Abstract
Evidence suggests that testicular cancer (TC) and its treatment are associated with cognitive impairment. However, the underlying neural substrate and biological mechanisms are poorly understood. This study aimed to investigate changes in cognition and brain grey matter (GM) morphology in TC patients undergoing treatment, and to explore associations with immune markers, endocrine markers, and genotype. Sixty-five patients with stage I-III TC underwent assessment after surgery but prior to further treatment and again 6 months after. Twenty-two patients received chemotherapy (+CT), while 43 did not (−CT). Assessments included neuropsychological testing, whole-brain magnetic resonance imaging, and blood samples. Twenty-five healthy controls (HCs) underwent neuropsychological testing with a matching time interval. A regression-based approach was used to determine cognitive changes and longitudinal voxel-based morphometry (VBM) was performed to investigate changes in GM density in the TC groups. Compared with the HCs, both TC groups showed higher rates of cognitive decline (p < 0.05). A trend towards greater decline was observed in + CT (63.6 %) compared with -CT patients (39.5 %) (p = 0.07). VBM revealed widespread GM reductions in both TC groups, but a group-by-time interaction analysis revealed prefrontal reductions specific to the + CT group (p = 0.02), which were associated with poorer cognitive performance. Poorer cognitive performance was also associated with an increase in tumor necrosis factor alpha in + CT patients. Furthermore, an interaction effect was found between the APOE ε4 genotype and chemotherapy on cognitive performance with ε4 carriers performing significantly worse. These findings provide novel evidence of changes in cognition and brain morphology in TC patients undergoing treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer-related cognitive impairment (CRCI) has gained increasing attention in recent years, and while research in high incidence cancer populations (i.e. breast cancer) has been steadily increasing, there are only a few studies investigating CRCI in the context of testicular cancer (TC). In western countries, TC is the most frequent type of cancer in men aged 15–34 (Trabert et al. 2014) with the highest prevalence found in Norway and Denmark (Dahl and Brydøy 2012; Groll et al. 2007). Treatment with the combined cytostatic regimens of bleomycin, etoposide, and cisplatin (platinum) (BEP) has dramatically reduced the mortality rate with current cure rates reaching well above 95 % (Dahl and Brydøy 2012). The young average age of TC patients, in combination with a favorable prognostic outlook, has led the majority of TC patients to return to normal life and work activities following successful treatment. Cisplatin-based chemotherapy, however, has well-known neurotoxic side effects such as peripheral sensory neuropathy and ototoxicity, which may persist several years post-treatment (Dahl and Brydøy 2012). Furthermore, elevated concentrations of plasma platinum have been observed in TC survivors more than 10 years post-treatment (Gietema et al. 2000). Although the exact underlying pathophysiology of platinum neurotoxicity is unclear, DNA damage, inflammation, alterations in cellular system repair, changes in mitochondria function, and apoptotic cell death are some of the proposed mechanisms involved (Carozzi et al. 2014; Li et al. 2015). So far, it has been assumed that cisplatin does not permeate the blood–brain-barrier (BBB) and thus the neurotoxic effects of BEP in the brain were considered to be minimal (Gregg et al. 1992). However, recent evidence suggests that cisplatin is in fact able to cross the BBB (Eiseman et al. 2014), and although the amount crossing may be small, neural populations including progenitor cells, oligodendrocytes, and hippocampal neurons are exceptionally vulnerable to even small concentrations with possible adverse effects on cognition (Andres et al. 2014; Dietrich et al. 2006; Gong et al. 2011; Rzeski et al. 2004).
The few available studies investigating the effects of BEP chemotherapy on neuropsychologically-assessed cognitive functions in TC populations have yielded mixed results (Pedersen et al. 2009; Schagen et al. 2008; Skaali et al. 2011; Wefel et al. 2014). It should be noted that in these studies, surgery-only TC patients were used as the only comparison group; however, recent findings suggest that a high proportion of these patients may also show signs of cognitive impairment (Amidi et al. 2015a, b; Wefel et al. 2011b), and may thus not serve as an adequate comparison condition. Cognitive impairment prior to the receipt of chemotherapy has also been observed in other cancer populations (e.g. Lange et al. 2014), and although the exact underlying pathophysiological mechanisms are still unknown, both endocrine and inflammatory pathways have been proposed as potential mediators (Janelsins et al. 2014). In our own previous work, we found that elevated cortisol levels were a predictor of pre-chemotherapy cognitive impairment (Amidi et al. 2015a). These findings highlight the need for including a healthy control (HC) group when investigating changes in cognitive functions in TC patients.
Findings from recent studies using magnetic resonance imaging (MRI) to examine the neural substrate underlying CRCI have revealed morphological changes in several brain regions associated with the receipt of chemotherapy (Kesler et al. 2013; Lepage et al. 2014; McDonald et al. 2010). For example, McDonald et al. (2010) showed reductions in grey matter (GM) density in breast cancer patients from pre-adjuvant chemotherapy to 1 month post-chemotherapy in bilateral frontal, temporal, and cerebellar regions, with some recovery by 1 year post-chemotherapy. Moreover, reductions in GM in cancer patients have been found to be negatively associated with cognitive functioning, including attention, learning, and memory (Conroy et al. 2013; Inagaki et al. 2007). Although most of the neuroimaging studies have been undertaken in breast cancer populations in which cisplatin-based chemotherapy is usually not administered, a recent cross-sectional study of long-term TC survivors investigated the effects of BEP chemotherapy on brain structural properties and found no indications of reduced GM volumes in TC survivors who had received BEP compared with those who had not. Interestingly, a difference in white matter microstructure was observed in the former group indicating higher radial kurtosis across widespread white matter areas in the brain (Stouten-Kemperman et al. 2015). In contrast, another recent cross-sectional study reported differences in GM density between small-cell lung cancer patients receiving platinum-based chemotherapy (including cisplatin) compared with non-small-cell lung cancer patients who did not receive such treatment (Simó et al. 2015). Specifically, reduced brain GM density was observed in the insula and the parahippocampal gyrus bilaterally, and in the left anterior cingulate cortex. To the best of our knowledge, the detrimental effects of BEP chemotherapy on brain morphology in TC patients are yet to be determined in a prospective study.
The role of various biomarkers in CRCI has been increasingly explored. For example, chemotherapy-related neurotoxicity may be caused indirectly through pro-inflammatory pathways via cytokine-mediated interactions between neurons and glia (Wilson et al. 2002). Cytokines mediate cellular mechanisms subserving cognition through cholinergic and dopaminergic pathways (Wilson et al. 2002). Circulating peripheral cytokines may induce brain cytokines synthesis by neuron and glial cells (Buller 2001). It has also been documented that cytokines may readily enter the CNS through active transport mechanisms (Osburg et al. 2002) or directly through a less stringent BBB in circumventricular regions (Buller 2001; Maier and Watkins 2003). Furthermore, the binding of cytokines to endothelial receptors may lead to impairment of BBB integrity (Wilson et al. 2002), potentially resulting in greater permeability of chemotherapeutic regimens into the CNS. There is evidence from research in breast cancer populations showing associations between proinflammatory cytokines, i.e., tumor necrosis factor alpha (TNFα) and interleukin 6 (IL-6), and both impaired cognitive functioning and morphological changes in specific brain regions (Kesler et al. 2013). Furthermore, the surrogate marker for TNFα, soluble TNF receptor type II (sTNF-RII) has been longitudinally associated with self-reported cognitive impairment as well as with changes in cerebral metabolism in breast cancer patients undergoing chemotherapy (Ganz et al. 2013). In addition, various proinflammatory cytokines, including TNFα, are reported to induce synthesis of C-reactive protein (CRP) (Sheldon et al. 1993), another biomarker associated with cognitive impairment (Roberts et al. 2009). Cortisol may be yet another biomarker of potential relevance to CRCI. Chronic stress may result in over-activation of the hypothalamic–pituitary–adrenal (HPA) axis causing elevations in glucocorticoid production, including cortisol. It is relatively well-established that increased cortisol levels may be associated with impaired cognition (Lee et al. 2007). Because cortisol secretion may be altered in cancer patients (Touitou et al. 1996), such alterations likely play a role in CRCI. Indeed, in a previous publication, we reported results indicating poorer cognitive performance to be related to higher cortisol levels in newly orchiectomized TC patients prior to systemic treatment (Amidi et al. 2015a). With respect to possible genetic vulnerability factors, a possible candidate could be the ε4 allele of the Apolipoprotein E (APOE ε4), which is a well-established genetic risk factor for Alzheimer’s disease (Lambert et al. 2013) and has been associated with cognitive dysfunction in both non-pathological and pathological conditions including the early phases of Alzheimer’s disease (Reinvang et al. 2013). There is evidence from cancer populations (i.e., breast cancer, lymphoma cancer) suggesting that carriers of the ε4 allele are at increased risk of post-treatment cognitive impairment (Ahles et al. 2003, 2014). The possible moderating role of APOE ε4 in chemotherapy-induced cognitive impairment is thought to be related to its role in neuronal repair and plasticity (Ahles and Saykin 2007).
Most of the above-mentioned biomarkers have primarily been explored in relation to CRCI in breast cancer populations, and so far, little work has been done in men with TC. The primary aims of the present study were thus: 1) to prospectively compare cognitive functioning in TC patients receiving surgery ± BEP chemotherapy and a HC group; 2) to compare possible morphological changes in brain GM between patients treated with and without BEP chemotherapy; 3) to examine associations between potential GM reductions and cognitive performance; and finally 4) to explore the role of immune, endocrine, and inflammatory markers, and the APOE genotype, in relation to CRCI.
Participants
From June 2012 to December 2013, recently orchiectomized TC patients were consecutively recruited at the Department of Oncology, Aarhus University Hospital, based on the following exclusion criteria: age < 18 years; time since orchiectomy > 30 days; previous cancer and central nervous system diseases; brain metastases; known mental disorders; inability to read and understand Danish, and any contraindications for MRI.
HC participants with no known confounding illnesses were recruited by means of advertisements in the local community. Informed consent was obtained from all participants upon enrollment. The regional scientific ethical committee approved the study and data were handled according to Danish Data Protection Agency guidelines.
Methods
Patients consisted of a surgery group that did not receive chemotherapy (−CT), and a chemotherapy group that, in addition to surgery, received 3 or 4 cycles of BEP chemotherapy (+CT). All patients were scheduled for two assessments: after orchiectomy, but before further treatment (baseline), and approximately 6-months later (follow-up). Assessments included neuropsychological testing, a structural MRI scan, a blood draw, and a questionnaire package. Participants in the HC group, matched on age and premorbid intellectual functioning with the + CT group, were scheduled for two assessments with a similar interval, but without MRI and blood draw.
Demographic and clinical data
Data for the demographic and clinical variables presented in Table 1, were extracted from medical records and questionnaires. Group differences were tested with ANOVAs and Kruskal-Wallis tests for continuous variables and Chi-square tests for categorical variables.
Neuropsychological assessment
An approximately 1.5 hour long neuropsychological test battery was used for the measurement of cognitive functioning consisting of eight standardized tests yielding 11 outcomes assessing reaction time (MOART Reaction Time Panel, Lafayette, Indiana), attention and working memory (Wechsler 2008; Wiens et al. 1997), processing speed (Reitan 1958; Wechsler 2008), auditory learning and memory (Schmidt 1996), verbal fluency (Benton and Hamsher 1989), and executive functions (Heaton et al. 1993; Reitan 1958). Premorbid intellectual functioning was estimated with the Wechsler Adult Intelligence Scale - Fourth Edition (WAIS-IV) Vocabulary subtest (Wechsler 2008). For details regarding the specific tests, please refer to Table 2 and our previous publication (Amidi et al. 2015a).
Neuropsychological change
As in previous prospective studies of TC (Skaali et al. 2011; Wefel et al. 2014), cognitive change was investigated at an individual level using a standardized regression-based (SRB) approach (McSweeny et al. 1993; Ouimet et al. 2009) which, compared with more traditional group-level statistical methods, allows us to take into account important factors such as regression towards the mean, practice effects, and important moderators such as age and premorbid intellectual functioning (Ouimet et al. 2009). Furthermore, because cognitive decline is usually only observed in a subset of patients, analysis at the group level tends to obscure important individual differences. Using the HC group as a reference group, follow-up scores for each neuropsychological outcome in this group were regressed on their baseline score, age, and premorbid intellectual functioning. Resulting regression equations were used to predict patients’ follow-up scores. Patient’s actual follow-up scores were then subtracted from the prediction scores and divided by the standard error of estimate of the HC group. This resulted in individual z-scores indicating directionality and magnitude of change for each neuropsychological test outcome. A global composite score (GCS), reflecting overall cognitive performance across time, was then computed for each patient as the average of all z-scores derived from the SRB approach.
As in previous publications (Ouimet et al. 2009; Skaali et al. 2011), significant decline on a given neuropsychological outcome was defined as a z-score ≤ −1.64, while significant improvement was defined as a score ≥1.64. These cut-offs indicated scores that fell in the extreme 5 % at either end of the normal distribution. Overall cognitive decline was defined as significant decline on at least two different cognitive tests (Wefel et al. 2011a). However, because some cognitive domains were represented by two separate tests, only patients with decline on two tests in two separate domains were considered to meet the criterion for overall cognitive decline. Similarly, overall improvement was defined as significant improvement on at least two outcomes in two domains. Proportions of patients with overall cognitive decline or improvement, and decline or improvement on individual outcomes, were compared across treatment groups with Fisher’s exact test.
MRI-scan acquisition
MRI data was collected from TC patients only, at baseline and follow-up, using the same 1.5 T scanner (Philips Ingenia) with a 32-channel phased-array head coil. Each scan took 45 minutes and included a diffusion-weighted sequence (not included here), a T1-weighted whole brain 3D-TFE sequence (200 continuous sagittal slices; slice thickness = 1.0 mm; FOV = 256x256 mm2; TR = 7.5 ms; TE = 3.4 ms; flip angle = 8°; matrix = 256x256; voxel size = 1.0 mm isotropic; number of excitations = 2) for voxel-based morphometry (VBM) analyses (Ashburner and Friston 2000) and a fluid-attenuated inversion recovery (FLAIR) sequence (22 transverse slices; slice thickness = 5.0 mm; FOV = 352 × 352 mm; TE = 140 ms; TR = 11.000 ms) to search for brain pathology that would imply study exclusion. Care was taken to ensure minimal movement artifacts by securing participants’ heads with custom made plastic foam and by visually quality-checking each individual scan. Two scans evidenced visible motion artifacts and these participants were rescanned immediately.
Voxel-based morphometry
Longitudinal VBM analysis was performed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm8/) for the Statistical Parametric Mapping software (SPM8; The Wellcome Trust Centre for Neuroimaging, University College London), which offers a fully automated preprocessing of longitudinal structural images. The processing steps include: (1) calculation of mean images based on realignment of each subjects’ two structural scans; (2) correction for signal inhomogeneities with the mean image as reference; (3) estimations of spatial normalization parameters through segmentation of the mean image using partial volume estimation and Dartel; (4) application of the normalization parameters to the segmentations of the bias-corrected images; (5) realignment of the normalized segmentations; and (6) smoothing of realigned and normalized images with an 8 mm full width half maximum Gaussian kernel.
A flexible factorial model was used to conduct repeated-measures ANCOVA with age as a covariate. To test for group × time interaction, the design matrix was set up with contrast weights for group, time, and their interaction. Within-group longitudinal analyses for each TC group were conducted within the same model. A between-group VBM analysis (−CT vs. +CT, two-sample t-test) at baseline was also performed with age and total intracranial volume (i.e., the sum of white matter, GM, and cerebrospinal fluid) as covariates to test for baseline group differences in brain morphology.
For all of the above VBM analyses, significance thresholds for voxel-wise comparisons were set at p < 0.001, uncorrected with an extent of threshold of 100 voxels. Clusters surviving a family-wise error correction (p < 0.05) were considered significant. Similar approaches have previously been used in studies with cancer patients (Lv et al. 2014; McDonald et al. 2013).
Region of interest analysis
Region of interest (ROI) analysis was used to investigate possible associations (Pearson’s r) between GM reductions and cognitive performance. ROIs were the statistically significant clusters based on the VBM analyses described above. ROI extraction was done with the xjView toolbox (http://www.alivelearn.net/xjview), and volumetric GM changes in each ROI were calculated using scripts developed for MATLAB (R2013a, MathWorks, Inc., Natick, MA). To reduce the risk of Type I error from multiple testing, our primary ROI analyses investigated correlations between ROIs and overall cognitive performance as captured by the GCS. Secondary exploratory analyses were performed to investigate possible correlations between ROIs and individual neuropsychological outcomes.
Biological specimens
Blood samples were collected from TC patients by venipuncture (non-fasting) for the assessment of various biomarkers (see below). Approximately 10 ml of blood was drawn from each patient between 9.00 AM – 2.00 PM at both time points prior to neuropsychological testing.
Immune and endocrine markers
High-sensitivity assessments of inflammatory and endocrine markers included plasma IL-6, TNFα, and CRP, and serum cortisol. Blood samples were processed according to marker-specific procedures and pipetted into appropriate tubes at the Department of Clinical Biochemistry (AUH) and stored at −80 °C before being batch analyzed at the Department of Clinical Biochemistry, Gentofte Hospital. Quantification of IL-6 and TNFα was done by ELISA (R&D systems, Abingdon, UK); CRP with Vitros 5.1 FS analyzer (Ortho Clinical Diagnostics, Birkerød, Denmark); and cortisol with ADVIA Centaur CP system (Siemens Healthcare, Ballerup, Denmark). All assessments were done according to the manufacturers’ instructions.
For each of the above markers, change values (Δ) for each participant were calculated by subtracting follow-up levels from baseline levels. For each treatment group, correlations between these change variables and overall cognitive performance (GCS) were explored with Pearson’s r. A formal test of interaction was then performed for any marker that showed a significant correlation with the GCS. To this end, a multiple linear regression was performed including the predictors: age, group (±CT), the marker of interest, and the mean centered interaction variable (group x marker).
APOE genotyping
Whole blood was collected at baseline in order to determine individual APOE genotypes. DNA extraction and genotyping was performed using the TaqMan® Sample-to-SNP™ Kit, following the protocol provided by the manufacturer, and genotyping was performed on a LightCycler 480 Real-Time PCR system (Roche Applied Science, Mannheim, Germany). Information about APOE genotype was obtained by TaqMan-genotyping of the two single nucleotide polymorphisms (SNPs), rs7412 and rs429358, which are the two SNPs responsible for the three isoforms of APOE referred to as APOE ε2, APOE ε3 and APOE ε4. Carriers of either one or two copies of the ε4 allele were classified as APOE ε4 positive. To test for the moderating effect of APOE ε4, a general linear model was used to examine the interaction effect of chemotherapy × APOE status on overall cognitive performance across time (GCS).
Results
Participants
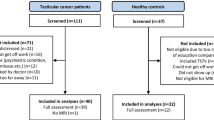
Of 94 eligible patients, a total of 66 patients (total participation rate = 70 %) with TC (seminoma and non-seminoma) at stages I-III agreed to participate at baseline. Of these, 22 patients received chemotherapy, while the remaining 42 did not (participation rates, respectively = 76 % vs. 69 %). There were no statistically significant differences between participating and non-participating patients regarding histology, cancer stage, chemotherapy ordination, occupational status, or age. Of the patients enrolled at baseline, one patient in the –CT group stopped attending his clinic visits and did not respond to our notices and was therefore excluded from the study. Of the initial 66 patients, 65 patients (98.5 %) participated at both assessment time points. The 22 + CT patients were assessed at baseline and 3 months after completing chemotherapy (follow-up), corresponding to an average of 6 months after baseline (SD = 1.6). The 43 -CT patients were tested at a similar interval (Mean = 6 months; SD = 0.8). Longitudinal MRI data was collected from all patients except one patient in the –CT group. For the HC group, 25 men were assessed at similar intervals (Mean = 6 months; SD = 1.0). There were no statistically significant differences between groups for any demographic variables other than age. As expected, patients in the –CT group were older than + CT patients and HCs (p < 0.01), and –CT patients differed from + CT patients with respect to cancer type, stage, and metastatic involvement (p < 0.01). Group characteristics are shown in Table 1.
Neuropsychological results
The frequencies of impairment and improvement for neuropsychological test outcomes, as defined in 3.2.1, are presented in Table 2 and will be explained in detail below.
TC groups compared with the HC group
Compared with the HC group, the frequency of decline was significantly higher in both TC groups for processing speed (Trail Making Test [TMT] - Part A) (p < 0.05), executive functioning (TMT -Part B) (p < 0.05), attention and working memory (Paced Auditory Serial Addition Test) (p < 0.05), and overall cognitive decline (p < 0.01). Furthermore, compared with the HC group, both TC groups showed decline on verbal learning and memory (Rey Auditory Verbal Learning Test total and retention); however, this only reached significance in the + CT group (p < 0.05). Compared with the HC group, verbal fluency declined in the –CT group (p < 0.05), and a measure of processing speed (WAIS-IV Coding) improved in the + CT group (p < 0.05).
Between-group comparisons of TC groups
There were no differences in the frequencies of domain-specific declines between + CT and -CT patients. However, the frequency of overall cognitive decline from baseline to follow-up was marginally higher (p = 0.07) in the + CT group (63.6 %) than in the –CT group (39.5 %). There were no between-groups differences in improvement on any outcomes.
Voxel-based morphometry
All results from the VBM analyses are presented in Table 3 and described in detail below.
Between-group VBM analyses
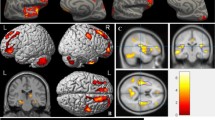
At baseline, a single significant between-group cluster was observed in which -CT patients showed greater GM volume compared with the + CT group subcortically in the right caudate. Group × time interaction analysis showed statistically significant GM reductions from baseline to follow-up in the + CT group relative to the –CT group in prefrontal regions including the right paracingulate gyrus (p = 0.02) (Fig. 1). The inverse contrast revealed no statistically significant reductions in the surgery group from baseline to follow-up relative to the chemotherapy group.
Within-group VBM analyses
In the + CT group, GM reductions were observed in six statistically significant clusters encompassing prefrontal and parieto-frontal regions. Prefrontal reductions, which overlapped with the cluster identified in the interaction analysis, included the right paracingulate gyrus extending to the right frontal pole. Reductions were also observed bilaterally in the central opercular cortex extending into the insula on the left, and to the parietal operculum cortex on the right. Finally, bilateral reductions were observed in clusters including the precentral gyrus and juxtapositional lobule cortex (Fig. 2).
Statistically significant GM reductions were also observed in the -CT group, pertaining to seven clusters encompassing the parieto-frontal, parieto-occipital, occipital, and subcortical areas. They included the right central opercular cortex extending anteriorly to the frontal operculum cortex, and subcortically into the putamen; bilateral pre- and postcentral gyrus; left lateral occipital cortex extending into the angular gyrus; and the right occipital pole. Subcortical areas also included the right parahippocampal gyrus (Fig. 3). Consistent with the interaction analysis results, there were no significant reductions in areas pertaining to the paracingulate gyrus in the -CT group.
There were generally no increases in GM from baseline to follow-up with the exception of an increase in a cluster encompassing the right Crus I and II in the cerebellum in the + CT group.
ROI analyses: associations between neuropsychological performance and grey matter reductions
For the +CT group two ROIs were extracted. One ROI was defined as the cluster in the prefrontal cortex that corresponded to the significant cluster observed in the interaction analysis. A second ROI contained all significant clusters from the within-group longitudinal analysis (including the prefrontal cluster) indicating global GM reductions. For the -CT group only one ROI was extracted containing all clusters from the within-group analysis indicating global GM reduction. The GCS showed a moderate negative correlation with prefrontal GM reductions in the + CT group (r = −0.49, p = 0.03; see Fig. 4), but not with global GM reductions (r = −0.23, p = 0.33). In the -CT group, there was no association between global GM reductions and GCS (r = −0.09, p = 0.63). Secondary ROI analyses did not reveal any significant correlations between ROIs and individual neuropsychological outcomes in any of the treatment groups.
Associations of cognitive performance and brain morphology with endocrine and inflammatory markers and APOE genotype
Overall cognitive performance, as captured by the GCS, was not associated with changes in any of the following inflammatory or endocrine biomarkers: IL-6, CRP, or cortisol.
Tumor-necrosis factor alpha
Poorer neuropsychological performance was significantly correlated with increased levels of TNFα in the +CT group (r = 0.46, p = 0.04), but not in the –CT group (r = −0.002, p = 0.99). Results of the regression analysis including age, group, ΔTNFα, and the interaction variable group × ΔTNFα revealed that the model significantly accounted for 23 % of the variability of GCS (p = 0.02). The strongest predictors were age (β = −0.35, p = 0.02) and the interaction variable (β = 0.31), although the latter did not reach statistical significance (p = 0.10). Increase in TNFα levels was not correlated with changes in GM density in either groups.
APOE ε4
APOE genotyping was successfully undertaken in 61 TC patients. Of these, 20 patients (32.8 %) carried at least one ε4 allele. This frequency was similar in both patient groups (−CT = 33.3 %, [n = 13]; +CT = 31.8 %, [n = 7], p = 0.90). The results of the age-adjusted ANCOVA revealed a significant interaction effect between APOE ε4 status and chemotherapy (F(1,46) = 4.50, p = 0.03), indicating that carriers of the ε4 allele who received chemotherapy had lower scores on the GCS compared with patients who did not carry the ε4 allele. However, there was no association between APOE ε4 status and changes in GM density.
Discussion
We previously reported data on baseline cognitive impairment in the present TC sample, indicating that an unexpected high proportion of TC patients evidenced cognitive impairment shortly after surgery but before further treatment (Amidi et al. 2015a). The present results add to this by suggesting that TC patients treated with BEP chemotherapy may exhibit a higher frequency of overall cognitive decline compared with those who did not receive BEP (63.6 % vs. 39.5 % respectively), although results did not quite reach statistical significance. We did not observe differences between TC groups on any of the domain-specific outcomes, suggesting that the detrimental effects of BEP on cognition may be more general. Our results support the prospective findings of Wefel et al. (2014) in which 52 % of TC patients who had received 2–3 cycles of chemotherapy evidenced overall cognitive decline at 12 months follow-up compared with a surgery-only group (0 %), but with no evidence of domain-specific declines. However, due to differences in type of TC and heterogeneity in cytotoxic regimens, their findings are not directly comparable to those of the present study. In contrast, Skaali et al. (Skaali et al. 2011) did not observe significant differences in neuropsychological change scores, or in the proportion of men with a significant decline between TC patients receiving multiple-cycles of BEP and those who did not. As we have argued, however, both previous studies used surgery-only TC patients as the control group, which may have confounded their results, as cognitive impairment may be evident in surgery-only patients as well (Amidi et al. 2015a; Wefel et al. 2011b). This was further corroborated in the present study with a significant proportion of the –CT group also showing cognitive decline over time. Domain-specific cognitive declines were observed in both TC groups relative to controls in the domains of attention and working memory, processing speed, verbal learning and memory, and executive functions. In contrast, decline in verbal fluency was only observed in the –CT group, which could possibly be due to statistical power issues related to group size differences. Domain-specific cognitive improvement was observed in the + CT group on a single measure of processing speed, while a second measure of processing speed indicated decline. Given the overall pattern of decline observed in both TC groups, it seems more likely that the observed improvement is due to random variation.
In support of the neuropsychological findings, the VBM analyses revealed both chemotherapy-specific as well as general brain GM reductions. The main VBM analysis revealed a group-by-time interaction with prefrontal GM reductions observed only in the + CT group. This provides the first evidence of prefrontal morphometric changes specifically related to BEP chemotherapy. The prefrontal cortex plays an important role in many higher cognitive functions such as attention, working memory, language, and executive functions (Szczepanski and Knight 2014) with greater volumes associated with better performance (Yuan and Raz 2014). This was further supported by the ROI analysis revealing that poorer overall cognitive performance was related to greater GM reduction in this region.
Additionally, widespread GM reductions were observed in both TC groups in anatomically overlapping regions related to parieto-frontal areas including the right central opercular cortex and bilaterally in the precentral gyrus. However, these global GM reductions were not associated with overall cognitive performance in either TC group. Surprisingly, reductions in right occipital and subcortical areas were specific to the –CT group. There is evidence linking higher testosterone levels with higher GM volumes in occipital cortex (Lentini et al. 2012) and an explanation of the occipital GM reductions in the –CT group could thus be related to decreased testosterone levels following orchiectomy (Bandak et al. 2011). Finally, an unexpected increase in GM in the cerebellum of the CT+ group was also observed. While an interpretation of this finding is not straightforward, an increase in cerebellum GM density related to chemotherapy could theoretically be the result of an indirect treatment of subclinical paraneoplastic limbic encephalitis (PLE), which has been associated with cerebellar atrophy and previously observed in men with testicular germ cell tumors (Gultekin et al. 2000). Interestingly, treatment with cisplatin-based chemotherapy has been shown to be beneficial for the treatment of PLE (Keime-Guibert et al. 1999).
To our knowledge, this is the first study to show morphological brain changes in TC patients undergoing chemotherapy, and the first to show such changes in the absence of cytotoxic treatment in surgery-treated TC patients. Although cognitive impairment has been observed in TC and other cancer populations without the use of chemotherapy (Amidi et al. 2015a; Lange et al. 2014; Wefel et al. 2011b), the underlying mechanisms remain unclear. In TC, endocrine disruption following orchiectomy may be of particular relevance and should be included in future studies, given the well-known association between testosterone, the brain, and cognitive functions (Beauchet 2006; Cherrier 2009; Holland et al. 2011). Stress-reactivity may also play an important role in explaining pre-chemotherapy cognitive dysfunction (Andreotti et al. 2014; Hermelink et al. 2015), and in a previous publication of the same TC sample, we reported negative associations between impaired cognitive functions and serum cortisol levels in the TC patients prior to systemic treatment (Amidi et al. 2015a). In the present prospective study, however, we did not detect an association between changes in cortisol levels and cognitive performance over time. This may suggest that the association between cognitive performance and cortisol is more pronounced around the time of diagnosis and/or surgery due to related stress responses (Hermelink et al. 2015). Regarding the included inflammatory markers, we did not detect any associations between cognitive performance and IL-6 or CRP. However, our results did reveal that increases in TNFα in the + CT group were associated with poorer cognitive performance. This is consistent with evidence from breast cancer populations (Ganz et al. 2013; Kesler et al. 2013). In a longitudinal study by Ganz et al. (2013) observed levels of sTNF-RII in breast cancer patients were significantly increased post-treatment. These levels gradually normalized over a 12-month period, and interestingly, changes in self-reported cognitive impairment followed the same normalization pattern of sTNF-RII. These lines of evidence seem to suggest that chemotherapy-related cognitive alterations may partly be mediated by changes in proinflammatory cytokines (Cheung et al. 2013). However, our results did not indicate an association between GM changes and TNFα levels, and thus more work is currently needed to clarify the potential mediating role of immune markers in treatment-induced brain changes in the context of testicular cancer.
Our exploratory analyses also revealed that TC patients undergoing chemotherapy who are heterozygous or homozygous for the APOE ε4 allele generally performed more poorly on cognitive tests. Early work by Ahles et al. (Ahles et al. 2003) in long-term breast cancer and lymphoma survivors who had undergone chemotherapy also showed preliminary evidence for the important role of APOE ε4. Survivors carrying at least one APOE ε4 allele scored more poorly on cognitive tests (i.e., visual memory and spatial ability) than those without the ε4 allele. Our findings expand on this research by showing that TC patients carrying the APOE ε4 allele who underwent BEP chemotherapy were more likely to show cognitive decline over time. Similarly, APOE ε4 has been found to be a risk factor for cognitive decline in both healthy and non-cancer clinical populations indicating that the allele may play an important role in brain physiology by increasing the chance of age-related pathological (such as Alzheimer’s disease), and non-pathological changes. Given that APOE ε4 is important for normal lipid homeostasis in the brain (Bu 2009; Holtzman et al. 2012), it has been suggested that the ε4 allele makes the brain less resilient to neurodegenerative processes (Ahles et al. 2003). It should be noted, however, that changes in brain morphology were not statistically related to APOE ε4 status in our TC population.
Overall, our most robust finding is that TC patients, irrespective of cytostatic treatment, evidence domain-specific, as well as overall cognitive decline compared with HCs. In support of this, we also show widespread reductions in cerebral grey matter in multiple brain regions of both TC treatment groups, although these were not statistically associated with the cognitive outcomes. Furthermore, our study adds to the literature by confirming previous findings indicating that TC patients treated with BEP have a higher frequency of overall cognitive decline (Wefel et al. 2014). Importantly, we add to the literature by showing a differential GM reduction pattern specifically in prefrontal cortical regions, which was associated with overall cognitive performance in chemotherapy-treated patients. Exploratory analyses revealed that poorer overall cognitive performance was associated with an increase in TNFα levels in the + CT group, and an interaction effect was found between the APOE ε4 genotype and chemotherapy on overall cognitive performance with ε4 carriers performing significantly worse. Future studies are clearly needed to replicate these findings and to further investigate biomarkers of CRCI that may contribute to cognitive and neurological deteriorations in TC patients.
Strengths of the present study include a prospective design with the longitudinal assessment of neuropsychological functioning, brain morphology, and relevant biomarkers. Other strengths include a low attrition rate (1.5 %) and the inclusion of a HC group. However, there are limitations that will need to be addressed in future studies. First, the HC group did not undergo MRI, which prevented us from controlling for possible morphometric changes due to measurement errors and aging. However, given the young age of the patients, and the relatively short time span between assessments, aging effects are expected to be minimal. Second, due to intrinsic differences between the –CT and +CT groups on medical variables such as cancer type, stage, and metastatic involvement, it cannot be ruled out that these variables are confounders of the chemotherapy-specific results. For example, the higher frequency of cognitive decline in the +CT group could statistically be explained by advanced cancer stage. Third, due to unequal group sizes, more statistical power was available to detect within-group brain changes in the –CT group, which could possibly explain the observed reductions in the occipital and subcortical regions in this group. Fourth, because endocrine and inflammatory markers as well as genotypes were only assessed in the two patient groups, we were unable to compare these with the healthy control group. Finally, to test for associations between different markers and cognitive performance, we performed multiple correlation tests. However, due to the dependent and exploratory nature of these analyses, correction for multiple testing was not undertaken and, thus, these results should be interpreted with caution.
References
Ahles, T. A., & Saykin, A. J. (2007). Candidate mechanisms for chemotherapy-induced cognitive changes. Nature Reviews Cancer, 7(3), 192–201. doi:10.1038/nrc2073.
Ahles, T. A., Saykin, A. J., Noll, W. W., Furstenberg, C. T., Guerin, S., Cole, B., & Mott, L. A. (2003). The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psycho-Oncology, 12(6), 612–9. doi:10.1002/pon.742.
Ahles, T. A., Li, Y., McDonald, B. C., Schwartz, G. N., Kaufman, P. A., Tsongalis, G. J., et al. (2014). Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: the impact of APOE and smoking. Psycho-Oncology, 23(12), 1382–90. doi:10.1002/pon.3545.
Amidi, A., Wu, L. M., Agerbaek, M., Larsen, P. L., Pedersen, A. D., Mehlsen, M., et al. (2015a). Cognitive impairment and potential biological and psychological correlates of neuropsychological performance in recently orchiectomized testicular cancer patients. Psycho-Oncology, 24(9), 1174–1180. doi:10.1002/pon.3804.
Amidi, A., Wu, L. M., Pedersen, A. D., Mehlsen, M., Pedersen, C. G., Rossen, P., et al. (2015b). Cognitive impairment in testicular cancer survivors 2 to 7 years after treatment. Supportive Care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer, 23(10), 2973–9. doi:10.1007/s00520-015-2663-3.
Andreotti, C., Root, J. C., Ahles, T. A., McEwen, B. S., & Compas, B. E. (2014). Cancer, coping, and cognition: a model for the role of stress reactivity in cancer-related cognitive decline. Psycho-Oncology. doi:10.1002/pon.3683.
Andres, A. L., Gong, X., Di, K., & Bota, D. A. (2014). Low-doses of cisplatin injure hippocampal synapses: a mechanism for “chemo” brain? Experimental Neurology, 255, 137–44. doi:10.1016/j.expneurol.2014.02.020.
Ashburner, J., & Friston, K. J. (2000). Voxel-based morphometry--the methods. NeuroImage, 11(6 Pt 1), 805–21. doi:10.1006/nimg.2000.0582.
Bandak, M., Aksglaede, L., Juul, A., Rørth, M., & Daugaard, G. (2011). The pituitary-Leydig cell axis before and after orchiectomy in patients with stage I testicular cancer. European Journal of Cancer, 47(17), 2585–2591. doi:10.1016/j.ejca.2011.05.026.
Beauchet, O. (2006). Testosterone and cognitive function: current clinical evidence of a relationship. European Journal of Endocrinology / European Federation of Endocrine Societies, 155(6), 773–81. doi:10.1530/eje.1.02306.
Benton, A. L., & Hamsher, K. D. S. (1989). Multilingual aphasia examination. Iowa City: AJA Associates.
Bu, G. (2009). Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nature Reviews Neuroscience, 10(5), 333–44. doi:10.1038/nrn2620.
Buller, K. M. (2001). Role of circumventricular organs in pro-inflammatory cytokine-induced activation of the hypothalamic-pituitary-adrenal axis. Clinical and Experimental Pharmacology & Physiology, 28(7), 581–9. http://www.ncbi.nlm.nih.gov/pubmed/11458886 . Accessed 5 June 2015.
Carozzi, V. A., Canta, A., Chiorazzi, A., & Cavaletti, G. (2014). Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neuroscience Letters. doi:10.1016/j.neulet.2014.10.014.
Cherrier, M. M. (2009). Testosterone effects on cognition in health and disease. Frontiers of Hormone Research, 37(0301–3073 (Print)), 150–62. doi:10.1159/000176051.
Cheung, Y. T., Lim, S. R., Ho, H. K., & Chan, A. (2013). Cytokines as mediators of chemotherapy-associated cognitive changes: current evidence, limitations and directions for future research. PLoS ONE, 8(12), e81234. doi:10.1371/journal.pone.0081234.
Conroy, S. K., McDonald, B. C., Smith, D. J., Moser, L. R., West, J. D., Kamendulis, L. M., et al. (2013). Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Research and Treatment, 137(1573–7217 (Electronic)), 493–502. doi:10.1007/s10549-012-2385-x.
Dahl, O., & Brydøy, M. (2012). Testicular germ cell tumours - still many challenges. Acta Oncologica (Stockholm, Sweden), 51(2), 147–50. doi:10.3109/0284186X.2011.653441.
Dietrich, J., Han, R., Yang, Y., Mayer-Pröschel, M., & Noble, M. (2006). CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. Journal of Biology, 5(7), 22. doi:10.1186/jbiol50.
Eiseman, J. L., Beumer, J. H., Rigatti, L. H., Strychor, S., Meyers, K., Dienel, S., & Horn, C. C. (2014). Plasma pharmacokinetics and tissue and brain distribution of cisplatin in musk shrews. Cancer Chemotherapy and Pharmacology. doi:10.1007/s00280-014-2623-5.
Ganz, P. A., Bower, J. E., Kwan, L., Castellon, S. A., Silverman, D. H. S., Geist, C., et al. (2013). Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain, Behavior, and Immunity, 30, S99–108. doi:10.1016/j.bbi.2012.07.015.
Gietema, J., Meinardi, M., Messerschmidt, J., Gelevert, T., Alt, F., Uges, D., & Th Sleijfer, D. (2000). Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. The Lancet, 355(9209), 1075–1076. doi:10.1016/S0140-6736(00)02044-4.
Gong, X., Schwartz, P. H., Linskey, M. E., & Bota, D. A. (2011). Neural stem/progenitors and glioma stem-like cells have differential sensitivity to chemotherapy. Neurology, 76(13), 1126–34. doi:10.1212/WNL.0b013e318212a89f.
Gregg, R. W., Molepo, J. M., Monpetit, V. J., Mikael, N. Z., Redmond, D., Gadia, M., & Stewart, D. J. (1992). Cisplatin neurotoxicity: the relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 10(5), 795–803. http://www.ncbi.nlm.nih.gov/pubmed/1569451 . Accessed 25 February 2015.
Groll, R. J., Warde, P., & Jewett, M. A. S. (2007). A comprehensive systematic review of testicular germ cell tumor surveillance. Critical Reviews in Oncology/Hematology, 64(3), 182–97. doi:10.1016/j.critrevonc.2007.04.014.
Gultekin, S. H., Rosenfeld, M. R., Voltz, R., Eichen, J., Posner, J. B., & Dalmau, J. (2000). Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain: a Journal of Neurology, 123(Pt 7), 1481–94. http://www.ncbi.nlm.nih.gov/pubmed/10869059 . Accessed 21 February 2016.
Heaton, R. K., Chelune, G. J., Talley, J. L., Kay, G. G., & Curtiss, G. (1993). Wisconsin card sorting test manual revised and expanded. Odessa: Psychological Assessment Resources.
Hermelink, K., Voigt, V., Kaste, J., Neufeld, F., Wuerstlein, R., Bühner, M., et al. (2015). Elucidating pretreatment cognitive impairment in breast cancer patients: the impact of cancer-related post-traumatic stress. Journal of the National Cancer Institute, 107(7). doi:10.1093/jnci/djv099
Holland, J., Bandelow, S., & Hogervorst, E. (2011). Testosterone levels and cognition in elderly men: a review. Maturitas, 69(1873–4111 (Electronic)), 322–337.
Holtzman, D. M., Herz, J., & Bu, G. (2012). Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine, 2(3), a006312. doi:10.1101/cshperspect.a006312.
Inagaki, M., Yoshikawa, E., Matsuoka, Y., Sugawara, Y., Nakano, T., Akechi, T., et al. (2007). Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer, 109(0008-543X (Print)), 146–156.
Janelsins, M. C., Kesler, S. R., Ahles, T. A., & Morrow, G. R. (2014). Prevalence, mechanisms, and management of cancer-related cognitive impairment. International Review of Psychiatry (Abingdon, England), 26(1), 102–13. doi:10.3109/09540261.2013.864260.
Keime-Guibert, F., Graus, F., Broët, P., Reñé, R., Molinuevo, J. L., Ascaso, C., & Delattre, J. Y. (1999). Clinical outcome of patients with anti-Hu-associated encephalomyelitis after treatment of the tumor. Neurology, 53(8), 1719–23. http://www.ncbi.nlm.nih.gov/pubmed/10563618 . Accessed 21 February 2016.
Kesler, S., Janelsins, M., Koovakkattu, D., Palesh, O., Mustian, K., Morrow, G., & Dhabhar, F. S. (2013). Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain, Behavior, and Immunity, 30(Suppl(1090–2139 (Electronic)), S109–16. doi:10.1016/j.bbi.2012.05.017.
Lambert, J. C., Ibrahim-Verbaas, C. A., Harold, D., Naj, A. C., Sims, R., Bellenguez, C., et al. (2013). Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nature Genetics, 45(12), 1452–8. doi:10.1038/ng.2802.
Lange, M., Giffard, B., Noal, S., Rigal, O., Kurtz, J.-E., Heutte, N., et al. (2014). Baseline cognitive functions among elderly patients with localised breast cancer. European Journal of Cancer (Oxford, England: 1990), 50(13), 2181–9. doi:10.1016/j.ejca.2014.05.026.
Lee, B. K., Glass, T. A., McAtee, M. J., Wand, G. S., Bandeen-Roche, K., Bolla, K. I., & Schwartz, B. S. (2007). Associations of salivary cortisol with cognitive function in the Baltimore memory study. Archives of General Psychiatry, 64(7), 810. doi:10.1001/archpsyc.64.7.810.
Lentini, E., Kasahara, M., Arver, S., & Savic, I. (2012). Sex differences in the human brain and the impact of sex chromosomes and sex hormones. Cerebral Cortex, 23(10), 2322–2336. doi:10.1093/cercor/bhs222.
Lepage, C., Smith, A. M., Moreau, J., Barlow-Krelina, E., Wallis, N., Collins, B., et al. (2014). A prospective study of grey matter and cognitive function alterations in chemotherapy-treated breast cancer patients. Springerplus, 3, 444. doi:10.1186/2193-1801-3-444.
Li, D.-W., Sun, J.-Y., Wang, K., Zhang, S., Hou, Y.-J., Yang, M.-F., et al. (2015). Attenuation of cisplatin-induced neurotoxicity by cyanidin, a natural inhibitor of ROS-mediated apoptosis in PC12 cells. Cellular and Molecular Neurobiology. doi:10.1007/s10571-015-0194-6.
Lv, X.-F., Zheng, X.-L., Zhang, W.-D., Liu, L.-Z., Zhang, Y.-M., Chen, M.-Y., & Li, L. (2014). Radiation-induced changes in normal-appearing gray matter in patients with nasopharyngeal carcinoma: a magnetic resonance imaging voxel-based morphometry study. Neuroradiology, 56(5), 423–30. doi:10.1007/s00234-014-1338-y.
Maier, S. F., & Watkins, L. R. (2003). Immune-to-central nervous system communication and its role in modulating pain and cognition: Implications for cancer and cancer treatment. Brain, Behavior, and Immunity, 17(Suppl 1), S125–31.
McDonald, B. C., Conroy, S. K., Ahles, T. A., West, J. D., & Saykin, A. J. (2010). Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Research and Treatment, 123(3), 819–28. doi:10.1007/s10549-010-1088-4.
McDonald, B. C., Conroy, S. K., Smith, D. J., West, J. D., & Saykin, A. J. (2013). Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain, Behavior, and Immunity, 30(Suppl(1090–2139 (Electronic)), S117–25. doi:10.1016/j.bbi.2012.05.007.
McSweeny, A. J., Naugle, R. I., Chelune, G. J., & Lüders, H. (1993). T scores for change”: an illustration of a regression approach to depicting change in clinical neuropsychology. Clinical Neuropsychologist, 7(3), 300–312. doi:10.1080/13854049308401901.
Osburg, B., Peiser, C., Dömling, D., Schomburg, L., Ko, Y. T., Voigt, K., & Bickel, U. (2002). Effect of endotoxin on expression of TNF receptors and transport of TNF-alpha at the blood–brain barrier of the rat. American Journal of Physiology Endocrinology and Metabolism, 283(5), E899–908. doi:10.1152/ajpendo.00436.2001.
Ouimet, L. A., Stewart, A., Collins, B., Schindler, D., & Bielajew, C. (2009). Measuring neuropsychological change following breast cancer treatment: an analysis of statistical models. Journal of Clinical and Experimental Neuropsychology, 31(1), 73–89. doi:10.1080/13803390801992725.
Pedersen, A. D., Rossen, P., Mehlsen, M. Y., Pedersen, C. G., Zachariae, R., & von der Maase, H. (2009). Long-term cognitive function following chemotherapy in patients with testicular cancer. Journal of the International Neuropsychological Society: JINS, 15(2), 296–301. doi:10.1017/S1355617709090316.
Reinvang, I., Espeseth, T., & Westlye, L. T. (2013). APOE-related biomarker profiles in non-pathological aging and early phases of Alzheimer’s disease. Neuroscience and Biobehavioral Reviews, 37(8), 1322–35. doi:10.1016/j.neubiorev.2013.05.006.
Reitan, R. M. (1958). Validity of the trail making test as an indicator of organic brain damage. Perceptual and Motor Skills, 8, 271–276.
Roberts, R. O., Geda, Y. E., Knopman, D. S., Boeve, B. F., Christianson, T. J. H., Pankratz, V. S., et al. (2009). Association of C-reactive protein with mild cognitive impairment. Alzheimer’s & Dementia: the Journal of the Alzheimer’s Association, 5(5), 398–405. doi:10.1016/j.jalz.2009.01.025.
Rzeski, W., Pruskil, S., Macke, A., Felderhoff-Mueser, U., Reiher, A. K., Hoerster, F., et al. (2004). Anticancer agents are potent neurotoxins in vitro and in vivo. Annals of Neurology, 56(3), 351–60. doi:10.1002/ana.20185.
Schagen, S. B., Boogerd, W., Muller, M. J., Huinink, W. T. B., Moonen, L., Meinhardt, W., & Van Dam, F. S. A. M. (2008). Cognitive complaints and cognitive impairment following BEP chemotherapy in patients with testicular cancer. Acta Oncologica (Stockholm, Sweden), 47(1), 63–70. doi:10.1080/02841860701518058.
Schmidt, M. (1996). Auditory and verbal learning test. A handbook. Los Angeles: Western Psychological Services.
Sheldon, J., Riches, P., Gooding, R., Soni, N., & Hobbs, J. R. (1993). C-reactive protein and its cytokine mediators in intensive-care patients. Clinical Chemistry, 39(1), 147–150.
Simó, M., Root, J. C., Vaquero, L., Ripollés, P., Jové, J., Ahles, T., et al. (2015). Cognitive and brain structural changes in a lung cancer population. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer, 10(1), 38–45. doi:10.1097/JTO.0000000000000345.
Skaali, T., Fosså, S. D., Andersson, S., Cvancarova, M., Langberg, C. W., Lehne, G., & Dahl, A. A. (2011). A prospective study of neuropsychological functioning in testicular cancer patients. Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO, 22(5), 1062–70. doi:10.1093/annonc/mdq553.
Stouten-Kemperman, M. M., de Ruiter, M. B., Caan, M. W. A., Boogerd, W., Kerst, M. J., Reneman, L., & Schagen, S. B. (2015). Lower cognitive performance and white matter changes in testicular cancer survivors 10 years after chemotherapy. Human Brain Mapping. doi:10.1002/hbm.22942.
Szczepanski, S. M., & Knight, R. T. (2014). Insights into human behavior from lesions to the prefrontal cortex. Neuron, 83(5), 1002–18. doi:10.1016/j.neuron.2014.08.011.
Touitou, Y., Bogdan, A., Levi, F., Benavibes, M., & Auzeby, A. (1996). Disruption of the circadian patterns of serum cortisol in breast and ovarian cancer patients: relationships with tumour marker antigens. British Journal of Cancer, 74(8), 1248–52. Nature Publishing Group. /pmc/articles/PMC2075940/?report = abstract. Accessed 2 September 2014.
Trabert, B., Chen, J., Devesa, S. S., Bray, F., & McGlynn, K. A. (2014). International patterns and trends in testicular cancer incidence, overall and by histologic subtype, 1973–2007. Andrology. doi:10.1111/andr.293.
Wechsler, D. (2008). Wechsler adult intelligence scale-fourth edition. San Antonio: Pearson.
Wefel, J. S., Vardy, J., Ahles, T., & Schagen, S. B. (2011a). International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. The Lancet Oncology, 12(7), 703–8. doi:10.1016/S1470-2045(10)70294-1.
Wefel, J. S., Vidrine, D. J., Veramonti, T. L., Meyers, C. A., Marani, S. K., Hoekstra, H. J., et al. (2011b). Cognitive impairment in men with testicular cancer prior to adjuvant therapy. Cancer, 117(1), 190–6. doi:10.1002/cncr.25298.
Wefel, J. S., Vidrine, D. J., Marani, S. K., Swartz, R. J., Veramonti, T. L., Meyers, C. A., et al. (2014). A prospective study of cognitive function in men with non-seminomatous germ cell tumors. Psycho-Oncology, 23(6), 626–33. doi:10.1002/pon.3453.
Wiens, A. N., Fuller, K. H., & Crossen, J. R. (1997). Paced auditory serial addition test: adult norms and moderator variables. Journal of Clinical and Experimental Neuropsychology, 19(4), 473–83. doi:10.1080/01688639708403737.
Wilson, C. J., Finch, C. E., & Cohen, H. J. (2002). Cytokines and cognition-the case for a head-to-toe inflammatory paradigm. Journal of the American Geriatrics Society, 50(12), 2041–2056. doi:10.1046/j.1532-5415.2002.50619.x.
Yuan, P., & Raz, N. (2014). Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neuroscience and Biobehavioral Reviews, 42, 180–92. doi:10.1016/j.neubiorev.2014.02.005.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was funded by grants from the Danish Cancer Society (R17-A698) and by Saværksejer Jeppe Juhls og Hustru Ovita Juhls Mindelegat. Lisa M. Wu’s was supported by the National Cancer Institute of the National Institutes of Health under Award Number #7K07CA184145-02. Anders D. Børglum and Ditte Demontis were financially supported by The Lundbeck Foundation.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study and performed procedures were in accordance with the ethical standards of The National Committee on Health Research Ethics and with the 1964 Helsinki declaration and its later amendments.
Rights and permissions
About this article
Cite this article
Amidi, A., Agerbæk, M., Wu, L.M. et al. Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging and Behavior 11, 769–783 (2017). https://doi.org/10.1007/s11682-016-9552-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-016-9552-3