9.42 ± 1.17 years, height was 137.4 ± 10.25 cm, weight was 31.88 ± 7.91 kg, and body fat was 19.21 ± 9.88%. BMI was 16.68 ± 2.66 kg/m2 and BMI-for-age-and-sex percentile was 41.27 ± 32.91. Of the boys, 14 were classified as “prepubertal” and 2 as “early pubertal.” Of the girls, 6 were classified as “prepubertal,” 1 as “midpubertal,” 2 as “late pubertal,” and 1 as “postpubertal.” Prior to the exercise condition, fullness was 1.27 ± 1.15 and prior to the sedentary condition fullness was 1.18 ± 1.07; the difference was not statistically significant (F (1,25) = 0.10; p = 0.759).
The whole-brain ANOVA revealed significant clusters of activation indicating a main effect of exercise condition in the left postcentral gyrus and the right insula (Table 1). There was less activity in the left postcentral gyrus following the exercise condition than following the sedentary condition (F (1,25) = 19.66; p < 0.05), while there was greater activity in the right anterior insula following the exercise condition than following the sedentary condition (F (1,25) = 17.67; p < 0.001). Figure 1 depicts the clusters of activation and the mean parameter estimates.
Significant clusters of activation for the main effect of exercise condition included (a) left postcentral gyrus and (b) right insula. Cool colors indicate greater activation after sedentary activity; warm colors indicate greater activation after exercise. Higher intensity is denoted by lighter colors
We next examined the whole-brain data for evidence of a stimulus type (low-calorie vs. high-calorie) × activity (exercise vs. sedentary) interaction, which would indicate a differential response to high- and low-calorie foods as a function of exercise condition. We observed two regions where there was an interaction between activity level and stimulus type in fMRI activation; one in the right medial temporal lobe (including right hippocampus and parahippocampal gyrus; F (1,25) = 23.52; p < 0.001) and one in the left hippocampus (F (1,25) = 14.06; p < 0.001). Figure 2 depicts these regions and their associated mean parameter estimates. In both regions, there was greater activation in response to high- compared to low-calorie food stimuli following exercise.
We also note a main effect of stimulus type (high-calorie vs. low-calorie pictures) (Table 1). In each region, there was greater activation for the high-calorie stimulus compared to the low-calorie stimulus (p < 0.001).
Discussion
This is the first study to examine the effect of an acute bout of exercise on neural responses to food stimuli using fMRI in 8 to 11 year-old children. Relative to the sedentary condition, our results showed decreased activation of the post-central gyrus following exercise and increased activation of the right anterior insula following exercise. This observation is consistent with a body of literature showing the insula activation may be related to cardiovascular control and increases in activity during physical exertion (particularly higher exertion) (Williamson et al. 1997, 1999). Studies focusing more specifically on exercise and food cues, however, report contradictory results. For example, two studies using exercise and food cues in adults show decreased response in the insula following both acute and chronic exercise (Cornier et al. 2012; Evero et al. 2012). Our results are supported by Crabtree and colleagues, who observed an increased response in the insula following an acute bout of exercise in adults (Crabtree et al. 2014). Crabtree et al. postulated that the greater insular response following exercise may be due to the need for increased water intake following exercise (Crabtree et al. 2014). Others suggest that reward areas, such as the insula, are affected by physical activity. However, changes in insular activity may be related to the processing of positive food images (whether high or low calorie) rather than the physical activity itself (Kinder et al. 2014). Given the connections of the insula throughout the limbic system and medial pre-frontal cortex (Dupont et al. 2003), emotion and motivational processing associated with food images and exercise is a likely reason for the increased insula activity following exercise. Overall, given that our insula activity was found when collapsed across high and low-calorie food images, it is most likely a reflection of increased physical exertion and cardiovascular control; however, these findings of increased insula activity should be considered in light of possible changes in thirst and improved contrasts with non-pleasant stimuli to better tease apart the specifics of the insular response.
The post-central gyrus decrease in activity following the exercise relative to sedentary condition when collapsed across high- and low-calorie foods is also of interest. The post-central gyrus has been implicated in the integration of emotion and sensory processing during exposure to food cues, particularly in females (Atalayer et al. 2014). More commonly, however, the post-central gyrus (primary sensory cortex) is associated with touch sensations and proprioception that may increase during exercise when there is a strong proprioceptive demand, but can be reduced immediately following exercise when those proprioceptive demands are no longer present (Brummer et al. 2011; Christensen et al. 2000). Thus, the decrease in post-central gyrus activity likely reflects the decrease in proprioceptive demand going immediately from exercising to laying in the scanner.
The second aim of this study was to compare fMRI responses by the type of food pictured (high-calorie vs. low-calorie). In each region, including the visual processing stream, medial prefrontal cortex, right middle frontal gyrus, left superior temporal gyrus, right superior temporal gyrus, right inferior frontal gyrus, and right post central gyrus, there was greater activation for the high-calorie pictures. The results of this study are consistent with previous research suggesting that brain activation is consistently higher in response to high-calorie food pictures compared to low-calorie food pictures regardless of activity condition (Killgore et al. 2003; Sweet et al. 2012; Yokum et al. 2011), including in adolescents (Jensen and Kirwan 2015). This is also consistent with our original hypothesis.
The interaction between exercise and high- versus low-calorie food pictures observed in the hippocampus is quite interesting in the context of food behaviors. The hippocampus is known to play a role in shaping motivated behaviors specifically those related to incentive motivation (Tracy et al. 2001). Davidson and colleagues have noted that although structures such as the hypothalamus and hindbrain are typically considered responsible for sensing hormonal satiety cues such as insulin and leptin, other structures such as they hippocampus may be involved in processing these cues and ultimately be responsible for control of behavior (Davidson et al. 2007). Furthermore, these authors have suggested that altering hippocampal activity may play a role in weight gain. In line with this hypothesis, a study by Wallner-Liebmann and colleagues have shown a link between insulin, hippocampal activation, and craving behavior to food cues in adolescents (Davidson et al. 2007). However, current theories behind hippocampal involvement related to eating behaviors are somewhat contradictory. Increased activation in the hippocampus has been shown in response to food stimuli (Yokum et al. 2011; Tsao et al. 2012; Stice et al. 2008; Masterson et al. 2016). Furthermore, individuals with obesity have shown greater hippocampal activation to food stimuli compared to their normal-weight counterparts (Stice et al. 2008; Pursey et al. 2014). Others have postulated that the hippocampus is crucial to the craving of foods due to its role in recalling food and related eating behaviors from cues, such as pictures (Pelchat et al. 2004). Increased hippocampal activation therefore has been thought to be a potential trigger for reward seeking behaviors (Pelchat et al. 2004; Porubska et al. 2006). However, others have suggested that the hippocampus may have several other important roles in food motivation including: serving as a negative feedback mechanism to appetite control, learning inhibitory associations between stimuli, inhibiting neural impulses entering the hypothalamus, and inhibiting memories that could contribute to the suppression of food intake (Jarrard 1973; Chan et al. 2001; Tracy et al. 2001; Davidson et al. 2005).
The right medial temporal lobe has been shown to be critical for future-oriented reward based decision making (Palombo et al. 2015). Furthermore, the hippocampus and its surrounding structures are located within the medial temporal lobe and therefore it has been implicated in similar food-related functions. It is considered important for memory and learning and is implicated in the food motivation responses listed above. These two regions are particularly interesting when considering that previous behavioral studies have shown that following an acute bout of exercise children do not increase energy intake and have even been shown to decrease energy intake in obese adolescents (Thivel et al. 2011, 2013, 2014; Fearnbach et al. 2016). Exercise and food intake studies combined with fMRI paradigms will allow us to understand the importance of these regions and their influences on complex eating behaviors. Such studies will also serve to illuminate and clarify the role of hippocampal activation when confronted with food stimuli and how exercise can modify this response. For now, however, these results support that the hippocampus and medial temporal lobes are affected by both the presentation of food cues and exercise. The interaction between food cues and exercise may be important for future developmental intervention studies, such as using go/no-go training, to change overall energy intake during development (Jones et al. 2016).
We note that the sample used in this study was fairly homogenous and this study only gives insight to the effect of an acute bout of exercise effects and not long term habitual exercise. One other limitation that should be noted is that subjective judgments of the food stimuli used were not collected. Child eating behaviors were measured using the Child Eating Behavior Questionnaire however there were no significant results likely due to lack of statistical power in the sample. Furthermore, actual energy intake following the exercise was not assessed and therefore we cannot make any direct conclusions about how these particular brain regions would predict energy intake. A final consideration is that all children in the study were healthy weight. Behavioral work by Thivel and colleagues have shown that children with obesity reduce intake following acute exercise but healthy weight adolescents do not adjust intake (Thivel et al. 2014). Therefore, future studies should examine how exercise may differentially effect those with obesity compared to healthy weight counterparts. Future studies may also consider evaluating differences in neural response between adolescent males and females as the post-central gyrus has been shown to be particularly important in processing food cues in females (Atalayer et al. 2014).
In summary, this study shows that an acute bout of exercise results in an interaction, greater activation to high-calorie foods and reduced activation for low-calorie foods, in response to food stimuli in both the left hippocampus and right medial temporal lobe. Understanding how exercise modulates brain response to food cues may inform the development of future interventions aimed at altering energy intake in developing adolescents. Further study of the acute and chronic effects of exercise on reward and cognitive control pathways and eating behaviors in children are needed.
References
Allsop, S., Green, B. P., Dodd-Reynolds, C. J., Barry, G., & Rumbold, P. L. (2016). Comparison of short-term energy intake and appetite responses to active and seated video gaming, in 8-11-year-old boys. The British Journal of Nutrition, 115(6), 1117–1125. https://doi.org/10.1017/S0007114515005437.
Atalayer, D., Pantazatos, S. P., Gibson, C. D., McOuatt, H., Puma, L., Astbury, N. M., et al. (2014). Sexually dimorphic functional connectivity in response to high vs. low energy-dense food cues in obese humans: an fMRI study. NeuroImage, 100, 405–413. https://doi.org/10.1016/j.neuroimage.2014.05.054.
Berridge, K. C. (2009). ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. [Research Support, N.I.H., Extramural Review]. Physiology and Behavior, 97(5), 537–550. https://doi.org/10.1016/j.physbeh.2009.02.044.
Bruce, A. S., Lepping, R. J., Bruce, J. M., Cherry, J. B., Martin, L. E., Davis, A. M., et al. (2013). Brain responses to food logos in obese and healthy weight children. The Journal of Pediatrics, 162(4), 759–764 e752. https://doi.org/10.1016/j.jpeds.2012.10.003.
Brummer, V., Schneider, S., Struder, H. K., & Askew, C. D. (2011). Primary motor cortex activity is elevated with incremental exercise intensity. [Research Support, Non-U.S. Gov’t]. Neuroscience, 181, 150–162. https://doi.org/10.1016/j.neuroscience.2011.02.006.
Buck, S. M., Hillman, C. H., & Castelli, D. M. (2008). The relation of aerobic fitness to stroop task performance in preadolescent children. [Validation Studies]. Medicine and Science in Sports and Exercise, 40(1), 166–172. https://doi.org/10.1249/mss.0b013e318159b035.
Carskadon, M. A., & Acebo, C. (1993). A self-administered rating scale for pubertal development. The Journal of Adolescent Health, 14(3), 190–195.
Chaddock, L., Erickson, K. I., Prakash, R. S., Kim, J. S., Voss, M. W., Vanpatter, M., et al. (2010a). A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Research, 1358, 172–183. https://doi.org/10.1016/j.brainres.2010.08.049.
Chaddock, L., Erickson, K. I., Prakash, R. S., VanPatter, M., Voss, M. W., Pontifex, M. B., et al. (2010b). Basal ganglia volume is associated with aerobic fitness in preadolescent children. Developmental Neuroscience, 32(3), 249–256. https://doi.org/10.1159/000316648.
Chan, K. H., Morell, J. R., Jarrard, L. E., & Davidson, T. L. (2001). Reconsideration of the role of the hippocampus in learned inhibition. Behavioural Brain Research, 119(2), 111–130.
Chaput, J. P., Tremblay, A., Pereira, B., Boirie, Y., Duclos, M., & Thivel, D. (2016). Food intake response to exercise and active video gaming in adolescents: effect of weight status. The British Journal of Nutrition, 115(3), 547–553. https://doi.org/10.1017/S0007114515004602.
Christensen, L. O., Johannsen, P., Sinkjaer, T., Petersen, N., Pyndt, H. S., & Nielsen, J. B. (2000). Cerebral activation during bicycle movements in man. [Research Support, Non-U.S. Gov’t]. Experimental Brain Research, 135(1), 66–72.
Cornier, M. A., Melanson, E. L., Salzberg, A. K., Bechtell, J. L., & Tregellas, J. R. (2012). The effects of exercise on the neuronal response to food cues. [Clinical Trial]. Research Support, N. I. H., & Extramural]. Physiology and Behavior, 105(4), 1028–1034, https://doi.org/10.1016/j.physbeh.2011.11.023.
Crabtree, D. R., Chambers, E. S., Hardwick, R. M., & Blannin, A. K. (2014). The effects of high-intensity exercise on neural responses to images of food. [Randomized Controlled Trial]. The American Journal of Clinical Nutrition, 99(2), 258–267. https://doi.org/10.3945/ajcn.113.071381.
Davidson, T. L., Kanoski, S. E., Schier, L. A., Clegg, D. J., & Benoit, S. C. (2007). A potential role for the hippocampus in energy intake and body weight regulation. Current Opinion in Pharmacology, 7(6), 613–616. https://doi.org/10.1016/j.coph.2007.10.008.
Davidson, T. L., Kanoski, S. E., Walls, E. K., & Jarrard, L. E. (2005). Memory inhibition and energy regulation. Physiology and Behavior, 86(5), 731–746. https://doi.org/10.1016/j.physbeh.2005.09.004.
Donnelly, J. E., Smith, B., Jacobsen, D. J., Kirk, E., Dubose, K., Hyder, M., et al. (2004). The role of exercise for weight loss and maintenance. Best Practice & Research Clinical Gastroenterology, 18(6), 1009–1029.
Dupont, S., Bouilleret, V., Hasboun, D., Semah, F., & Baulac, M. (2003). Functional anatomy of the insula: new insights from imaging. Surgical and Radiologic Anatomy, 25(2), 113–119. https://doi.org/10.1007/s00276-003-0103-4.
Ekelund, U., Tomkinson, G., & Armstrong, N. (2011). What proportion of youth are physically active? Measurement issues, levels and recent time trends. [Review]. British Journal of Sports Medicine, 45(11), 859–865. https://doi.org/10.1136/bjsports-2011-090190.
Evero, N., Hackett, L. C., Clark, R. D., Phelan, S., & Hagobian, T. A. (2012). Aerobic exercise reduces neuronal responses in food reward brain regions. [Comparative Study]. Journal of Applied Physiology, 112(9), 1612–1619. https://doi.org/10.1152/japplphysiol.01365.2011.
Fearnbach, S. N., Masterson, T. D., Schlechter, H. A., Ross, A. J., Rykaczewski, M. J., Loken, E., et al. (2016). Impact of imposed exercise on energy intake in children at risk for overweight. Nutrition Journal, 15(1), 92.
Fearnbach, S. N., Silvert, L., Keller, K. L., Genin, P. M., Morio, B., Pereira, B., et al. (2016). Reduced neural response to food cues following exercise is accompanied by decreased energy intake in obese adolescents. International Journal of Obesity, 40(1), 77–83. https://doi.org/10.1038/ijo.2015.215.
Hanlon, B., Larson, M. J., Bailey, B. W., & LeCheminant, J. D. (2012). Neural response to pictures of food after exercise in normal-weight and obese women. [Comparative Study]. Medicine and Science in Sports and Exercise, 44(10), 1864–1870. https://doi.org/10.1249/MSS.0b013e31825cade5.
Hillman, C. H., Kamijo, K., & Scudder, M. (2011). A review of chronic and acute physical activity participation on neuroelectric measures of brain health and cognition during childhood. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review]. Preventive Medicine, 52 Suppl 1, S21–28. https://doi.org/10.1016/j.ypmed.2011.01.024.
Hillman, C. H., Pontifex, M. B., Raine, L. B., Castelli, D. M., Hall, E. E., & Kramer, A. F. (2009). The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. [Research Support, N.I.H., Extramural]. Neuroscience, 159(3), 1044–1054. https://doi.org/10.1016/j.neuroscience.2009.01.057.
Hirshkowitz, M., Whiton, K., Albert, S. M., Alessi, C., Bruni, O., DonCarlos, L., et al. (2015). National sleep foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health, 1(1), 40–43.
Jarrard, L. E. (1973). The hippocampus and motivation. Psychological Bulletin, 79(1), 1–12.
Jensen, C. D., & Kirwan, C. B. (2015). Functional brain response to food images in successful adolescent weight losers compared with normal-weight and overweight controls. Obesity (Silver Spring), 23(3), 630–636. https://doi.org/10.1002/oby.21004.
Jensky-Squires, N. E., Dieli-Conwright, C. M., Rossuello, A., Erceg, D. N., McCauley, S., & Schroeder, E. T. (2008). Validity and reliability of body composition analysers in children and adults. The British Journal of Nutrition, 100(4), 859–865. https://doi.org/10.1017/S0007114508925460.
Jones, A., Di Lemma, L. C., Robinson, E., Christiansen, P., Nolan, S., Tudur-Smith, C., et al. (2016). Inhibitory control training for appetitive behaviour change: a meta-analytic investigation of mechanisms of action and moderators of effectiveness. Appetite, 97, 16–28.
Keller, K. L., Assur, S. A., Torres, M., Lofink, H. E., Thornton, J. C., Faith, M. S., et al. (2006). Potential of an analog scaling device for measuring fullness in children: development and preliminary testing. Appetite, 47(2), 233–243. https://doi.org/10.1016/j.appet.2006.04.004.
Killgore, W. D., Kipman, M., Schwab, Z. J., Tkachenko, O., Preer, L., Gogel, H., et al. (2013). Physical exercise and brain responses to images of high-calorie food. [Research Support, U.S. Gov’t, Non-P.H.S.]. Neuroreport, 24(17), 962–967, https://doi.org/10.1097/WNR.0000000000000029.
Killgore, W. D., Young, A. D., Femia, L. A., Bogorodzki, P., Rogowska, J., & Yurgelun-Todd, D. A. (2003). Cortical and limbic activation during viewing of high- versus low-calorie foods. NeuroImage, 19(4), 1381–1394.
Kinder, M., Lotze, M., Davids, S., Domin, M., Thoms, K., Wendt, J., et al. (2014). Functional imaging in obese children responding to long-term sports therapy. [Research Support, Non-U.S. Gov’t]. Behavioural Brain Research, 272, 25–31. https://doi.org/10.1016/j.bbr.2014.06.037.
Kist, C., Gier, A., Tucker, J., Barbieri, T. F., Johnson-Branch, S., Moore, L., et al. (2015). Physical activity in clinical pediatric weight management programs: current practices and recommendations. Clinical Pediatrics. https://doi.org/10.1177/0009922815620772.
Kwon, S., Janz, K. F., Letuchy, E. M., Burns, T. L., & Levy, S. M. (2015). Developmental trajectories of physical activity, sports, and television viewing during childhood to young adulthood: Iowa bone development study. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. JAMA Pediatrics, 169(7), 666–672. https://doi.org/10.1001/jamapediatrics.2015.0327.
Lawrence, N. S., Hinton, E. C., Parkinson, J. A., & Lawrence, A. D. (2012). Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. NeuroImage, 63(1), 415–422.
LeCheminant, J. D., Heden, T., Smith, J., & Covington, N. K. (2009). Comparison of energy expenditure, economy, and pedometer counts between normal weight and overweight or obese women during a walking and jogging activity. European Journal of Applied Physiology, 106(5), 675–682. https://doi.org/10.1007/s00421-009-1059-9.
Lenard, N. R., & Berthoud, H. R. (2008). Central and peripheral regulation of food intake and physical activity: pathways and genes. [Research Support, N.I.H., Extramural Review]. Obesity (Silver Spring), 16 Suppl 3, S11–22. https://doi.org/10.1038/oby.2008.511.
Mahon, A. D., Marjerrison, A. D., Lee, J. D., Woodruff, M. E., & Hanna, L. E. (2010). Evaluating the prediction of maximal heart rate in children and adolescents. [Comparative Study]. Research Quarterly for Exercise and Sport, 81(4), 466–471. https://doi.org/10.1080/02701367.2010.10599707.
Masterson, T. D., Kirwan, C. B., Davidson, L. E., & LeCheminant, J. D. (2016). Neural reactivity to visual food stimuli is reduced in some areas of the brain during evening hours compared to morning hours: an fMRI study in women. Brain Imaging and Behavior, 10(1), 68–78. https://doi.org/10.1007/s11682-015-9366-8.
Murdaugh, D. L., Cox, J. E., Cook, E. W. 3rd, & Weller, R. E. (2012). fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. [Research Support, N.I.H., Extramural]. NeuroImage, 59(3), 2709–2721.
Ng, J., Stice, E., Yokum, S., & Bohon, C. (2011). An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Appetite, 57(1), 65–72. https://doi.org/10.1016/j.appet.2011.03.017.
Palombo, D. J., Keane, M. M., & Verfaellie, M. (2015). The medial temporal lobes are critical for reward-based decision making under conditions that promote episodic future thinking. Hippocampus, 25(3), 345–353. https://doi.org/10.1002/hipo.22376.
Pelchat, M. L., Johnson, A., Chan, R., Valdez, J., & Ragland, J. D. (2004). Images of desire: food-craving activation during fMRI. NeuroImage, 23(4), 1486–1493. https://doi.org/10.1016/j.neuroimage.2004.08.023.
Physical Activity Guidelines Advisory Committee (2009). Physical activity guidelines advisory committee report, 2008. To the secretary of health and human services. Part A: executive summary. Nutrition Reviews, 67(2), 114–120, https://doi.org/10.1111/j.1753-4887.2008.00136.x.
Porubska, K., Veit, R., Preissl, H., Fritsche, A., & Birbaumer, N. (2006). Subjective feeling of appetite modulates brain activity: an fMRI study. NeuroImage, 32(3), 1273–1280. https://doi.org/10.1016/j.neuroimage.2006.04.216.
Pursey, K. M., Stanwell, P., Callister, R. J., Brain, K., Collins, C. E., & Burrows, T. L. (2014). Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Front Nutrition, 1:7. https://doi.org/10.3389/fnut.2014.00007.
Spence, C., Okajima, K., Cheok, A. D., Petit, O., & Michel, C. (2015). Eating with our eyes: from visual hunger to digital satiation. Brain and Cognition. https://doi.org/10.1016/j.bandc.2015.08.006.
Stice, E., Spoor, S., Bohon, C., Veldhuizen, M. G., & Small, D. M. (2008). Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. Journal of Abnormal Psychology, 117(4), 924–935. https://doi.org/10.1037/a0013600.
Sweet, L. H., Hassenstab, J. J., McCaffery, J. M., Raynor, H. A., Bond, D. S., Demos, K. E., et al. (2012). Brain response to food stimulation in obese, normal weight, and successful weight loss maintainers. Obesity (Silver Spring), 20(11), 2220–2225. https://doi.org/10.1038/oby.2012.125.
Talma, H., Chinapaw, M. J., Bakker, B., HiraSing, R. A., Terwee, C. B., & Altenburg, T. M. (2013). Bioelectrical impedance analysis to estimate body composition in children and adolescents: a systematic review and evidence appraisal of validity, responsiveness, reliability and measurement error. Obesity Reviews, 14(11), 895–905. https://doi.org/10.1111/obr.12061.
Thivel, D., Aucouturier, J., Doucet, E., Saunders, T. J., & Chaput, J. P. (2013). Daily energy balance in children and adolescents. Does energy expenditure predict subsequent energy intake? Appetite, 60(1), 58–64. https://doi.org/10.1016/j.appet.2012.09.022.
Thivel, D., Isacco, L., Rousset, S., Boirie, Y., Morio, B., & Duche, P. (2011). Intensive exercise: a remedy for childhood obesity? Physiology and Behavior, 102(2), 132–136. https://doi.org/10.1016/j.physbeh.2010.10.011.
Thivel, D., Metz, L., Julien, A., Morio, B., & Duche, P. (2014). Obese but not lean adolescents spontaneously decrease energy intake after intensive exercise. Physiology and Behavior, 123, 41–46. https://doi.org/10.1016/j.physbeh.2013.09.018.
Tompkins, C. L., Flanagan, T., Lavoie, J., 2nd, & Brock, D. W. (2015). Heart rate and perceived exertion in healthy weight and obese children during a self-selected physical activity program. [Research Support, Non-U.S. Gov’t]. Journal of Physical Activity & Health, 12(7), 976–981, https://doi.org/10.1123/jpah.2013-0374.
Tracy, A. L., Jarrard, L. E., & Davidson, T. L. (2001). The hippocampus and motivation revisited: appetite and activity. Behavioural Brain Research, 127(1–2), 13–23.
Tsao, S., Adam, T. C., Goran, M. I., & Singh, M. (2012). Application of fMRI to obesity research: Differences in reward pathway activation measured with fMRI BOLD during visual presentation of high and low calorie foods. In Medical imaging 2012: Biomedical applications in molecular, structural, and functional imaging, 8317. https://doi.org/10.1117/12.911854.
Williamson, J. W., McColl, R., Mathews, D., Ginsburg, M., & Mitchell, J. H. (1999). Activation of the insular cortex is affected by the intensity of exercise. [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov’t]. Journal of Applied Physiology, 87(3), 1213–1219.
Williamson, J. W., Nobrega, A. C., McColl, R., Mathews, D., Winchester, P., Friberg, L., et al. (1997). Activation of the insular cortex during dynamic exercise in humans. [Clinical Trial Research Support, Non-U.S. Gov’t Research Support Gov’t, P.H.S.]. Journal of Physiology, 503(Pt 2), 277–283.
Yokum, S., Ng, J., & Stice, E. (2011). Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. [Research Support, N.I.H., Extramural]. Obesity (Silver Spring), 19(9), 1775–1783. https://doi.org/10.1038/oby.2011.168.
Acknowledgements
The authors would like to acknowledge the assistance of Paula Johnson, Rick LeCheminant, Jessica Taylor, Nathanial Tanner and Rachel Wirthlin.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Masterson, T.D., Kirwan, C.B., Davidson, L.E. et al. Brain reactivity to visual food stimuli after moderate-intensity exercise in children. Brain Imaging and Behavior 12, 1032–1041 (2018). https://doi.org/10.1007/s11682-017-9766-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-017-9766-z