Abstract
Posttraumatic stress disorder (PTSD) is associated with abnormalities in functional connectivity of a specific cortico-limbic network; however, less is known about white matter abnormalities providing structural connections for this network. This study investigated whether the diagnosis and symptoms of PTSD are associated with alterations in fractional anisotropy (FA), an index reflecting white matter organization, across six, a priori-defined tracts. White matter FA was quantified by diffusion tensor imaging using 3 T-MRI among 57 male, combat-exposed veterans with no history of moderate to severe head injuries or current alcohol dependence: 31 met criteria for PTSD and 26 were demographically comparable, combat-exposed controls without PTSD. Clinician-administered and self-report questionnaires assessed PTSD severity, dissociation, and mood. PTSD + veterans had significantly higher FA than exposed controls in the superior fronto-occipital fasciculus (SFOF) and borderline higher FA in the anterior corona radiata (ACR) and cingulum (CGC), controlling for age and neurovascular comorbidities. When lifetime alcohol use disorders was included, only the association of PTSD with SFOF-FA remained significant. Among PTSD + veterans, higher SFOF-FA was associated with greater mood disturbance, dissociative symptoms, and re-experiencing, while lower FA of the uncinate fasciculus (UF) was associated with greater mood disturbance symptoms. Compared to combat-exposed controls without PTSD, veterans with PTSD exhibited higher white matter FA in the SFOF, and a similar tendency in the ACR and CGC, tracts involved in conflict-processing and spatial attention. Prior alcohol use might explain the associations of PTSD with ACR-FA and CGC-FA but not the association with SFOF-FA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posttraumatic stress disorder (PTSD) is prevalent among combat-exposed veterans (Ramchand et al. 2010) and is associated with increased risk of mortality (Schlenger et al. 2015). In a meta-analysis of functional neuroimaging studies, Etkin and Wager (2007) identified abnormal connectivity associated with PTSD across a neural network that includes the anterior cingulate cortex (ACC), medial prefrontal cortex (PFC), thalamus, and amygdala. However, relatively little is known about the association between PTSD and the white matter tracts that form the structural basis for these functional interactions (Sanjuan et al. 2013; Schuff et al. 2011; Daniels et al. 2013).
DTI is a powerful technique for investigating structural brain connectivity. DTI yields indices of fractional anisotropy (FA), based on the fact that neuronal axons restrict the movement of water molecules (Jones et al. 2013). Higher FA values can reflect increased fiber organization, axonal number and membrane structure, and myelination (Beaulieu 2002). Although FA is frequently reported as an index of “white matter integrity” and connectivity, this may over-interpret what FA can definitively index in samples without clear vascular pathology (Jones et al. 2013). In addition, “More is not always better” (Hoeft et al. 2007); psychiatric disorders including PTSD are related to both lower and higher FA.
To select white matter tracts a priori, we identified long association tracts implicated by the literature (Sanjuan et al. 2013; Schuff et al. 2011; Daniels et al. 2013; Kim et al. 2005; Admon et al. 2013; Bierer et al. 2015), and connected to key structures in PTSD-associated neural circuits (e.g., the ACC, thalamus, and amygdala)(Etkin and Wager 2007). The most consistent adult-onset PTSD-associated white matter alterations include the cingulum (CGC) and superior longitudinal fasciculus (SLF)(Daniels et al. 2013). In veterans specifically, PTSD is associated with altered FA of the CGC (Schuff et al. 2011; Bierer et al. 2015) and anterior corona radiata (ACR)(Sanjuan et al. 2013). Lower FA was more common, but higher FA values were also reported. In a pre-post study, combat-exposed paramedics whose PTSD symptoms increased exhibited longitudinal decreases in FA of the uncinate fasciculus (UF), a fronto-amygdalar tract implicated in anxiety (Phan et al. 2009). The current study selected ana priori-defined set of tracts, which connect the prefrontal and anterior cingulate regions of the cortex with the thalamus and amygdala: the CGC, ACR, and the UF. Secondarily, we included three other long association tracts implicated by the literature on white matter alterations in PTSD, or relevant to threat biases (an RDoc-phenotypic lens): the SLF, and the superior and inferior fronto-occipital fasciculi (SFOF and IFOF)(Daniels et al. 2013; Philippi et al. 2009; Pessoa and Adolphs 2010).
The criteria for PTSD per the Diagnostic and Statistical Manual (DSM) have been revised since this study began (American Psychiatric Association 2013). The DSM-IV-TR PTSD diagnostic criteria included exposure to a traumatic event and symptoms from three clusters: re-experiencing, avoidance, and hyperarousal. The DSM-5 revision reconfigures the symptoms into four clusters, assesses negative alterations in cognition and mood, and adds a dissociative subtype (American Psychiatric Association 2013). Some evidence suggests that patients with the dissociative subtype exhibit prefrontal over-regulation of emotion (Wolf et al. 2012; Lanius et al. 2010). Hence, the current study explored whether FA in tracts with substantial frontal/cingulate connectivity (the ACR, CGC, SFOF) might exhibit positive associations with dissociative symptoms. As both hyperarousal and dissociative symptoms can co-occur, this study took a dimensional approach to exploring whether different symptom types (e.g., re-experiencing/hyperarousal versus dissociative/numbing) would exhibit unique associations with white matter tracts.
In sum, the current study investigates: (1) group differences between combat-exposed veterans with and without PTSD on key white matter tracts, and, (2) exploratory symptom-tract associations in PTSD. The current study provides one of the largest characterizations to date of white matter quantified by diffusion tensor imaging (DTI), among male, OEF/OIF combat-exposed veterans with and without PTSD, while excluding those with moderate to severe traumatic brain injury.
Methods & materials
Participants
The current study included a subsample of 57 male veterans exposed to combat during Operation Enduring Freedom and Operation Iraqi Freedom, who were recruited by New York University (NYU) Langone Medical Center (NYULMC) and Icahn School of Medicine at Mount Sinai/James J. Peters Veterans Administration (ISMMS/JJPVAMC) to participate in a larger systems biology study of PTSD (Lindqvist et al. 2014). Participants were recruited from the Mental Health Services of Manhattan, the Bronx and Brooklyn Veterans Affairs Medical Centers, other regional VA medical centers, Veterans Service Organizations, the National Guard, reservist agencies, and from the community using clinician referrals, presentations, flyers, online postings, and other advertisements. The current study focused on the subsample of 60 male participants with data available from diffusion tensor imaging. Of these 60 participants, three were excluded from analyses, leaving 57 participants in total. Two participants were excluded due to missing data on medical comorbidities, and one control participant was excluded due to meeting SCID criteria for current MDD.
Inclusion and exclusion criteria
A diagnosis of war zone-related PTSD was made according to DSM-IV-TR criteria, based on the Clinician-Administered PTSD Scale (CAPS) (Blake et al. 1990), in conjunction with the Structured Clinical Interview for DSM-IV (SCID-IV). The CAPS was administered using the “F1/I2 rule”, meaning that a symptom is considered present if it is scored 1 or higher for frequency and 2 or higher for intensity (Blake et al. 1990). Per the inclusion criteria, all participants had experienced a combat-related trauma per criterion A of the DSM-IV-TR definition of PTSD, but only the PTSD group met criteria for the remaining DSM-IV diagnostic criteria. Further inclusion criteria for the PTSD group consisted of CAPS scores > 40 and the presence of a PTSD diagnosis for a minimum duration of 3 months. Inclusion criteria for combat-exposed controls consisted of CAPS scores < 20 without any previous or current PTSD diagnosis. The SCID was used to assess, and exclude from the control group, participants with any non-warzone lifetime history of meeting PTSD criterion. The SCID modules administered included: mood disorders, psychotic screener, substance use disorders, and anxiety disorder. The psychotic screener and substance use disorders modules were used to assist with inclusion and exclusion criteria. The AUDIT was additionally included to assess current alcohol use frequency and intensity (Saunders et al. 1993). Exclusion criteria for all participants included: classification with a moderate or severe traumatic brain injury (TBI) using the Ohio State University TBI Identification Method; loss of consciousness for more than 10 min; any neurologic disorder; anemia; medication changes over the previous 2 months on psychiatric, anticonvulsant, antihypertensive or sympathomimetic medications; alcohol dependence within the past 8 months (SCID DSM-IV); drug abuse or dependence within the previous year (SCID DSM-IV); exposure to traumatic events within the previous 3 months prior to assessment; history of schizophrenia, bipolar disorder, or obsessive–compulsive disorder; and prominent suicidal or homicidal ideation (as described in Lindqvist et al. 2014). Major Depressive Disorder as determined by the SCID was not an exclusion criterion for participants in the PTSD group, due to its high comorbidity with PTSD (Flory and Yehuda 2015; Marmar et al. 2015), and was therefore examined in post-hoc analyses. Participant characteristics and statistical group comparisons are described in Table 1. The study was approved by the Institutional Review Boards of ISMMS, the JJPVAMC, New York University Langone Medical Center, and the University of California, San Francisco, Medical Center. All participants provided written informed consent to participate and were compensated for participation.
Clinician-administered PTSD scale (CAPS)
The CAPS is a gold-standard, clinician-administered assessment tool used to assess PTSD symptom severity (Blake et al. 1990). The CAPS for DSM-IV provides a total score for current symptoms and three subscales corresponding to criteria for re-experiencing (B), avoidance (C), and hyperarousal (D).
Symptom checklist-90-revised (SCL-90-R)
The current analyses included the Global Severity Index (GSI) overall score and the mood subscales from the SCL (symptoms of depression, anxiety, and hostility). The SCL is a widely-used self-report measure of general psychopathology that quantifies 9 separate symptom dimensions (Derogatis 1992), and reveals differences between veterans with and without war-zone related PTSD (Weathers et al. 1996). The anxiety and depression subscales of the SCL have been validated against the SCID as a gold-standard and correlate with clinical diagnoses (Schmitz et al. 1999).
Peritraumatic dissociative experiences questionnaire
The 10-item Peritraumatic Dissociation Experiences Questionnaire (PDEQ) is a well-validated assessment of symptoms of derealization and depersonalization (Candel and Merckelbach 2004; Marmar et al. 1994). The PDEQ asks participants to report on symptoms experienced “during the most distressing incident you discussed with the clinician during your interview.” Though the PDEQ is not a substitute for the CAPS DSM-5 items that help assess the dissociative subtype, it may nonetheless provide useful insights into this symptom spectrum. Responses are scored using a 5-point scale ranging from “Not at all true” to “Extremely true.”
Diffusion tensor imaging
Imaging
All subjects were studied on a 3 T Trio Siemens magnet (Erlangen, Germany) at NYU equipped with a 32 channel receive head coil. The following sequences, which were part of a larger research imaging protocol, were obtained: 1) T1-weighted 3D whole brain gradient echo MRI TR/TE/TI = 2300/2.98/900 ms, 1.0 × 1.0 × 1.0 mm3 resolution (cortical thickness measurements). 2. EPI-based DTI (TR/TE = 8000/97, 2.2 × 2.2 × 2.2 mm3 resolution, 64 diffusion encoding directions with b = 1000s/ mm2.
Post-processing
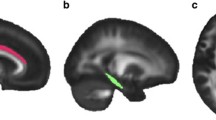
The DTI data were motion and eddy current corrected using the FLIRT and FUGUE algorithms from Free-Surfer Library (FSL) (http://www.fmrib.ox.ac.uk/fsl), and an additional geometric distortion correction was performed. The DTI images were then co-registered to the T1 image and up-sampled to the T1 resolution. Fractional anisotropy (FA) maps were calculated using the teem algorithms (teem.sourceforge.net). An unbiased project specific FA atlas in MNI152 space was generated from the all images using the fast, high degree diffeomorphic image registration algorithm (DARTEL) implemented in SPM8 (http://www.fil.ion.ucl.ac.uk/spm). The FA maps of all subjects were warped onto the FA atlas using DARTEL. Labels from the ICBM DTI81 atlas (created by hand segmentation of a standard-space average of diffusion MRI tensor maps from 81 healthy subjects) were used to extract mean FA values from the six white matter tracts of interest (Fig. 1), voxels with FA < 0.2 were masked out to reduce partial volume effects from neighboring gray matter regions. The mean FA values from the left and right tract were combined after excluding a hemispheric effect.
Medical comorbidities
Based on inclusion screening with the Ohio Traumatic Brain Injury scale, we created a variable coded 1 for participants who reported any previous injury to the head or neck that involved loss of consciousness (LOC) < 10 min (n = 13), regardless of whether the participant reported that the event affected his cognition (e.g., feeling dazed or experiencing memory loss). Note that LOC > = 10 min was already an exclusion criteria. In addition, an aggregate medical comorbidity variable was formed, such that a score of 1 indicated the presence of any of the following self-reported conditions: diabetes (n = 2), hypertension (n = 6), angina (n = 1), heart murmur or leaky valve (n = 2), or atrial fibrillation (n = 1). No participants reported a history or diagnosis of heart attack, heart failure, stroke, transient ischemic attack, rheumatic fever, or cancer.
Data analysis
Variables were screened for normality and potential outliers prior to analyses. Group differences in white matter FA were tested using separate Analyses of Covariance (ANCOVA) for each tract. Exploratory symptom-FA associations within the PTSD group were tested using regression analyses, in which symptoms and covariates were entered as independent variables with white matter FA values as the dependent variables. Symptom scores were Blom-transformed to improve the normality of the distributions prior to correlating them with tract FA values. Major Depressive Disorder (MDD) commonly co-occurs with PTSD, and it may constitute an integral part of the disorder in this population. As a post-hoc analysis to explore whether comorbid depression might have impacted the PTSD group differences in white matter, group difference models were additionally run with dummy coded variables to compare PTSD-only and comorbid PTSD/MDD subgroups with the control, applying a Bonferroni-corrected critical alpha of 0.025.
Initial covariates included age and the presence of comorbidities (cardiovascular problems, diabetes, or a history of a brief loss of consciousness < 10 min) (Davis et al. 2009; Raz et al. 2007). To retain power, comorbidities were combined into a single factor (present/ absent). Greater use of antidepressants (AD) and history of prior alcohol or drug use disorders in veterans with PTSD (Table 1) may reflect the fact that AD are often prescribed to treat PTSD, while alcohol and drugs to “self-medicate.” Hence, the initial models present the results without AD or substance use, and then secondarily examine how these factors impact group effects. Daily smoking and BMI were not significant predictors of these white matter outcomes; hence, they were not included as covariates.
Participant characteristics
Participant characteristics are given in Table 1. Veterans with a diagnosis of PTSD were significantly more likely to take antidepressants and to meet SCID criteria for a prior history of alcohol or substance use disorders, compared to combat-exposed controls without PTSD. The primary racial/ethnic identification for the sample was as follows: White (n = 16, 28.1%), Black (n = 13, 22.8%), Asian (n = 2, 3.5%)Footnote 1 and Hispanic ethnicity (n = 31, 54.4%).
PTSD group differences on white matter FA
Veterans with PTSD were compared to combat-exposed controls on six, long associational white matter tracts using ANCOVA (Table 2 and Fig. 2), covarying for age and comorbidities (LOC < 10 min and cardiometabolic diagnoses). The PTSD group exhibited significantly higher FA values relative to controls in the SFOF tract (p = .034) and borderline higher FA in the ACR (p = .054) and CGC (p = .077), while controlling for age and comorbidities. No differences were present in the UF, IFOF or SLF. Age was not a significant predictor, potentially due to relative youth of the sample (mean age: 33 years; range: 22–55). Medical comorbidities, including LOC and cardiometabolic diagnoses, were a significant predictor of higher SFOF FA, independent of PTSD.
Effects of covarying for alcohol, substances, and medication use
When additionally controlling for four classes of medications (i.e., anti-depressant, anti-inflammatory, statin, anti-diabetic drugs), PTSD group differences in ACR and SFOF-FA were both significant (all p’s < 0.05). When controlling for lifetime alcohol use disorders (AUD) assessed by the SCID, PTSD remained significantly related to higher SFOF-FA (p = .037), but was no longer marginally associated with ACR-FA or CGC-FA (p’s > 0.280). Lifetime AUD was marginally related to ACR-FA (p = .097). When controlling for current alcohol use severity per the AUDIT, the association of PTSD with ACR-FA remained borderline (p = .097) while the association with CGC-FA did not (p = .121). Inclusion of the AUDIT had a lesser effect on the association of PTSD with SFOF-FA (p = .056). Higher AUDIT scores were marginally related to higher ACR-FA (p = .080) but not SFOF-FA (p = .256) or CGC-FA (p = .210). Inclusion of SUD did not substantially impact the pattern of significance; the association of PTSD with ACR and SFOF-FA became marginal (p’s = 0.051 and 0.054 respectively).
Post-hoc analyses: comorbid PTSD and major depression (PTSD/MDD)
The definition of PTSD overlaps with the criteria for MDD (e.g., anhedonia, sleep disturbance, trouble concentrating)(Flory and Yehuda 2015). Therefore, when MDD occurs comorbidly with PTSD, it is unclear whether it should be considered a “separate” disorder with unique pattern of structural connectivity. Eleven PTSD group participants (35.5%) met criteria for a concurrent diagnosis of MDD on the SCID. To clarify how comorbid depression might impact group differences, exploratory post-hoc ANCOVA analyses were conducted including separate group variables for PTSD only and PTSD plus MDD and employing a critical alpha of 0.025. Consistent with the a priori PTSD group differences, with post-hoc corrections, FA values in the PTSD-only group remained non-significantly, but numerically, higher for the ACR (p = .052) and the CGC (p = .079) compared to controls. The PTSD plus MDD group had significantly higher FA in the IFOF (F(1,52) = 7.400, p = .009) and borderline higher FA in the SFOF (F(1,52) = 5.066, p = .029) compared to controls.
Exploratory analyses: symptom-tract associations
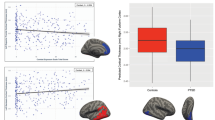
Symptom-tract associations were exploratory. Table 3 presents symptom-tract associations within the PTSD group (n = 31), analyzed by regression analyses, controlling for covariates (age and comorbidities). Notably, greater CAPS re-experiencing symptoms were significantly related to higher SFOF-FA (p = .031) and a borderline relationship to higher ACR-FA (p = .059). Neither tract was related to total CAPS scores. Interestingly, higher UF-FA was significantly associated with lower scores on GSI and mood disturbance, whereas higher SFOF-FA correlated with higher scores on GSI, mood disturbance, and dissociation (Fig. 3). The remaining tracts showed no significant relationships with symptoms. Comorbidities (loss of consciousness < 10 min and vascular disease) were significant predictors of higher SFOF-FA (all p’s < 0.05), independent of psychological symptoms.
Symptom-tract associations among veterans with PTSD. **p ≤ .01, *p ≤ .05. The data visualized is unadjusted and use the untransformed symptom scores for interpretability. The regression coefficients and p-values, given in each graph, are from full regression analyses that adjust for age and medical comorbidities (see Table 3)
Discussion
This is one of the largest studies to date of white matter abnormalities associated with combat-related posttraumatic stress disorder (PTSD). This study excluded participants meeting criteria for moderate to severe traumatic brain injury (TBI) or for alcohol dependence in the prior 8 months. Relative to combat-exposed controls, veterans with combat-related PTSD exhibited higher fractional anisotropy (FA) in the superior fronto-occipital fasciculus (SFOF) and borderline higher FA in the anterior corona radiata (ACR) and cingulum (CGC), controlling for age and neurovascular comorbidities. When we additionally controlled for lifetime history of alcohol use disorders (AUD), which was elevated in veterans with PTSD, it reduced the association of PTSD with higher FA in the ACR and cingulum, but did not explain the association with higher FA in the SFOF. These tracts provide structural connections for a corticolimbic network, previously identified by a meta-analysis of abnormal functional connectivity in PTSD (Etkin and Wager 2007). Higher FA is typically thought to be associated with greater axon diameter, density, or myelination, indicating relative enhancements of white matter integrity (Beaulieu 2002). However, important caveats exist (Jones et al. 2013), and higher FA is also found in the context of psychiatric and neurocognitive pathology (Hoeft et al. 2007; Abe et al. 2006; Davenport et al. 2010). In addition, exploratory tract-symptom relationships were present among veterans with PTSD. Due the cross-sectional nature of these findings, it is unclear whether white matter alterations may play a precipitating role in PTSD or reflect a compensatory response to neuroinflammatory stimuli such as psychological trauma and heavy alcohol use.
It is difficult to contrast this study’s findings with previous literature on white matter and PTSD due to substantial heterogeneity in the demographics, trauma models, comorbid conditions, and methods. Few prior studies have investigated white matter in combat-related PTSD, and all but one (Kennis et al. 2015) were comparatively small samples (Sanjuan et al. 2013; Schuff et al. 2011; Hedges et al. 2007; Bierer et al. 2015). The most prominent finding in a prior meta-analysis of white matter alterations in PTSD was lower FA of the cingulum (Daniels et al. 2013). In contrast, the current study found a non-significant trend toward higher FA in the dorsal cingulum. Lower FA in PTSD is more common in the literature; nonetheless, two other studies have reported higher FA (Abe et al. 2006; Bierer et al. 2015). Moreover, a recent study reported that persistent, treatment-resistant PTSD is associated with increases in FA of the right cingulum over time (Kennis et al. 2015). The authors proposed that these FA increases could be a marker of neural plasticity related to “fear learning.” Finally, it is worth noting that many of the studies contributing to the meta-analysis by Daniels et al., employed Asian samples. Hence, differing racial/ethnic compositions of samples could conceivably contribute to heterogeneity of results.
A second, common finding in the PTSD-white matter meta-analysis was alterations in the SLF (both higher and lower levels)(Daniels et al. 2013; Kennis et al. 2015). In the current study, PTSD was associated with significantly higher FA values in the SFOF, but not the SLF. However, some consider the SFOF to be an “extension” of the SLF (Forkel et al. 2014). The SFOF and the SLF together form the “dorsal visual stream,” which is involved in visual aspects of goal-related motor activity, attention, and vigilance (Milner and Goodale 2008; Maheshwari et al. 2011). In the current study, higher SFOF-FA was significantly associated with greater symptoms of re-experiencing, mood disturbance and dissociation among veterans with PTSD. Neurovascular comorbidities were also significantly correlated with higher SFOF FA, independent of PTSD. PTSD group differences were only slightly reduced by the inclusion of antidepressants. Post-hoc analyses of the subgroup comorbid for PTSD and MDD suggests that this subgroup may have contributed to higher FA values. First, this could reflect the overlap of anhedonia, numbing, and dissociation in the definitions of PTSD and MDD. Second, MDD, as an independent disorder, has generally been associated with lower FA in meta-analytic research (Liao et al. 2013) and recent studies of first-episode, treatment naive MDD patients (Srivastava et al. 2015). In sum, higher FA values in the SFOF of veterans with PTSD are not likely due merely to MDD or antidepressant use, but may reflect the fact that symptoms of numbing and anhedonia can present in both PTSD and MDD.
We found a borderline significant trend (p = .054) toward higher ACR-FA values among combat-exposed veterans with PTSD, compared to combat-exposed veterans without PTSD, when controlling for age and neurovascular comorbidities. Higher ACR-FA also exhibited a borderline association with greater symptoms of re-experiencing among veterans with PTSD. However, once we accounted for a lifetime history of AUD, the association of ACR-FA with PTSD was no longer significant. The ACR connects regions of the prefrontal and cingulate cortex with the thalamus, and indirectly, with the amygdala. The ACR has a distinct role in conflict resolution, attention, and emotion regulation (Niogi et al. 2010). Our results contrast with one prior study of male, OIF/OEF veterans with a history of AUD, which reported that comorbid PTSD and AUD was associated with lower ACR-FA (Sanjuan et al. 2013) compared to a control group with AUD alone. Both studies implicate the ACR as involved in PTSD with comorbid AUD; however, it is unclear why the directionality of the associations with white matter differ.
One potentially relevant factor may be the duration of recovery from heavy alcohol use. Excess alcohol can cause oxidative stress, which induces neuronal death and impairs white matter; however, with reductions in alcohol use, regeneration can occur (Crews and Nixon 2009). Both extreme psychological stress and AUD constitute potent oxidative and neuroinflammatory stressors (Lindqvist et al. 2014; Aschbacher et al. 2013; Crews and Nixon 2009). Though speculative, future studies might explore the possibility that oxidative and neuroinflammatory processes could contribute to FA abnormalities among veterans with PTSD, many of which also have a lifetime history of comorbid AUD.
It may seem counter-intuitive that PTSD would be associated with higher FA values. While higher FA is often interpreted as an index of better integrity, experts caution against doing so (Jones et al. 2013). Both higher and lower FA levels have been associated with psychopathology (Hoeft et al. 2007; Abe et al. 2006), and several potential interpretations may explain why higher FA values would be related to a psychopathological condition. First, increased FA in the ACR and SLF could potentially result from decreased dendritic branching (Hoeft et al. 2007; Davenport et al. 2010). Alternately, Montag et al. (2012), suggest that, “Higher white matter tract integrity in the temporal lobe could strengthen anxiety signals from phylogenetically older brain areas to the cortex.” Immune and growth factor changes are other potential causes for increases in white matter (Setzu et al. 2006; Zatorre et al. 2012). The mechanisms that underlie the association of FA values and PTSD merit further investigation.
It has been suggested that DSM diagnoses do not map on well to neurobiology, and should be complemented with dimensional investigations of specific symptoms (Insel 2014). Intriguingly, UF was consistently negatively associated with psychopathology symptoms (i.e., the SCL global severity index and mood disturbance subscales), whereas the SFOF was positively associated with symptoms of re-experiencing, dissociation and mood disturbance. While exploratory, these data raise the question of whether PTSD might be better understood through dimensional analyses of separate symptom clusters than as a categorical syndrome. While exploratory, these data raise the question of whether symptom-tract associations might reflect dissociable dimensions. Functional neuroimaging studies suggest that prototypical PTSD is associated with under-regulated affect (e.g., hypervigilance), whereas a “dissociative subtype” of PTSD has been associated with over-regulated affect (Lanius et al. 2010). However, the data visualizations in this study (Fig. 2) imply dimensional relationships. Future studies pairing structural and functional connectivity might elucidate whether different networks underlie the symptom expression of hyperarousal versus numbing/dissociation.
The limitations to generalizability should be considered, particularly in light of the fact that meta-analytic research reveals high heterogeneity across studies (Daniels et al. 2013). As this study focused on male veterans and employed a combat-exposure model, these results may not generalize to populations of women, non-veterans, or alternative PTSD models. Many PTSD models in the meta-analysis by Daniels et al. study natural disasters. Combat models may differ in terms of potentially exposing individuals to repeated events over time and including a social threat dimension – a key driver of biological stress reactivity (Dickerson and Kemeny 2004; Aschbacher et al. 2013). Veteran samples may have a higher prevalence of lifetime AUD. Six tracts were examined, and due to the relatively small sample size, we did not correct for multiple comparisons. This study utilized a region of interest approach, which focuses on a certain part of the white matter tract. Hence, PTSD-related alterations might exist in aspects of these tracts not quantified by this approach.
This is the largest study to date to demonstrate that combat-related PTSD is associated with alterations in major white matter tracts, which connect the major self-regulatory and threat-responsive regions of the brain. Understanding these alterations in brain networks may provide insights that ultimately enhance the understanding and treatment of PTSD.
Notes
Note: Percentages do not sum to 100% as some participants indicated both race and Hispanic ethnicity, and some participants checked “Other” for race.
References
Abe, O., Yamasue, H., Kasai, K., Yamada, H., Aoki, S., Iwanami, A., et al. (2006). Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Research, 146(3), 231–242. doi:10.1016/j.pscychresns.2006.01.004.
Admon, R., Leykin, D., Lubin, G., Engert, V., Andrews, J., Pruessner, J., et al. (2013). Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Human Brain Mapping, 34(11), 2808–2816. doi:10.1002/hbm.22100.
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (5th edn.). Washington, DC: ManMag.
Aschbacher, K., O’Donovan, A., Wolkowitz, O. M., Dhabhar, F. S., Su, Y., & Epel, E. (2013). Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology, 38(9), 1698–1708. doi:10.1016/j.psyneuen.2013.02.004.
Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in Biomedicine, 15(7–8), 435–455. doi:10.1002/nbm.782.
Bierer, L. M., Ivanov, I., Carpenter, D. M., Wong, E. W., Golier, J. A., Tang, C. Y., et al. (2015). White matter abnormalities in Gulf War veterans with posttraumatic stress disorder: a pilot study. Psychoneuroendocrinology, 51, 567–576. doi:10.1016/j.psyneuen.2014.11.007.
Blake, D., Weathers, F., Nagy, L., Kaloupek, D., Klauminzer, G., Charney, D., et al. (1990). A clinician rating scale for assessing current and lifetime PTSD: The CAPS-1.
Candel, I., & Merckelbach, H. (2004). Peritraumatic dissociation as a predictor of post-traumatic stress disorder: a critical review. Comprehensive Psychiatry, 45(1), 44–50. doi:10.1016/j.comppsych.2003.09.012.
Crews, F. T., & Nixon, K. (2009). Mechanisms of Neurodegeneration and Regeneration in Alcoholism. Alcohol and Alcoholism, 44(2), 115–127. doi:10.1093/alcalc/agn079.
Daniels, J. K., Lamke, J. P., Gaebler, M., Walter, H., & Scheel, M. (2013). White matter integrity and its relationship to PTSD and childhood trauma–a systematic review and meta-analysis. Depression and Anxiety, 30(3), 207–216. doi:10.1002/da.22044.
Davenport, N. D., Karatekin, C., White, T., & Lim, K. O. (2010). Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Research, 181(3), 193–198. doi:10.1016/j.pscychresns.2009.10.012.
Davis, S. W., Dennis, N. A., Buchler, N. G., White, L. E., Madden, D. J., & Cabeza, R. (2009). Assessing the effects of age on long white matter tracts using diffusion tensor tractography. NeuroImage, 46(2), 530–541.
Derogatis, L. R. (1992). SCL-90-R: administration, scoring and procedures manual for the R (evised) version and other instruments of the psychopathology rating scale series: clinical psychometric research.
Dickerson, S. S., & Kemeny, M. E. (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. doi:10.1037/0033-2909.130.3.355.
Etkin, A., & Wager, T. D. (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry, 164(10), 1476–1488. doi:10.1176/appi.ajp.2007.07030504.
Flory, J. D., & Yehuda, R. (2015). Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues in Clinical Neuroscience, 17(2), 141–150.
Forkel, S. J., Thiebaut de Schotten, M., Kawadler, J. M., Dell’Acqua, F., Danek, A., & Catani, M. (2014). The anatomy of fronto-occipital connections from early blunt dissections to contemporary tractography. Cortex, 56, 73–84. doi:10.1016/j.cortex.2012.09.005.
Hedges, D. W., Thatcher, G. W., Bennett, P. J., Sood, S., Paulson, D., Creem-Regehr, S., et al. (2007). Brain integrity and cerebral atrophy in Vietnam combat veterans with and without posttraumatic stress disorder. Neurocase, 13(5), 402–410. doi:10.1080/13554790701851551.
Hoeft, F., Barnea-Goraly, N., Haas, B. W., Golarai, G., Ng, D., Mills, D., et al. (2007). More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. Journal of Neuroscience, 27(44), 11960–11965. doi:10.1523/JNEUROSCI.3591-07.2007.
Insel, T. R. (2014). The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry. The American Journal of Psychiatry, 171(4), 395–397. doi:10.1176/appi.ajp.2014.14020138.
Jones, D. K., Knosche, T. R., & Turner, R. (2013). White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. NeuroImage, 73, 239–254. doi:10.1016/j.neuroimage.2012.06.081.
Kennis, M., van Rooij, S. J., Tromp do, P. M., Fox, A. S., Rademaker, A. R., Kahn, R. S., et al. (2015). Treatment outcome-related white matter differences in veterans with posttraumatic stress disorder. Neuropsychopharmacology, 40(10), 2434–2442. doi:10.1038/npp.2015.94.
Kim, M. J., Lyoo, I. K., Kim, S. J., Sim, M., Kim, N., Choi, N., et al. (2005). Disrupted white matter tract integrity of anterior cingulate in trauma survivors. Neuroreport, 16(10), 1049–1053.
Lanius, R. A., Vermetten, E., Loewenstein, R. J., Brand, B., Schmahl, C., Bremner, J. D., et al. (2010). Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. The American Journal of Psychiatry, 167(6), 640–647. doi:10.1176/appi.ajp.2009.09081168.
Liao, Y., Huang, X., Wu, Q., Yang, C., Kuang, W., Du, M., et al. (2013). Is depression a disconnection syndrome? meta-analysis of diffusion tensor imaging studies in patients with MDD. Journal of Psychiatry & Neuroscience: JPN, 38(1), 49–56. doi:10.1503/jpn.110180.
Lindqvist, D., Wolkowitz, O. M., Mellon, S., Yehuda, R., Flory, J. D., Henn-Haase, C., et al. (2014). Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behavior and Immunity, 42, 81–88. doi:10.1016/j.bbi.2014.06.003.
Maheshwari, M., Klein, A., & Ulmer, J. L. (2011). White matter: Functional anatomy of key tracts. In S. H. Faro, F. B. Mohamed, M. Law & J. L. Ulmer (Eds.), Functional neuroradiology: Principles and clinical applications (pp. xxi, 1029 pages). New York: Springer.
Marmar, C. R., Schlenger, W., Henn-Haase, C., Qian, M., Purchia, E., Li, M., et al. (2015). Course of posttraumatic stress disorder 40 years after the vietnam war: findings from the national vietnam veterans longitudinal study. JAMA Psychiatry, 72(9), 875–881. doi:10.1001/jamapsychiatry.2015.0803.
Marmar, C. R., Weiss, D. S., Schlenger, W. E., Fairbank, J. A., Jordan, B. K., Kulka, R. A., et al. (1994). Peritraumatic dissociation and posttraumatic stress in male Vietnam theater veterans. The American Journal of Psychiatry, 151(6), 902–907. doi:10.1176/ajp.151.6.902.
Milner, A. D., & Goodale, M. A. (2008). Two visual systems re-viewed. Neuropsychologia, 46(3), 774–785. doi:10.1016/j.neuropsychologia.2007.10.005.
Montag, C., Reuter, M., Weber, B., Markett, S., & Schoene-Bake, J. C. (2012). Individual differences in trait anxiety are associated with white matter tract integrity in the left temporal lobe in healthy males but not females. Neuroscience, 217, 77–83. doi:10.1016/j.neuroscience.2012.05.017.
Niogi, S., Mukherjee, P., Ghajar, J., & McCandliss, B. D. (2010). Individual differences in distinct components of attention are linked to anatomical variations in distinct white matter tracts. Front Neuroanat, 4, 2. doi:10.3389/neuro.05.002.2010.
Pessoa, L., & Adolphs, R. (2010). Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience, 11(11), 773–783. doi:10.1038/nrn2920.
Phan, K. L., Orlichenko, A., Boyd, E., Angstadt, M., Coccaro, E. F., Liberzon, I., et al. (2009). Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biological Psychiatry, 66(7), 691–694. doi:10.1016/j.biopsych.2009.02.028.
Philippi, C. L., Mehta, S., Grabowski, T., Adolphs, R., & Rudrauf, D. (2009). Damage to association fiber tracts impairs recognition of the facial expression of emotion. Journal of Neuroscience, 29(48), 15089–15099. doi:10.1523/JNEUROSCI.0796-09.2009.
Ramchand, R., Schell, T. L., Karney, B. R., Osilla, K. C., Burns, R. M., & Caldarone, L. B. (2010). Disparate prevalence estimates of PTSD among service members who served in Iraq and Afghanistan: possible explanations. Journal of Traumatic Stress, 23(1), 59–68. doi:10.1002/jts.20486.
Raz, N., Rodrigue, K. M., Kennedy, K. M., & Acker, J. D. (2007). Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology, 21(2), 149–157. doi:10.1037/0894-4105.21.2.149.
Sanjuan, P. M., Thoma, R., Claus, E. D., Mays, N., & Caprihan, A. (2013). Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: a diffusion tensor imaging study. Psychiatry Research, 214(3), 260–268. doi:10.1016/j.pscychresns.2013.09.002.
Saunders, J. B., Aasland, O. G., Babor, T. F., de la Fuente, J. R., & Grant, M. (1993). Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction, 88(6), 791–804.
Schlenger, W. E., Corry, N. H., Williams, C. S., Kulka, R. A., Mulvaney-Day, N., DeBakey, S., et al. (2015). A prospective study of mortality and trauma-related risk factors among a nationally representative sample of vietnam veterans. American Journal of Epidemiology, American Journal of Epidemiology, 182(12), 980–990
Schmitz, N., Kruse, J., Heckrath, C., Alberti, L., & Tress, W. (1999). Diagnosing mental disorders in primary care: the general health questionnaire (GHQ) and the symptom check list (SCL-90-R) as screening instruments. Social Psychiatry and Psychiatric Epidemiology, 34(7), 360–366.
Schuff, N., Zhang, Y., Zhan, W., Lenoci, M., Ching, C., Boreta, L., et al. (2011). Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: an MRI study. NeuroImage, 54 Suppl 1, S62-68. doi:10.1016/j.neuroimage.2010.05.024.
Setzu, A., Lathia, J. D., Zhao, C., Wells, K., Rao, M. S., Ffrench-Constant, C., et al. (2006). Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia, 54(4), 297–303. doi:10.1002/glia.20371.
Srivastava, S., Bhatia, M. S., Bhargava, S. K., Kumari, R., & Chandra, S. (2015). A diffusion tensor imaging study using a voxel-based analysis, region-of-interest method to analyze white matter abnormalities in first-episode, treatment-naive major depressive disorder. The Journal of Neuropsychiatry and Clinical Neurosciences. doi:10.1176/appi.neuropsych.15050120.
Weathers, F. W., Litz, B. T., Keane, T. M., Herman, D. S., Steinberg, H. R., Huska, J. A., et al. (1996). The utility of the SCL-90-R for the diagnosis of war-zone related posttraumatic stress disorder. Journal of Traumatic Stress, 9(1), 111–128.
Wolf, E. J., Miller, M. W., Reardon, A. F., Ryabchenko, K. A., Castillo, D., & Freund, R. (2012). A latent class analysis of dissociation and posttraumatic stress disorder: evidence for a dissociative subtype. Archives of General Psychiatry, 69(7), 698–705. doi:10.1001/archgenpsychiatry.2011.1574.
Zatorre, R. J., Fields, R. D., & Johansen-Berg, H. (2012). Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nature Neuroscience, 15(4), 528–536. doi:10.1038/nn.3045.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the following grants: U.S. Department of Defense, W81XWH-11-2-0223 (PI: Charles R. Marmar); U.S. Department of Defense, W81XWH-10-1-0021 (PI: Owen M. Wolkowitz); The Mental Illness Research, Education and Clinical Center (MIRECC). Dr. Kirstin Aschbacher received support from The Institute of Integrative Health (TIIH), The Hellman Foundation, and the NIH/NHLBI: K23 HL112955.
Conflict of interest
No authors have conflicts of interest to disclose. This publication arises from collaborative activities among eight institutions under the U.S. Department of Defense contract “Systems Biology Studies of PTSD”: University of California San Francisco, New York University Langone Medical Center, Icahn School of Medicine at Mount Sinai, US Army Medical Command (MEDCOM), University of California Santa Barbara, Institute for Systems Biology, Emory University and the Veterans Administration Health Care System. The first author works with Jawbone/Aliph; however, that company had no role in the design, funding, or writing of this article.
Ethical approval
This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Aschbacher, K., Mellon, S.H., Wolkowitz, O.M. et al. Posttraumatic stress disorder, symptoms, and white matter abnormalities among combat-exposed veterans. Brain Imaging and Behavior 12, 989–999 (2018). https://doi.org/10.1007/s11682-017-9759-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-017-9759-y