Abstract
Previous studies suggest obesity is associated with altered function within the insula and dorsomedial frontal cortex (including dorsal anterior cingulate cortex; DMFC/dACC), reflecting abnormal reward processing and reduced sensitivity to feelings of satiety. Given the proposed roles of DMFC/dACC in monitoring response conflict and reward-based decision making, the present study examined DMFC/dACC activation, and functional connectivity between the DMFC/dACC and the anterior insula (AI), during food-related decision-making. Twenty participants recruited from the general population (10 Female) performed a decision task while undergoing functional magnetic resonance imaging. They were instructed to “choose the healthier option” when simultaneously shown pairs of images of different foods. Significant DMFC/dACC activation was observed during food-related decision-making, and activation levels also positively correlated with self-reported cravings for high-fat foods (r = 0.57, p = 0.009) and self-reported desire to eat the high-fat foods depicted in the images (r = 0.48, p = 0.032). Negative functional connectivity estimates between the right AI and DMFC/dACC were also associated with self-reported control over eating (r = −0.50, p = 0.025). These results suggest that (1) more intense cravings for unhealthy foods are associated with greater response conflict when deciding between healthy and unhealthy food options, and (2) lack of eating-related control may involve a reduced influence of insula-mediated bodily signals on decision-making. This task may offer a neuroimaging-based probe for identifying individuals vulnerable to eating-related disorders and should be replicated in clinical populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the World Health Organization (WHO), roughly 500 million adults are affected by obesity worldwide (Candeias et al. 2010). As such, obesity is considered a significant health concern, and there may be important benefits associated with gaining a greater understanding of its neurological basis. Existing neuroimaging data have suggested that unhealthy weight gain may relate in part to maladaptive brain function – specifically with regard to interactions between prefrontal cortical regions (associated with cognitive control and decision-making) and cortical/subcortical regions implicated in reward processing, addiction, and the representation of afferent signals from the body (Carnell et al. 2012; Kaye et al. 2009; Stice et al. 2013; van der Laan et al. 2011; Volkow et al. 2008). For example, one recent meta-analysis (Brooks et al. 2013) found that obese individuals, when compared to those of normal weight, showed greater activation within the dorsomedial frontal cortex (DMFC), anterior cingulate cortex (ACC), parahippocampal gyrus, and precentral gyrus in response to food images; reduced activations in obese individuals were also observed in this study within the dorsolateral prefrontal cortex and insular cortex. It is suggested that such findings indicate aberrant cognitive control (i.e., from dorsal frontal structures) and a reduced sensitivity to bodily signals of satiety (i.e., from insular regions representing bodily feelings) within obese individuals. This interpretation is based on the known role of the insula in representing bodily feelings (Craig 2009), including hunger/satiety, as well as the known role of dorsal frontal structures in cognitive control (Domenech and Koechlin 2015). However, while such studies of neural responses in obese individuals are important, their correct interpretation is constrained by limited understanding of the neural basis of food image processing, food cravings, and food-related decision-making in non-clinical populations. As such, there is still considerable controversy regarding the appropriate model of neural processes contributing to obesity (Ziauddeen et al. 2012; Ziauddeen and Fletcher 2013). Further consideration of food-related processing in non-clinical populations may therefore remain important in making progress in this area.
Current work on food-related perceptual processing in non-clinical populations has tended to implicate some, but not all, of the same regions as those associated with obesity. For example, one recent meta-analysis of neuroimaging studies of the visual processing of food images found that the insula, lateral orbitofrontal cortex (OFC), and fusiform gyrus were most consistently activated across studies using healthy participants (van der Laan et al. 2011). This is consistent with the known strong biological saliency of high-calorie visual food stimuli and with previous observations suggesting that automatic visual attention is biased toward such stimuli (Werthmann et al. 2013). However, agreement between the findings across studies in this meta-analysis was only moderate, and dorsal frontal regions implicated in obesity were not observed. On the other hand, more recent animal work has found results within the DMFC (including adjacent dorsal ACC [dACC]) consistent with its role in the neural processing of food stimuli in obesity. For example, one study in mice provided evidence that the DMFC/dACC plays an important role in regulating the rewarding effects of a high-fat diet (Del Rio et al. 2015). Specifically, they observed that the strength of conditioning induced by high-fat food exposure (in a conditioned place preference task) correlated with upregulation of genes involved in dopaminergic transmission within this region. Such findings are also consistent with other animal work demonstrating the role of DMFC/dACC in discriminating the palatability of food (Gaykema et al. 2014; Jezzini et al. 2013) and in food seeking behavior (Kelley 2004). Together, these results suggest that the role of the DMFC/dACC in food perception may relate specifically to its reward- and/or motivation-related influences on decision-making. Differences in the rewarding/motivating properties of food images might therefore explain why DMFC/dACC is not found across all neuroimaging studies of food perception.
In human studies, the DMFC/dACC has also been implicated in cognitive control functions (Domenech and Koechlin 2015), such as action selection (Ridderinkhof et al. 2004), estimating the likelihood of errors (Brown and Braver 2005), and monitoring for (and facilitating the resolution of) conflicting response options (Botvinick et al. 2004; Zavala et al. 2014). Recent computational models within the framework of reinforcement learning have also been constructed in order to integrate these results into a unifying model of this region (Silvetti et al. 2014), and such models can account for the aforementioned findings regarding its role in dopamine-related reward processing, conflict/error processing, and decision-making and cognitive control. While the details of these models are beyond the scope of the present article, they generally predict that, in the context of a goal-directed decision-making task, such as the goal of choosing healthy food options, the DMFC/dACC should respond more strongly when a competing high-calorie (unhealthy) food option is more salient/rewarding (and hence represents a strong competing response option).
Based on the role of DMFC/dACC in food-related reward in animals (Del Rio et al. 2015; Gaykema et al. 2014; Jezzini et al. 2013; Kelley 2004) and in obesity (Brooks et al. 2013) and conflict-related cognitive control in humans (Ridderinkhof et al. 2004; Silvetti et al. 2014; Zavala et al. 2014), we reasoned that, when choosing between healthy and unhealthy food options, greater DMFC/dACC activation should be associated with stronger motivation to choose the competing unhealthy option. For example, when shown both a healthy and an unhealthy food option, and given the goal of choosing the healthy option, individuals who find the unhealthy food option more enticing (i.e., salient/rewarding) would be expected to have greater response conflict (and hence greater DMFC/dACC activation) – reflecting the greater demand to inhibit automatic attention to, and desire for, the unhealthy option. If this hypothesis were supported, it could help clarify the role of this structure in current models of the neural basis of obesity. It could also provide a neural signature capable of predicting vulnerability to unhealthy food decisions. Therefore, in conjunction with the other literature discussed above (Brooks et al. 2013; van der Laan et al. 2011), we predicted that, when compared to a non-food-related decision-making task, food-related decision-making would activate the DMFC/dACC and insula, as well as ventral visual regions associated with food perception. More specifically, however, since it has been particularly related to the rewarding properties of high-fat food (Del Rio et al. 2015), we predicted that greater DMFC/dACC activation would be associated with greater self-reported desire/craving for high-fat foods.
Secondly, as previous models have suggested that poor food-related decision-making may involve an insensitivity of decision-making to bodily signals (Brooks et al. 2013), we also predicted that differences in functional connectivity between the DMFC/dACC and the insula would be associated with self-reported differences in eating-related self-control. Specifically, we hypothesized that eating-related self-control would be diminished as connectivity between the insula and DMFC/dACC became more negative, as this would imply that the insula is more inhibited whenever DMFC/dACC is engaged, potentially leading cognitive decision-making/control processes to be less sensitive to bodily signals of satiety.
Materials/Subjects and Methods
Participants
Twenty adults (10 female) were recruited from the general population within the Boston, MA metropolitan area via flyers, newspapers, and internet/radio advertisements to participate in the present study. These participants ranged in age from 21 to 43 years (M = 30.85, SD = 6.3), and had a body mass index (BMI) ranging from 20.37–35.96 (M = 25.64, SD = 3.46). In order to permit generalization of our findings to the broader population, no attempt was made to exclude participants based on weight status, which resulted in 50% of the sample falling within the normal weight range (BMI: 18–24.9), 40% in the overweight range (BMI: 25–29.9), and 10% in the obese range (BMI: ≥ 30). Participants did not have any history of psychiatric, neurological, eating-related, or substance use disorders (assessed via a phone screen questionnaire based on criteria within the Diagnostic and Statistical Manual for Mental Disorders, 4th addition; DSM-IV-TR), and all provided written informed consent prior to participation. The research protocol of the present study was also reviewed and approved by the US Army Human Research Protections Office, as well as by the Institutional Review Board of McLean Hospital.
Procedure
Pre-task measures
In the morning portion of the study visit, and prior to performing the food decision task, participants filled out a survey that included the following questions:
“When hungry, how much do you crave fats (e.g., fried food, red meats, dairy)?”
“Do you feel you eat more than you intend to (circle one)?”
Participants answered these questions on a scale from 1 (not at all/never) to 10 (always). The question about fat cravings and the question about over-eating (i.e., also used in our previous studies of food processing; e.g., Killgore et al. 2013b; Killgore et al. 2013c) were both based specifically on our hypotheses stated in the introduction.
After completing these pre-task measures, and other assessments unrelated to the food decision task (i.e., assessments relevant to the larger funded study of which the food decision task was one part), all participants were provided an unregulated lunch period. Immediately following lunch, each participant was then escorted to the scanner, and underwent a series of structural and functional neuroimaging scans. The food decision task was initiated one hour after the end of the lunch period. This was done to help maintain relatively stable hunger/satiety levels across participants during task performance. Across participants, the average (mean) start time for this task was 13:40 (SD = 00:38); thus all participants completed the food decision task at roughly the same time of day.
Food decision task
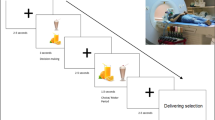
In this task, and while undergoing functional magnetic resonance imaging (fMRI), participants were shown a series of paired images and were instructed to “choose the healthier option” (via button press) for each pair. In the food condition, the two images each contained a food option, with one of the images presenting a food that was clearly healthier (i.e., less calorie-dense, lower fat content, higher nutritional value) than the other (e.g., fresh salad vs. cheeseburger, shrimp vs. hotdog, etc.). The food images for these pairs of stimuli were identical to those in several of our previous studies (Killgore et al. 2003; Killgore et al. 2013a; Killgore and Yurgelun-Todd 2005, 2010). In the activities condition, each image pair displayed people engaged in various activities, and one of the images depicted an activity that was clearly healthier than the other (e.g., exercising vs. sitting on a couch). The activities condition was therefore matched with the food condition in terms of the goal/decision process (i.e., “choose the healthier option”) and in terms of visual processing complexity (i.e., both sets of images contained complex, meaningful objects). However, relative to the “unhealthy” activity images, the “unhealthy” high-calorie/high-fat food images were expected to be more biologically salient (e.g., more powerful at attracting attention, engaging motivation, etc.) (Werthmann et al. 2013), and therefore more potent for promoting response conflict and the need for cognitive control; as described above, we predicted this effect would be greater in participants with stronger cravings/desires for such unhealthy foods.
Finally, there was a visual control condition, in which pixelated images created from the food or activity images were shown. While similar in color and luminance to the previous stimuli, the pixilation process degraded the objects so as to be unrecognizable. In this condition, participants were instructed to choose the image in the pair that was composed of smaller pixel fragments. This condition was only included to aid in further interrogation/interpretation of our resulting imaging data in the food and activities conditions (i.e., if this became necessary).
For all conditions, each pair of images was shown for 2.75 s (followed by a 0.25 s black screen). The image pairs were also counterbalanced such that healthy and unhealthy images (or large and small pixel images) were each displayed on each side of the screen on half of the trials. A block design was used in which 10 image pairs from a given condition were displayed sequentially, followed by 10 image pairs from the next condition, and so forth. The condition block order was: pixels, activities, foods, pixels, foods, activities, pixels. Thus, in total, 20 different image sets were shown for the food and activities conditions, and 30 different image sets were shown for the pixels condition. The healthy/unhealthy food images were selected from a set used in a previous study (Killgore et al. 2003), and paired healthy/unhealthy food images were matched (to the extent possible) with regard to color and object number. Activities images were drawn from an Internet search, and paired healthy/unhealthy activities images were also matched for colors in the image as well as the gender and number of individuals depicted (e.g., two men running vs. two men sitting on a couch). Pixel images were constructed by filtering the images within the food and activities condition using the “stained glass” filter within Adobe Photoshop (CS4 for Macintosh), resulting in unrecognizable shape fragments containing the colors in the original images.
This task therefore represents an adaptation of our previously published food image processing tasks (Killgore et al. 2003; Killgore et al. 2013a; Killgore and Yurgelun-Todd 2005, 2010), in which we added a decision-making component. By placing the healthy and unhealthy food images side-by-side, and adding this decision component, our updated task resembles many other widely used stroop tasks that are often used to study response conflict (e.g., Kerns et al. 2004). In such tasks, as also in our task, the stated goal is to choose a response based on one of the presented stimuli, while another simultaneously presented stimulus strongly competes for control of cognition/behavior. Therefore, although many other response conflict tasks have been used in the literature (e.g., other stroop tasks, flanker tasks, etc.; see Botvinick et al. 2004), we chose to modify a previous food image processing task to incorporate a stroop-like element (i.e., instead of modifying one of these other available conflict tasks to include food images).
Post-task measures
After exiting the scanner, to further confirm individual differences in the types of foods participants had strong tendencies to crave/desire, they were then shown each of the healthy and unhealthy food images from the decision task one at a time, and were asked on a 7-point scale how much they would currently like to eat each food item depicted (1 = do not want to eat it, 7 = strongly desire to eat it). These were intermixed with pictures of non-edible items (e.g., rocks, bricks, trees, flowers), in order to ensure compliance. Participants were also asked to indicate how hungry they were at that moment on a 7-point scale (1 = not at all hungry; 7 = extremely hungry). Given that this measure was always administered directly after exiting the scanner (and before participants could consume any food/water), we considered it a reliable indicator of any individual differences in hunger that may have been present across participants during task performance. Each of these post-task measures have also been used in our previous studies on the neural basis of food processing (e.g., Killgore et al. 2013b; Killgore et al. 2013c).
Neuroimaging methods
Neuroimaging was performed using a 3 T (Siemens Tim Trio scanner, Erlangen, Germany) with a 12-channel head coil. T1-weighted structural 3D MPRAGE images were acquired (TR/TE/flip angle = 2.1 s/ 2.25 ms/ 12 degree) covering 128 sagittal slices (256 × 256) with a slice thickness of 1.33 mm (voxel size = 1.33 × 1 × 1). Functional T2*-weighted scans were acquired over 42 transverse slices (3.5 mm thickness). Volumes were collected with an interleaved sequence (TR/TE/flip angle = 2.5 s/ 30 ms/ 90 degree). The voxel size of the T2* sequence was 3.5 × 3.5 × 3.5 mm. The field of view (FOV) was 22.4 cm, with a 64 × 64 acquisition matrix.
Image processing
Preprocessing steps on all MRI scans, as well as subsequent statistical analyses, were performed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm ). Raw functional images were realigned, unwarped, and coregistered to each subject’s MPRAGE image in accordance with standard algorithms. Images were then normalized to Montreal Neurological Institute (MNI) coordinate space, spatially smoothed (6 mm full-width at half maximum), and resliced to 2 × 2 × 2 mm voxels. The standard canonical hemodynamic response function in SPM was used, low-frequency confounds were minimized with a 128-s high-pass filter, and serial autocorrelation was corrected using the AR(1) function. The Artifact Detection Tool (ART; http://www.nitrc.org/projects/artifact_detect/ ) was also used to regress out scans as nuisance covariates in the first-level analysis exceeding 3 SD in mean global intensity and scan-to-scan motion that exceeded 1.0 mm.
Statistical analysis
For each participant, a general linear model was specified to contrast activation between the food condition and the activities condition. Each trial was modeled as a 2.75 s interval. Motion regressors (generated by ART – see image processing above) were also added to each of these 1st-level designs. These contrast images were then entered into a second-level SPM analysis (one-sample T-test) to assess the main effect of our contrast of interest (i.e., Food > Activities).
For these analyses we used a whole-brain peak significance threshold of p < 0.001 (uncorrected), along with a cluster extent threshold of p < 0.05 (family-wise error [FWE] corrected). For more extensive data analysis, the first eigenvariate across subjects was also extracted from the DMFC/dACC cluster found in this analysis (see results section), based on our a priori hypothesis, and correlated with our pre- and post-task measures of unhealthy/high-fat food craving to test whether activation differences in this region were related to the rewarding properties of unhealthy/high-fat foods (and thus, the amount of decision-conflict) in our participants.
The first-level results for each participant were then imported into the publicly available CONN functional connectivity toolbox (version 15.C; https://www.nitrc.org/projects/conn) in order to perform a generalized form of seed-to-voxel psychophysiologic interaction (gPPI) analyses. This method was chosen because of its improved specificity/sensitivity in detecting connectivity effects (McLaren et al. 2012). The CONN toolbox uses the following equations to estimate PPI effects:
In these equations, H refers to the Hemodynamic Response Function (HRF) in Toeplitz matrix form; Y k refers to the blood oxygenation level dependent (BOLD) signal observed in the seed region; x a refers to the estimated neural activity from the BOLD signal in the seed region (Gitelman et al. 2003); Y i refers to the BOLD signal observed at each voxel in the brain; β i is a matrix of the beta estimates of the psychophysiological interaction terms; β G is a matrix of the beta estimates of the seed region BOLD signal (Y k ), covariates of no interest (G), and convolution of psychological vectors H(g p ); e i is a vector of the residuals of model; and g p is a matrix of N columns, where N refers to the number of conditions in the experiment (formed by separating the time when the conditions are present into separate columns).
For our gPPI analysis, we chose the DMFC/dACC cluster found within the second-level contrast of the Food and Activities decision conditions described above (see results section) as a seed region for a whole-brain seed-to-voxel analysis. This cluster was extracted from SPM (by saving the individual cluster image and importing it into the CONN toolbox as a region-of-interest) and used as a seed region within gPPI, in order to assess changes in connectivity between Food and Activities decision task conditions. For this connectivity analysis we used a whole-brain peak threshold of p < 0.001 (uncorrected) and a cluster-level extent threshold of p < 0.05 (FWE-corrected).
Results
Behavioral measures
Our pre-task self-report ratings for fat cravings had a mean rating (on a 1–10 scale, with 10 = highest) of 5.4 (SD = 2.62). Our pre-task self-report ratings for eating-related self-control problems (i.e., eating more than you intend to) had a mean rating (on a 1–10 scale) of 4.65 (SD = 2.18). Our post-task self-report ratings for desire to eat the foods depicted in the images were divided according to high-fat/high-calorie (“unhealthy”) and low-fat/low-calorie (“healthy”) foods. The mean rating (on a 1–7 scale) for desire to eat the high-fat/high-calorie foods was 4.65 (SD = 1.86). The mean rating (on a 1–7 scale) for desire to eat the low-fat/low-calorie foods was 4.14 (SD = 1.38). The mean rating (on a 1–7 scale) for desire to eat the non-food control items (e.g., flowers, rocks, etc.) was 1.03 (SD = 0.07), suggesting participants were answering the questions honestly/appropriately. The mean rating for post-scan hunger level (on a 1–7 scale) was 4.65 (SD = 1.69). No significant correlation was observed between BMI and any of these ratings (−0.25 ≤ r ≤ 0.01; 0.28 ≤ p ≤ .97, 2-tailed).
Within the food decision task, the average median reaction time (RT) in the food condition was 1098.83 ms (SD = 149.66 ms). Accuracy in selecting the healthier option in this condition was 95.0% (SD = 4.9%). Average median RT for the activities condition was 1177.45 ms (SD = 194.88 ms). Accuracy in selecting the healthier option in this condition was 97.8% (SD = 4.1%). Average median RT for the pixels condition was 884.18 ms (SD = 174.67 ms). Accuracy in selecting the correct pixel image in this condition was 95.5% (SD = 7.0%). These results confirm that all participants performed the task successfully and as instructed.
fMRI activation contrasts
Food > Activities
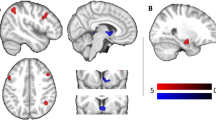
As predicted, this contrast revealed significant activations within a large cluster encompassing the DMFC/dACC region bilaterally, as well as clusters within the right anterior insula (AI), left dorsolateral prefrontal cortex (DLPFC), two left hemisphere regions of the ventral visual cortex, the left AI, the left motor/premotor cortex, and the right posterior insula (PI) (See Table 1; Fig. 1a).
a Displays clusters within the DMFC/dACC and left/right insula (listed in Table 1) that were significantly activated by the “Healthy Food Decisions > Healthy Activities Decisions” contrast. b Displays the right insula cluster (listed in Table 2) observed within the gPPI analysis to have significant negative connectivity with the DMFC/dACC cluster in the “Healthy Food Decisions > Healthy Activities Decisions” contrast. DMFC = dorsomedial frontal cortex; dACC = dorsal anterior cingulate cortex
To ensure these results were not influenced by differences in BMI across the group, we also re-ran this analysis using BMI as a covariate. We observed no substantial change in these results when accounting for BMI (i.e., all of these clusters were still observed and no new clusters were found).
Correlations with high-fat food desire
Based on a priori hypotheses, we extracted the eigenvariate of the DMFC/dACC cluster (using SPM8’s built-in volume-of-interest [VOI] time-series extraction tool) and correlated this with self-reported pre- and post-measures of desire for high-fat food. As predicted, DMFC/dACC activation was significantly positively correlated with both pre-task self-reported cravings for high-fat foods (r = 0.57, p = 0.009, 2-tailed; See Fig. 2, top panel) and post-task self-reported desire to eat the high-fat/high-calorie foods depicted in the “unhealthy” food images (r = 0.48, p = 0.032, 2-tailed).
(Top) Scatterplot illustrating the positive relationship between self-reported high-fat food cravings and DMFC/dACC activation (i.e., first eigenvariates) in the “Healthy Food Decisions > Healthy Activities Decisions” contrast. (Bottom) Scatterplot illustrating the negative relationship between self-reported tendencies to eat more than intended and connectivity estimates (from gPPI analysis) between the right insula and the DMFC/dACC cluster (seed region). DMFC = dorsomedial frontal cortex; dACC = dorsal anterior cingulate cortex
Next, to increase confidence that the DMFC/dACC activation we observed did not simply reflect a greater saliency of food images generally (i.e., compared to images of activities), we ran correlation analyses between DMFC/dACC activation and self-reported desire to eat the low-fat/low-calorie foods shown in the “healthy” food images. As expected, using a significance threshold of p < 0.05, this correlation was also found to be nonsignificant (r = −0.04, p = .87, 2-tailed). Thus, while we expected that the “unhealthy” (high-calorie/high-fat) food images would be more salient (van der Laan et al. 2011; Werthmann et al. 2013), and would therefore promote more conflict in individuals with strong cravings/desires for such foods, this nonsignificant correlation between DMFC/dACC activity and desire for low-fat/low-calorie foods supports the idea that the DMFC/dACC activation we observed cannot be explained by exposure to food images vs. activities images in general.
To ensure that these results were not confounded by age, post-scan hunger level, or BMI, we also ran additional correlation analyses between DMFC/dACC activation and these variables. Using a significance threshold of p < 0.05, no significant correlation was observed between DMFC/dACC activation and any of these variables (age: r = 0.07; hunger: r = 0.10; BMI: r = −0.12; 0.61 ≤ p ≤ 0.77). A two-sample t-test also did not reveal a significant difference in DMFC/dACC activation between males and females (t = 0.290, p = .76).
Finally, as a further test of our assumption that individual differences in DMFC/dACC activation in the ‘Food > Activities’ contrast reflected individual differences in response conflict (i.e., greater activation associated with greater conflict), we ran a partial correlation analysis between median RTs in the Food condition and DMFC/dACC activation values across participants, controlling for median RTs in the Activities condition. In support of this assumption, this analysis revealed a significantly positive partial correlation (rp = .489, p = .03, 2-tailed), indicating longer RTs in the Food condition in participants displaying greater DMFC/dACC activation (i.e., consistent with the idea of greater response conflict).
gPPI analyses
For the contrast of Food > Activities, our seed-to-voxel gPPI analysis using the DMFC/dACC cluster (i.e., from the standard fMRI analysis reported in Table 1) as a seed region revealed significantly negative connectivity between the DMFC/dACC cluster and both the right AI and the right precuneus (see Table 2 and Fig. 1b).
Correlation with eating-related self-control
To test the idea that healthy-food-related decision-making ability was associated with sensitivity to bodily signals, we ran correlation analyses between right AI-DMFC/dACC connectivity estimates and self-reported eating-related self-control. We observed a significant negative correlation (r = −0.50, p = 0.025, 2-tailed), suggesting that the stronger the negative relationship was between the right AI and the DMFC/dACC, the more likely an individual was to report generally eating more than they intend (See Fig. 2, bottom panel).
To ensure that these results were not confounded by age, post-scan hunger level, or BMI, we also ran additional correlation analyses between right AI-DMFC/dACC connectivity estimates and these variables. Using a significance threshold of p < 0.05, no significant correlation was observed between right AI-DMFC/dACC connectivity estimates and any of these variables (age: r = −0.22; hunger: r = 0.02; BMI: r = 0.24; 0.31 ≤ p ≤ 0.93). A two-sample t-test also did not reveal a significant difference in right AI-DMFC/dACC connectivity estimates between males and females (t = −2.05, p = 0.06), although there did appear to be a trend suggesting males tended to have stronger negative connectivity between these regions than females.
Discussion
DMFC/dACC activation differences
In this study we tested the hypothesis that DMFC/dACC activation within the context of food-related decision-making would act as a reliable indicator of desire/craving for high-fat food in a non-clinical sample. Consistent with this hypothesis, we observed that greater DMFC/dACC activation was associated with greater self-reported cravings for high-fat foods generally (i.e., a self-reported trait), and that it was also associated with greater average self-reported desire to eat the specific high-fat/high-calorie foods depicted in images on the day of the assessment. Therefore two different measures of an individual’s tendency/disposition to desire high-fat foods showed the same relationship to activity in this region during task performance. Crucially, activation within this region was not significantly associated with self-reported desires for low-fat foods, suggesting that DMFC/dACC responses do not simply reflect increased sensitivity to the saliency or rewarding properties of food images generally.
As the DMFC/dACC is known to activate more strongly in the context of a conflict between goal-directed and automatic responses (Botvinick et al. 2004), we suggest that there is a straightforward interpretation of this result. Specifically, when the task-specific goal is to choose the healthier food options, it is reasonable to expect that those who find the competing unhealthy food options more salient/rewarding (i.e., those with a stronger desire for, and therefore a stronger motivation to choose, the unhealthy/high-fat foods) will also have a stronger conflict between response options. In other words, the unhealthy food images would be expected to compete more strongly for further cognitive/behavioral processing in such individuals, and therefore greater response conflict management would be required for them to perform the task successfully. Based on this reasoning, these individuals would therefore be expected to demonstrate stronger conflict-related DMFC/dACC responses in the food condition. This interpretation is also supported by our finding that greater DMFC/dACC responses in the ‘Food > Activities’ contrast were associated with longer reaction times in the Food condition (i.e., when controlling for reaction times in the Activities condition), as might be expected with greater response conflict.
The fact that such individuals have greater response conflict is also theoretically related to stronger reward properties of high-fat food, in that reward-related decision-making specifically seeks to make decisions that are predicted to maximize expected reward (Silvetti et al. 2014). This conceptual/theoretical connection between reward and response conflict thus also allows these findings to relate to animal work illustrating a relationship between DMFC/dACC and high-fat food reward (Del Rio et al. 2015; Gaykema et al. 2014; Jezzini et al. 2013; Kelley 2004), as well as to recent theoretical models of the DMFC/dACC that explain conflict-related responses in terms of the necessary computations underlying reward-based decision-making (Silvetti et al. 2014). Essentially, individuals who find unhealthy/high-fat food more rewarding will also have greater response conflict in healthy food-related decision-making, and it therefore makes sense why the DMFC/dACC activation observed within the present experimental paradigm would be reliably associated with the desire/craving for high-fat food.
In conjunction with the aforementioned interpretation, many studies have also suggested that, in the context of high conflict, the DMFC/dACC and the DLPFC may both play a role in resolving conflict via adjustment of top-down attentional modulation (e.g., Kerns et al. 2004; Weissman et al. 2004). For healthy food choice specifically, related studies (Hare et al. 2009, 2011) have also suggested that DLPFC aids in this process by attentionally modulating the values assigned to different food options in ventromedial prefrontal cortex (VMPFC). Therefore, when considering these previous results, it is important to recognize that the greater DMFC/dACC activation we observed in participants with strong cravings/desires for high-fat foods likely only represents one part of a larger process. For example, since our accuracy and reaction time data confirm that all participants performed the task successfully, one might plausibly expect that, after DMFC/dACC engagement, top-down attentional control processes were also deployed to modulate represented food values (i.e., in a manner that facilitated the recognition and selection of the healthy food images). Future studies should therefore attempt to replicate the individual differences in conflict-related activation found in the present study, while also using a paradigm designed to simultaneously assess the value-related VMPFC responses to each food image during the decision process.
Aside from this specific, hypothesized result, we also observed other regional brain activation clusters in the ‘Food > Activities’ contrast that we expected based on the fact that these two conditions contained pictures of food and non-food items, respectively. That is, we also observed activations within ventral visual cortical regions, and in the insula, which overlap with the results of a recent meta-analysis of neuroimaging studies examining the visual processing of food images (van der Laan et al. 2011). Thus, it is plausible that our results within these areas relate to differences between food and non-food images (and are therefore not specific to making food-related decisions). In contrast, the clusters we observed within dorsal frontal regions are not reliably associated with food perception in past studies, and thus are more plausibly associated with food-related decision-making specifically. Interestingly, it is the DMFC/dACC, DLPFC, and motor/premotor regions we observed that overlap with results of a recent meta-analysis of neuroimaging studies of obesity (Brooks et al. 2013), which is consistent with the possibility that obesity is more strongly associated with abnormalities in decision-making than in perception. As described above, the observed activity in the DLPFC would also be expected given its known complimentary role in cognitive control and goal-directed attentional modulation processes (e.g., Hare et al. 2009, 2011). In summary, while we have focused on our hypothesized results within DMPFC/dACC, the rest of our reported fMRI results are also consistent with previous findings regarding food perception and food-related decision-making.
DMFC/dACC functional connectivity differences
In this study, we also used functional connectivity analysis to test the previously proposed hypothesis (Brooks et al. 2013) that unhealthy food-related decision-making may relate to an insensitivity to represented bodily signals associated with hunger/satiety. Given the role of DMFC/dACC in decision-making, we predicted that connectivity differences between this structure and the insula would be associated with differences in self-reported eating-related self-control. This was based on the idea that bodily signals represented in the insula (Craig 2003, 2009) may need to have an appropriate influence on decision-making within DMFC/dACC in order to drive healthy decisions regarding when to stop eating. Consistent with this line of reasoning, we observed that those who reported a greater tendency to eat more than intended also showed a stronger inverse relationship (i.e., stronger negative connectivity) between the right insula and the DMFC/dACC. This suggests that, in the context of food decisions, and specifically in participants who often eat more than they intend, the right insula tends to be inhibited whenever the DMFC/dACC is engaged. One plausible interpretation of this result is thus that, in those who report more eating-related self-control problems, representations of bodily signals are inhibited/activated in a pattern that is more “out of sync” with neural systems subserving decision-making and cognitive control. This may therefore represent a plausible neural signature underlying the idea that unhealthy food decisions, and eating-related self-control problems specifically, might be explained by the insensitivity of decision-making to bodily signals.
This finding/interpretation also appears to find support from other previous work on the role of the insula in self-control within the context of substance addiction (Naqvi et al. 2014). Such studies support a role for the insula in representing bodily states associated with drug use, and engaging motivational processes that promote addictive behavior and that conflict with more adaptive goals; this proposed role for the insula explains, for example, why insula lesions have been found to disrupt addiction to cigarette smoking (Naqvi et al. 2007). In the context of the present results, it would also help explain why, if the insula tends to be inhibited whenever the DMFC/dACC is activated, insula-mediated representations of bodily satiety would tend to fail to motivate the cessation of eating behavior – leading to the greater self-reported levels of over-eating we observed.
Limitations and conclusions
It is important to highlight the limitations of the methodology and interpretations used in the present study. First, our data were obtained from a non-clinical sample that was recruited to reflect the general pattern of weight variation within the larger population. Recent data from the CDC suggest that 69% of the U.S. population is overweight or obese (http://www.cdc.gov/nchs/fastats/obesity-overweight.htm), while our sample only included 50% in these weight ranges. Thus, further replication of these findings will need to include a more representative proportion of individuals in the extreme low and high ends of the BMI continuum. The low number of participants at the extreme low and high ends of this continuum may also explain why we did not observe any influence of BMI on our neuroimaging results, despite the known difference in brain activity patterns in obese individuals (e.g., Brooks et al. 2013). It should also be kept in mind that the present study was designed to look at brain activation during decisions between healthy and unhealthy food options, while brain activity differences previously observed in obese individuals have not been in the specific context of a food decision task (i.e., neural associations with weight status have mainly been assessed in tasks involving food perception and food anticipation; reviewed in van Meer et al. 2016). Given that our stimuli included equal numbers of calorie-dense and calorie-lean foods (presented simultaneously), we would therefore not expect to see the same types of relationships with obesity/BMI as seen in simple food perception tasks or in other tasks that lack an explicit choice component.
Another potential limitation of the present study pertains to our gPPI functional connectivity analysis. It is important to highlight that gPPI analyses do not justify conclusions about the direction of influence between two regions (McLaren et al. 2012); gPPI methodology accounts for task-related changes when assessing the relationship between activity in two regions (thus only detecting a relationship over and above those attributable to task-related changes in activation), but it does not provide causal information. Therefore, while we can conclude that the negative relationship we observed between the right insula and the DMFC/dACC is independent of activation differences between the food and activities conditions, we are unable to conclude that the right insula inhibits the DMFC/dACC (or vice-versa). Future work will therefore be required to better understand these aspects of the relationship between DMFC/dACC, the insula, and food-related decision-making.
A third limitation pertains to the fact that we used self-report measures to assess the desire/craving for high-fat food and to assess tendencies toward eating more than intended. It is well known that self-reports, even when provided honestly, can sometimes be only modestly associated with actual behavior (Dunning et al. 2004). Thus, we cannot be sure that our participants’ actual food-related desires, decisions, and behaviors are accurately reflected in the self-reports they provided, and future work should attempt to replicate our results using behavioral and/or other objective measures to assess individual differences in eating-related self-control and in the desire for, and rewardingness of, high-fat foods. Despite this limitation, we are unaware of any evidence suggesting that self-reports in this context should be expected to be inaccurate; we also consider it promising that the relationship we observed between DMFC/dACC and high-fat food desire was consistent across two distinct self-report measures of the same construct (i.e., general self-reported cravings for high-fat foods and specific desire ratings across many high-fat/high-calorie food images).
A fourth limitation pertains to the novel task we used. While it was adapted from previously used food image processing paradigms (Killgore et al. 2003; Killgore et al. 2013a; Killgore and Yurgelun-Todd 2005, 2010), some aspects of the present task have not been used before. Future studies should therefore attempt to replicate our findings using this task, and perhaps attempt to address some of its potential limitations as well. For example, stronger and more realistic levels of conflict may have been achieved if actual eating choices would have been included, and future studies might attempt to improve the task along these lines. Another possible task limitation is that identifying “activities” in the control condition might plausibly have been a more abstract/complex task than identifying simple food objects. Further, although images of unhealthy activities were expected to be much less biologically salient in comparison to unhealthy (high-calorie) food images, we cannot rule out the possibility that some conflict was engendered by choosing healthier activities as well; and as we did not collect information about our participants’ desires to engage in healthy vs. unhealthy activities, we also cannot rule out the possibility that the salience of unhealthy food images and that of unhealthy activity images could be somewhat correlated across individuals. Thus, while the activities condition was well-matched with the food condition with regard to the presentation of complex, recognizable objects, and with regard to the general goal/decision process, its limitations should be acknowledged.
Finally, it is important to acknowledge that, while we asked participants to choose the “healthier” food option in our study, this term does not have an unambiguous, context-independent meaning. For example, while a salad may be healthier than a cheeseburger for most individuals, the exact opposite might be true of someone who was malnourished or suffering from anorexia nervosa. However, given that accuracy was near ceiling for choosing healthier food options in our task, and given that our participants were screened out for these types of clinical health issues, we do not believe the context-dependent meaning of the term “healthy” represents a confound to our proposed interpretations.
In conclusion, we report findings that support the possibility that the pattern of DMFC/dACC activation (and connectivity) provoked by the present food decision paradigm might serve as a reliable predictor of clinically important aspects of food consumption behavior. Conflict-related DMFC/dACC activation differences appear capable of predicting the relative extent to which individuals desire high-fat foods, and the connectivity patterns between this region and the insula also appear capable of predicting relative tendencies toward eating more than intended. Although these results come from a community sample, they support the possibility that differences in connectivity between the insula and DMFC/dACC, and differences in the efficiency of response conflict resolution processes in the DMFC/dACC, might represent mechanisms contributing to poor cognitive control over eating in clinically obese populations as well. Given that these processes are both important potential contributors to obesity, we suggest the present paradigm might be usefully extended to clinical contexts such as morbid obesity and eating disorders. Future studies should examine, for example, whether food-related decision-making, and DMFC/dACC activation, is abnormal under conditions of high response conflict in such populations. Such work might also examine whether interventions designed to increase attention to interoceptive bodily signals might improve food-related decisions among individuals with eating disorders or excessive weight gain. Such examples illustrate potential ways the neural signatures identified in the present study might be usefully harnessed in future research in clinical populations.
References
Botvinick, M., Cohen, J., & Carter, C. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8(12), 539–546. doi:10.1016/j.tics.2004.10.003.
Brooks, S., Cedernaes, J., & Schiöth, H. (2013). Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: A meta-analysis of fMRI studies. PloS One, 8(4), e60393. doi:10.1371/journal.pone.0060393.
Brown, J., & Braver, T. (2005). Learned predictions of error likelihood in the anterior cingulate cortex. Science, 307(5712), 1118–1121. doi:10.1126/science.1105783.
Candeias, V., Armstrong, T., & Xuereb, G. (2010). Diet and physical activity in schools: Perspectives from the implementation of the WHO global strategy on diet, physical activity and health. Canadian Journal of Public Health, 101(Suppl), S28–S30.
Carnell, S., Gibson, C., Benson, L., Ochner, C. N., & Geliebter, A. (2012). Neuroimaging and obesity: Current knowledge and future directions. Obesity Reviews, 13(1), 43–56. doi:10.1111/j.1467-789X.2011.00927.x.
Craig, A. D. (2003). Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology, 13(4), 500–505.
Craig, A. D. (2009). How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10(1), 59–70.
Del Rio, D., Cano, V., Martín-Ramos, M., Gómez, M., Morales, L., Del Olmo, N., & Ruiz-Gayo, M. (2015). Involvement of the dorsomedial prefrontal cortex in high-fat food conditioning in adolescent mice. Behavioural Brain Research, 283, 227–232. doi:10.1016/j.bbr.2015.01.039.
Domenech, P., & Koechlin, E. (2015). Executive control and decision-making in the prefrontal cortex. Current Opinion in Behavioral Sciences, 1, 101–106. doi:10.1016/j.cobeha.2014.10.007.
Dunning, D., Heath, C., & Suls, J. (2004). Flawed self-assessment: Implications for health, education, and the workplace. Psychological Science in the Public Interest, 5(3), 69–106. doi:10.1111/j.1529-1006.2004.00018.x.
Gaykema, R., Nguyen, X.-M., Boehret, J., Lambeth, P., Joy-Gaba, J., Warthen, D., & Scott, M. (2014). Characterization of excitatory and inhibitory neuron activation in the mouse medial prefrontal cortex following palatable food ingestion and food driven exploratory behavior. Frontiers in Neuroanatomy, 8, 60. doi:10.3389/fnana.2014.00060.
Gitelman, D., Penny, W., Ashburner, J., & Friston, K. (2003). Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. NeuroImage, 19(1), 200–207. doi:10.1016/S1053-8119(03)00058-2.
Hare, T., Camerer, C., & Rangel, A. (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 324(5927), 646–648. doi:10.1126/science.1168450.
Hare, T., Malmaud, J., & Rangel, A. (2011). Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. Journal of Neuroscience, 31(30), 11077–11087. doi:10.1523/JNEUROSCI.6383-10.2011.
Jezzini, A., Mazzucato, L., La Camera, G., & Fontanini, A. (2013). Processing of hedonic and chemosensory features of taste in medial prefrontal and insular networks. The Journal of Neuroscience, 33(48), 18966–18978. doi:10.1523/JNEUROSCI.2974-13.2013.
Kaye, W., Fudge, J., & Paulus, M. (2009). New insights into symptoms and neurocircuit function of anorexia nervosa. Nature reviews. Neuroscience, 10(8), 573–584. doi:10.1038/nrn2682.
Kelley, A. (2004). Ventral striatal control of appetitive motivation: Role in ingestive behavior and reward-related learning. Neuroscience and Biobehavioral Reviews, 27(8), 765–776. doi:10.1016/j.neubiorev.2003.11.015.
Kerns, J., Cohen, J., MacDonald III, A., Cho, R., Stenger, V., & Carter, C. (2004). Anterior cingulate conflict monitoring and adjustments in control. Science, 303(5660), 1023–1026. doi:10.1126/science.1089910.
Killgore, W., & Yurgelun-Todd, D. (2005). Body mass predicts orbitofrontal activity during visual presentations of high-calorie foods. Neuroreport, 16(8), 859–863.
Killgore, W., & Yurgelun-Todd, D. (2010). Sex differences in cerebral responses to images of high versus low-calorie food. Neuroreport, 21(5), 354–358. doi:10.1097/WNR.0b013e32833774f7.
Killgore, W., Young, A., Femia, L., Bogorodzki, P., Rogowska, J., & Yurgelun-Todd, D. (2003). Cortical and limbic activation during viewing of high- versus low-calorie foods. NeuroImage, 19(4), 1381–1394. doi:10.1016/S1053-8119(03)00191-5.
Killgore, W., Kipman, M., Schwab, Z., Tkachenko, O., Preer, L., Gogel, H., et al. (2013a). Physical exercise and brain responses to images of high-calorie food. Neuroreport, 24(17), 962–967. doi:10.1097/WNR.0000000000000029.
Killgore, W., Schwab, Z., Weber, M., Kipman, M., DelDonno, S., Weiner, M., & Rauch, S. (2013b). Daytime sleepiness affects prefrontal regulation of food intake. NeuroImage, 71, 216–223. doi:10.1016/j.neuroimage.2013.01.018.
Killgore, W., Weber, M., Schwab, Z., Kipman, M., DelDonno, S., Webb, C., & Rauch, S. (2013c). Cortico-limbic responsiveness to high-calorie food images predicts weight status among women. International Journal of Obesity, 37(11), 1435–1442. doi:10.1038/ijo.2013.26.
van der Laan, L., de Ridder, D., Viergever, M., & Smeets, P. (2011). The first taste is always with the eyes: A meta-analysis on the neural correlates of processing visual food cues. NeuroImage, 55(1), 296–303. doi:10.1016/j.neuroimage.2010.11.055.
McLaren, D., Ries, M., Xu, G., & Johnson, S. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61(4), 1277–1286. doi:10.1016/j.neuroimage.2012.03.068.
van Meer, F., Charbonnier, L., & Smeets, P. (2016). Food decision-making: Effects of weight status and age. Current Diabetes Reports, 16(9), 84. doi:10.1007/s11892-016-0773-z.
Naqvi, N., Rudrauf, D., Damasio, H., & Bechara, A. (2007). Damage to the insula disrupts addiction to cigarette smoking. Science, 315(5811), 531–534. doi:10.1126/science.1135926.
Naqvi, N., Gaznick, N., Tranel, D., & Bechara, A. (2014). The insula: A critical neural substrate for craving and drug seeking under conflict and risk. Annals of the New York Academy of Sciences, 1316, 53–70. doi:10.1111/nyas.12415.
Ridderinkhof, K., Ullsperger, M., Crone, E., & Nieuwenhuis, S. (2004). The role of the medial frontal cortex in cognitive control. Science, 306(5695), 443–447. doi:10.1126/science.1100301.
Silvetti, M., Alexander, W., Verguts, T., & Brown, J. (2014). From conflict management to reward-based decision making: Actors and critics in primate medial frontal cortex. Neuroscience and Biobehavioral Reviews, 46(Pt 1), 44–57. doi:10.1016/j.neubiorev.2013.11.003.
Stice, E., Figlewicz, D., Gosnell, B., Levine, A., & Pratt, W. (2013). The contribution of brain reward circuits to the obesity epidemic. Neuroscience and Biobehavioral Reviews, 37(9 Pt a), 2047–2058. doi:10.1016/j.neubiorev.2012.12.001.
Volkow, N., Wang, G.-J., Fowler, J., & Telang, F. (2008). Overlapping neuronal circuits in addiction and obesity: Evidence of systems pathology. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 363(1507), 3191–3200. doi:10.1098/rstb.2008.0107.
Weissman, D., Gopalakrishnan, A., Hazlett, C., & Woldorff, M. (2004). Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cerebral Cortex, 15(2), 229–237. doi:10.1093/cercor/bhh125.
Werthmann, J., Roefs, A., Nederkoorn, C., Mogg, K., Bradley, B., & Jansen, A. (2013). Attention bias for food is independent of restraint in healthy weight individuals—An eye tracking study. Eating Behaviors, 14(3), 397–400. doi:10.1016/j.eatbeh.2013.06.005.
Zavala, B., Tan, H., Little, S., Ashkan, K., Hariz, M., Foltynie, T., et al. (2014). Midline frontal cortex low-frequency activity drives subthalamic nucleus oscillations during conflict. The Journal of Neuroscience, 34(21), 7322–7333. doi:10.1523/JNEUROSCI.1169-14.2014.
Ziauddeen, H., & Fletcher, P. (2013). Is food addiction a valid and useful concept? Obesity Reviews, 14(1), 19–28. doi:10.1111/j.1467-789X.2012.01046.x.
Ziauddeen, H., Farooqi, I., & Fletcher, P. (2012). Obesity and the brain: How convincing is the addiction model? Nature reviews. Neuroscience, 13(4), 279–286. doi:10.1038/nrn3212.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by a USAMRAA grant to WDSK (grant number W81XWH-09-1-0730).
Conflict of interest
Ryan Smith declares that he has no conflict of interest. Anna Alkozei declares that she has no conflict of interest. W.D. “Scott” Killgore declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Smith, R., Alkozei, A. & Killgore, W.D.S. Conflict-related dorsomedial frontal cortex activation during healthy food decisions is associated with increased cravings for high-fat foods. Brain Imaging and Behavior 12, 685–696 (2018). https://doi.org/10.1007/s11682-017-9726-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-017-9726-7