Abstract
Growing evidence suggests that sports-related concussions (SRC) may lead to acute changes in intrinsic functional connectivity, although most studies to date have been cross-sectional in nature with relatively modest sample sizes. We longitudinally assessed changes in local and global resting state functional connectivity using metrics that do not require a priori seed or network selection (regional homogeneity; ReHo and global brain connectivity; GBC, respectively). A large sample of collegiate athletes (N = 43) was assessed approximately one day (1.74 days post-injury, N = 34), one week (8.44 days, N = 34), and one month post-concussion (32.47 days, N = 30). Healthy contact sport-athletes served as controls (N = 51). Concussed athletes showed improvement in mood symptoms at each time point (p’s < 0.05), but had significantly higher mood scores than healthy athletes at every time point (p’s < 0.05). In contrast, self-reported symptoms and cognitive deficits improved over time following concussion (p’s < 0.001), returning to healthy levels by one week post-concussion. ReHo in sensorimotor, visual, and temporal cortices increased over time post-concussion, and was greatest at one month post-injury. Conversely, ReHo in the frontal cortex decreased over time following SRC, with the greatest decrease evident at one month post-concussion. Differences in ReHo relative to healthy athletes were primarily observed at one month post-concussion rather than the more acute time points. Contrary to our hypothesis, no significant cross-sectional or longitudinal differences in GBC were observed. These results are suggestive of a delayed onset of local connectivity changes following SRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The analysis of slow oscillations in the blood-oxygen-level dependent (BOLD) signal via resting state functional magnetic resonance imaging (fcMRI) has emerged as one method to assess potential changes in intrinsic functional brain architecture following mild traumatic brain injury (mTBI) and sports-related concussion (SRC). To date, most studies have focused on a priori selection of seed-regions or intrinsic connectivity networks, which may not accurately capture the known heterogeneity of mTBI. For example, mTBI likely affects connectivity on a global level due to diffuse white matter injuries (V. E. Johnson et al. 2013) and microvasculature damage (Park et al. 2009). In addition, previous studies of SRC, specifically, have been primarily cross-sectional in nature and/or have included relatively modest sample sizes. Thus, well-powered studies that prospectively track brain-wide changes in fcMRI across the time frame of typical neurobehavioral symptom recovery (one week to one month) are needed to pin down the neurophysiological consequences of SRC.
Previous fcMRI studies on SRC have documented abnormalities in a variety of brain regions at various times following injury (for review, see (Mayer et al. 2015a)). For example, increased connectivity between bilateral hippocampus, as well as between the precuneus and ventromedial prefrontal cortex (PFC), has been reported 3–6 days following SRC (Militana et al. 2015). In addition, reduced interhemispheric connectivity has been observed in the primary visual cortex, dorsolateral PFC, and hippocampus 10-days post-injury in asymptomatic athletes compared to non-injured athletes at rest and following a physical stress challenge (Slobounov et al. 2011). The same group also reported increased connectivity in the medial prefrontal cortex and reduced connectivity in the posterior cingulate at 10-days post-injury in concussed athletes relative to controls (B. Johnson et al. 2012). Increased functional connectivity was reported at one month following SRC in the attentional and executive function networks relative to controls, with both increases and decreases in default mode network (DMN) connectivity (Borich et al. 2015). Even up to an average of over 3 months post-concussion asymptomatic athletes had increased connectivity of the anterior cingulate cortex (ACC) and dorsolateral PFC to several regions relative to healthy athletes (Czerniak et al. 2015). Finally, one recent longitudinal study observed a reduction of functional connectivity within the DMN from one day to one week post-injury, with evidence of partial return toward healthy control levels by one month post-concussion in a sample of eight collegiate football players (D. C. Zhu et al. 2015).
An alternative approach is to use fcMRI metrics that do not require a priori selection of seed-regions or networks to assess the recovery from SRC. One such method involves characterizing the connectivity of single voxels with all other voxels in the brain, providing a voxel-wise measure of global brain connectivity (GBC). Assessing GBC is of particular interest for mTBI patients due to diffuse axonal injury that occurs following traumatic brain injuries of all severity (V. E. Johnson et al. 2013). This method has previously been used to characterize differences in disorders exemplified by functional disconnection, such as in autism-spectrum disorders (Gotts et al. 2012). A second fcMRI analysis method that does not require a priori seed or network selection is regional homogeneity (ReHo), which measures the similarity of the BOLD signal in a single voxel with its nearest neighboring voxels (Zang et al. 2004). In contrast to GBC, ReHo is a measure of local, short-range connectivity, and can potentially be used to identify discrete, localized regions with mTBI-induced abnormalities (e.g., lesions or contusions). ReHo abnormalities have been observed in populations typified by functional disconnection, including mild cognitive impairment and Alzheimer’s disease (Z. Zhang et al. 2012). In addition, a recent study found decreased ReHo in the left insula, pre- and postcentral gyri, and supramarginal gyrus in mTBI patients on average 3 days post-injury relative to healthy controls (Zhan et al. 2015). Thus, GBC and ReHo can provide complimentary information regarding the pathophysiological effects of SRC.
The current study examined global and local connectivity in a sample of collegiate athletes at approximately one day, one week, and one month following SRC. A sample of healthy contact-sport collegiate athletes served as controls to account for potential confounds associated with collegiate athletics such as the potential effects of previous head hit exposure, including previous concussions and sub-concussive head injuries, on fcMRI (Abbas et al. 2015; B. Johnson et al. 2014). We hypothesized that both local (ReHo) and global connectivity (GBC) would be reduced acutely (i.e., days to week post-concussion), and would show partial recovery toward healthy athletes levels at the sub-acute phase (i.e., one month post-concussion) similar to measures of cerebral perfusion (Meier et al. 2015b). A secondary hypothesis was that SRC would have a greater effect on GBC than ReHo, due to the diffuse nature of mTBI.
Methods
Participants
NCAA Division I student-athletes (N = 94) were referred by sports-medicine professionals and provided written informed consent for this study, which was approved by an institutional review board. All participants self-reported no past or current mood disorders, anxiety disorders, alcohol abuse, or substance abuse. Forty-three concussed athletes participated in at least one of three visits that occurred approximately one day (T1: 1.74+/−0.93 days post-concussion; N = 34), one week (T2: 8.44+/−2.15 days post; N = 34), and one month (T3: 32.47+/−4.68 days post-concussion; N = 30) following SRC. Nineteen concussed athletes participated in all three visits and 36 participated in at least 2 visits. Physicians trained in sports medicine diagnosed concussions independently of the study at the time of injury following recommend guidelines (McCrory et al. 2013) based on a clinical exam assessing symptoms, a cranial nerve check, manual muscle testing for strength deficits, the Rhomberg’s test for balance deficits, on-field cognitive testing developed by the UPMC Center for Sports Medicine, and the King-Devick test. Fifty-one healthy, collegiate contact-sport athletes (HA) served as a control group. Behavioral information from a subset of athletes included in this study has been reported elsewhere (Meier et al. 2015b; Singh et al. 2015; Meier et al. 2015c). Demographic information for all participants and the final number of participants included in each analysis can be found in Table 1.
Behavioral data
Structured interviews for the Hamilton Depression (HAM-D) and Anxiety (HAM-A) Rating Scales served as the primary behavioral measures. The Automated Neuropsychological Assessment Metrics 4 Sports Medicine Battery (ANAM) was used to collect secondary measures of interest, including computerized assessments of self-reported concussion symptoms, mood scales, and cognitive tests (Cernich et al. 2007). Overall symptom frequency, overall symptom severity, concussion symptom inventory frequency, and concussion symptom inventory severity were strongly correlated (mean r = 0.93, range 0.90–0.98) and thus only overall symptom frequency was used. ANAM mood scales were not assessed because the primary behavioral measures were structured interviews for mood symptoms. Throughput, which is the number of correct responses per minute, was used to measure performance on each cognitive task included in the ANAM (Thorne 2006). Scores on the cognitive battery were converted to standard scores by using a gender and age matched sample of collegiate athletes available in as part of the ANAM suite. Reference group scores for the go-no-go and the mathematical processing tests were not available and these measures were not assessed.
All other cognitive measures were positively correlated (average r = 0.50, range 0.16–0.84) and averaged to create a single cognitive composite measure in order to limit the number of comparisons performed. The ANAM Validity Indicator Report was used to flag individual tests with scores indicating misunderstanding of subtest instructions or cognitive batteries with questionable effort (Roebuck-Spencer et al. 2013). These scores were excluding from analyses. Similarly, athletes reporting zeroes on all self-reported symptoms and mood scale questions (including positive measures) were also excluded from analyses. Not every participant completed ANAM testing (final n can be found in Table 1). An independently determined, real-world measure of outcome was defined as the number of days that athletes were withheld from competition (i.e., return-to-play decisions) by clinicians trained in sports medicine following recommended guidelines (McCrory et al. 2013).
Imaging parameters and processing
MRI was performed using a General Electric Healthcare Discovery MR750 3-Tesla whole body MRI scanner and brain-dedicated receive-only 32-element coil array optimized for parallel imaging (Nova Medical, Inc.). A six minute gradient-echo echo-planar image (EPI) sequence was used to collect 180 volumes of fcMRI data with the following parameters: FOV = 240 mm, acquisition matrix = 96 × 96, slice thickness = 3 mm, inter-slice spacing = 0.2 mm, 37 axial slices, TR/TE = 2 s/30 ms, flip angle = 90 degrees, sampling bandwidth = 250 kHz, acceleration factor R = 2, acquired voxel size = 2.5 × 2.5 × 3.2 mm interpolated to 1.875 × 1.875 × 3.2 mm. Participants were instructed to fixate on a cross and think of nothing in particular during the resting state scan. For anatomical reference, T1-weighted structural images were collected using a parallelized magnetization-prepared rapid gradient-echo sequence with sensitivity encoding and the following parameters: FOV = 240 mm, 130 axial slices, slice thickness = 1.1 mm, image matrix = 256 × 256, TR/TE = 5/1.948 ms, acceleration factor R = 2, flip angle = 8 degrees, TI = 725 ms, sampling bandwidth = 31.25 kHz, voxel size =0.9375 × 0.9375 × 1.1 mm.
Image processing was performed using a combination of neuroimaging analysis software. Each participant’s structural image was skull stripped and transformed to standard space using the Advanced Normalization Tools (ANTS) by applying a 12-parameter affine transformation followed by a non-linear warp (Avants et al. 2011). Tissue segmentation was subsequently performed to create an eroded white matter mask. Finally, a bilateral lateral ventricle mask was extracted in native space following automated segmentation via Freesurfer v5.1 (Fischl et al. 2002), transformed to standard space, and eroded by 1 voxel along the x, y, and z directions to ensure exclusion of non-CSF voxels.
Processing and analyses of EPI volumes were conducted using AFNI (Cox 1996). The first four EPI volumes were removed and any anomalous time-series data were replaced using AFNI’s despike program. Images were slice-time corrected, and volumes were registered to the first volume to account for head motion using a 6-degree of freedom transformation. The resulting volumes were then aligned to standard space using both the affine transformation and non-linear warp calculated for the structural image and resampled to 1.75 mm isotropic voxel size using ANTS.
GBC pipeline
Resampled EPI images were spatially smoothed using a Gaussian kernel with full-width at half-maximum (FWHM) of 4 mm. Signals of no-interest were regressed from the spatially smoothed time-series data, including six rigid-body motion parameters and their derivatives, the average lateral ventricle signal, the averaged local white matter signal (Jo et al. 2010), and bandpass filter frequencies (0.01–0.10 Hz). The Euclidian norm of the six motion parameters was calculated (Jones et al. 2010), and time-points with total motion >0.30 were censored from the analyses along with the preceding time-point.
The AFNI program 3dTcorrMap was used to calculate voxel-wise GBC from the smoothed time series data. Specifically, the Pearson correlation between time series data from each voxel with all other voxels was calculated, Fisher z-transformed, and averaged to create one GBC measure for each voxel.
ReHo pipeline
The preprocessing pipeline for ReHo analyses was identical to the GBC pipeline with the exception that nuisance regression was performed prior to spatial smoothing (4 mm FWHM kernel) to prevent smoothing-related increases in ReHo amplitude (Zuo et al. 2013). ReHo is the Kendall’s coefficient of concordance for a time series relative to time series in a defined neighborhood of adjacent voxels (Zang et al. 2004). This method is an alternative, complementary approach to analyze time series data that specifically assesses similarity in local connectivity of voxels rather than connectivity to other voxels across the brain, including those in anatomically distant regions (i.e., traditional seed-based analyses). Specifically, ReHo was calculated with 3dReHo (Taylor and Saad 2013) across a neighborhood of 27 voxels in resampled space using the processed time series without spatial smoothing (additional analyses using different thresholds for defining local neighborhoods can be found in the supplement). A 4 mm FWHM smoothing kernel was subsequently applied and voxel-wise ReHo maps were then converted to z-values by subtracting the mean global ReHo and dividing by the standard deviation of the mean at each voxel.
Participants with image artifacts or less than 128 usable time-points following motion censoring were excluded for all imaging analyses (final n with usable data found in Table 1). Analyses were limited to grey matter for which all participants had EPI coverage by using an inclusion mask created from a tissue segmentation of the standard template (GMP > 0.25), combined with binary masks of normalized anatomical images from each participant.
Statistics
The number of prior concussions and the number of censored time points were square root transformed following the addition of a constant prior to statistical analyses. Linear mixed-effects (LME) models with time as a fixed factor and a random intercept to account for missing data were used to investigate changes in time as a function of recovery. Voxel-wise LMEs were performed using the AFNI program 3dLME (Chen et al. 2013). Two-tailed independent samples t-tests were used to characterize cross-sectional differences against HA. The estimated smoothness of the residuals (GBC FWHM = 6.6) and the unsmoothed residuals plus blurring kernel (ReHo = 2.7 + 4 mm kernel) were similar across both analytic pipelines. Therefore, the more conservative kernel was adopted to correct for false positives. Monte Carlo simulations (10,000 iterations in native voxel space) determined the necessary correction for family-wise error rate at p < 0.05 for all voxel-wise analyses (p = 0.005, minimum cluster volume = 660 μl). Anatomical localization for significant clusters was determined in part by the use of the macro-labels of the Eickhoff-Zilles brain atlas that is distributed with AFNI (Eickhoff et al. 2005).
Results
Behavioral analyses
Two-tailed independent samples t-tests confirmed that concussed athletes did not differ from HA in age or education (p’s > 0.10). However, there was a non-significant trend for concussed athletes self-reporting more previous concussions than HA (t(92) = 1.72, p = 0.088.) The median length of return-to-play decisions for concussed athletes was 13 days (range 3 to indefinite). The mean number of months between the study date and the last reported concussion for healthy athletes with a previous concussion was 34.06 (range 3 to 134 months).
There was a significant effect of time on HAM-D (F(2,53) = 37.69, p < 0.001) and HAM-A scores (F(2,53) = 43.52, p < 0.001; Fig. 1 ; Table 2). Both HAM-D and HAM-A scores were significantly higher at T1 and T2 relative to T3 (p’s < 0.001), and were significantly higher at T1 relative to T2 (p = 0.024 and p < 0.001). Similarly, there was a significant main effect of time on self-reported symptom frequency (F(2,40) = 31.28, p < 0.001) and cognitive performance (F(2,41) = 13.97, p < 0.001). Self-reported symptoms and cognitive performance were worse at T1 relative to T2 (p’s < 0.001) and T3 (p’s < 0.001), while symptoms and cognitive performance were not different at T3 relative to T2 (p’s > 0.10).
Behavioral Measures: Scores from structured interviews for depression (HAM-D) and anxiety (HAM-A) as well as the average scores from the cognitive battery and frequency of self-reported symptoms are shown for concussed athletes (CA) at one day (T1; light grey), one week (T2; grey), and one month (T3; dark grey) post-concussion, as well as in healthy athletes (HA; white). Notches indicate 95 % confidence interval of the median and whiskers represent data within 1.5 interquartile range
Two-tailed independent samples t-tests confirmed that concussed athletes had higher HAM-D scores relative to HA at T1 (t(83) = 9.98, p < 0.001), T2 (t(83) = 8.86, p < 0.001), and T3 (t(79) = 3.03, p = 0.003). Similarly, HAM-A scores were higher in concussed athletes relative to HA at T1 (t(83) = 9.93, p < 0.001), T2 (t(83) = 6.84, p < 0.001), and T3 (t(79) = 2.17, p = 0.033; Fig. 1). In contrast, self-reported symptoms (t(64) = 5.63, p < 0.001) and cognitive scores (t(61) = −3.22, p = 0.002) were worse at T1 relative to HA and, but not at T2 (p’s > 0.10). Finally, cognitive performance at T3 was not different from HA (p > 0.10), and concussed athletes at T3 had fewer self-reported symptoms than HA (t(57) = −2.97, p = 0.004).
Resting state functional connectivity quality assurance
There was no significant main effect of time on head motion or the number of censored time points across concussed athletes (all p’s > 0.10). There were also no significant differences in head motion on the number of censored TRs between HA and concussed athletes at T1, T2, or T3 (all p’s > 0.10).
Global connectivity differences
There was no significant main effect of time on voxel-wise GBC following appropriate correction for multiple comparisons in concussed athletes. A voxel-wise comparison of GBC between HA and concussed athletes at T1 also found no significant differences following appropriate correction for multiple comparisons.
Local connectivity differences
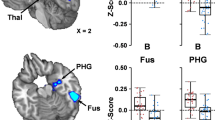
Longitudinal analyses indicated two main patterns of ReHo change as a function of time in the concussed athletes (see Table 2 , Fig. 2; Supplementary Fig. 1 ). The first pattern indicated a general increase in ReHo at sub-acute (i.e., one week and one month) relative to acute (i.e., one day) assessment points, and included the left paracentral lobule and postcentral gyrus (PCL/PoG), the right PoG, right lingual and fusiform gyri (LgG/FuG), right superior temporal gyrus (STG), bilateral supplementary motor areas (SMA), right LgG, and the right middle and superior temporal gyri (MTG/STG). For each of these clusters, ReHo was significantly higher at T3 relative to T1 (all p’s < 0.001). ReHo for the right STG cluster was also significantly higher at T2 relative to T1 (p < 0.001), with no difference between T2 and T3. ReHo was higher for all other clusters at T3 relative to T2 (all p’s < 0.005), while T1 and T2 did not differ. In addition to these longitudinal effects, regions with increased ReHo were also significantly higher at T3 relative to HA (all p’s < 0.01). ReHo in the right STG was significantly higher at T2 compared to HA (p < 0.05), and ReHo in the right LgG/FuG was significantly lower at T1 relative to HA (p < 0.05). No other differences relative to HA were observed at T1 or T2.
Local Connectivity Differences: a Clusters that showed a significant difference in regional homogeneity over time post-concussion are shown. Warm color scheme represents regions in which regional homogeneity increased over time post-concussion. Cool color scheme represents regions in which regional homogeneity decreased over time post-concussion. b Box plots illustrating the average z-scored regional homogeneity (ReHo) for each cluster are shown for concussed athletes (CA) at one day (T1; light grey), one week (T2; grey), and one month (T3; dark grey) post-concussion, as well as in healthy athletes (HA; white). Notches indicate 95 % confidence interval of the median and whiskers represent data within 1.5 interquartile range. Regions with increasing ReHo over time are labeled in red and regions with decreasing ReHo over time are labeled in blue. L = left, R = Right, PoG = postcentral gyrus, PCL = paracentral lobule, LgG = lingual gyrus, FuG = fusiform gyrus, STG = superior temporal gyrus, SMA = supplementary motor area, MTG = middle temporal gyrus, MFG = middle frontal gyrus, SFG = superior frontal gyrus, SMFG = superior medial frontal gyrus
In contrast, the bilateral superior medial frontal gyrus (SMFG) and the right middle and superior frontal gyri (MFG/SFG) also exhibited a significant effect of time but with a pattern of reduced ReHo as a function of time (Table 2). For both regions, ReHo was significantly lower at T3 relative to both T1 and T2 (p’s < 0.001). There was no difference between ReHo in the bilateral SMFG at T1 and T2, but ReHo in the right MFG/SFG was higher at T2 than at T1 (p < 0.05). Relative to HA, ReHo in both regions was lower in concussed athletes at T3 (p’s < 0.05). Finally, ReHo in the right MFG/SFG was significantly higher at T2 relative to HA (p < 0.05), while no other differences were observed at T1 or T2 relative to HA.
Exploratory analyses
Finally, exploratory Spearman correlations were performed to determine if post-concussion abnormalities in functional connectivity were associated with primary behavioral (i.e., HAM-A and HAM-D) or outcome (i.e., days withheld from competition) measures. To limit the number of comparisons and control for Type I error, clusters with similar patterns over time (i.e., separated into clusters with either increased ReHo at one month or clusters with decreased ReHo at one month) were averaged. This resulted in two ReHo clusters with either increasing or decreasing ReHo over the one-month assessment period. However, there were no significant correlations between behavior and connectivity measures at T1, T2, or T3 (all p’s > 0.05).
Discussion
To our knowledge, the current study represents the first attempt to prospectively characterize abnormalities in local (ReHo) and global (GBC) connectivity following SRC. Both self-report and clinical assessments indicated increased neurobehavioral symptomatology in the acute phase that either completely (self-report and cognitive testing) or partially (clinical assessment) abated as athletes transitioned from the acute (i.e., one day) to sub-acute (i.e., one week or one month) post-injury stages. Contrary to our hypothesis, there were no longitudinal changes or cross-sectional abnormalities on global metrics of connectivity. However, results indicated evidence of both longitudinal and cross-sectional changes in local connectivity that were more prominent in the semi-acute phase of SRC. This pattern of findings is contrary to current understanding of typical recovery characterized by rapid resolution of symptoms followed by return to play, and potentially suggests a complex and temporally varying pattern of neurophysiological changes following SRC.
The recovery-rate of post-concussion symptoms was similar to our previous work in subsets of the current sample of athletes and thus is only briefly described here (Meier et al. 2015b; Singh et al. 2015; Meier et al. 2015c). Self-report symptoms and cognitive deficits showed recovery by one week post-concussion, which is consistent with large-scale clinical studies of typical symptom recovery following SRC (McCrea et al. 2003). Although we observed partial recovery of clinically assessed mood symptoms by one week and one month relative to one-day post-concussion, mood symptoms remained elevated throughout our sub-acute assessment phase. Importantly, these findings suggest that mood symptoms may remain elevated beyond the typical recovery period, and that the method of mood symptom assessment (i.e., self-report vs. clinically assessed) may play a role in determining recovery. However, structured interviews were not conducted blinded to concussion status and thus rater bias cannot be ruled out.
Longitudinal changes in local connectivity (ReHo) following SRC showed two general patterns. The first pattern, present in two rostral PFC regions, was a reduction in ReHo at one month post-concussion relative to the acute assessment. The superior medial frontal gyrus corresponds to the anterior node of the DMN, which is thought to mediate internally oriented processing (Buckner et al. 2008). Altered functional connectivity in the DMN following mTBI is consistent with numerous previous studies (reviewed in (Mayer et al. 2015a)). For example, alterations in DMN connectivity have been previously reported in concussed athletes at one month post-concussion (Borich et al. 2015). Other groups have observed reduced DMN connectivity within the first week (D. C. Zhu et al. 2015) as well as both reductions and increases in DMN connectivity at 10 days following SRC (B. Johnson et al. 2012).
The fact that differences in DMN connectivity are consistently observed in SRC could be due a several factors. First, the DMN is one of the most functionally connected networks, both locally and globally, in the brain (Buckner et al. 2009; Cole et al. 2010; Sepulcre et al. 2010), and thus potentially one of the most susceptible to the effects of SRC. In addition, computational modeling suggests that midline structures, such as the main hubs of the DMN (i.e., anterior and posterior cingulate cortices), are particularly sensitive to shear strains associated with rotational forces (L. Zhang et al. 2004). Finally, similar to hypothesized alterations of DMN in depression (Sheline et al. 2009), changes in DMN connectivity may be epiphenomenal in nature, resulting from abnormal self-referential processing following SRC rather than being a direct result of injury.
In contrast, the rostrolateral PFC is a part of the frontoparietal network, or executive control network, and is associated with decision-making and cognitive control (Seeley et al. 2007; Vincent et al. 2008; Yeo et al. 2011). As with the DMN, several studies have also demonstrated abnormalities in the frontoparietal network following mTBI. For example, increased connectivity to the dorsolateral PFC was observed in a variety of regions, including other frontal regions and parietal cortex, on average over three months post-injury (Czerniak et al. 2015). Increased activity in frontoparietal regions has also been documented during a working memory task up to two months post-injury (Dettwiler et al. 2014). Acute deficits in executive function are common following mTBI (Karr et al. 2014), and current and retired football players with histories of previous concussion self-report executive dysfunction relative to standard normative data (Seichepine et al. 2013). Although the clinical significance of the current findings remains to be elucidated, to our knowledge they represent the first demonstration of altered local connectivity in the frontal cortex following SRC.
In contrast to prefrontal cortex, sensorimotor, visual, and temporal regions exhibited an increase in local connectivity as concussed athletes transitioned from the acute to semi-acute injury phases. Previous studies have reported various metabolic abnormalities in primary motor cortex within one week of injury in concussed relative to healthy athletes (Henry et al. 2010). In addition, a recent study in an emergency room cohort of mTBI patients at approximately 3 days post-injury found decreased ReHo during the resting state in the left pre- and postcentral gyri, as well as the left supramarginal gyrus and insula (Zhan et al. 2015). Disrupted motor network connectivity during a unilateral finger movement task has also been reported in TBI patients of varying severity at least 6 months post-injury (Kasahara et al. 2010), and reduced interhemispheric coherence of the motor cortex during a finger movement task was recently reported in pediatric mTBI patients with post-concussion syndrome (Urban et al. 2015). A reduction in cortical thickness in the right motor cortex has also been observed in collegiate football players with a history of concussion relative to players without a concussion history (Meier et al. 2015a). Furthermore, decreased interhemispheric connectivity has been previously observed both at rest and following a physical stress test in the visual cortex following SRC (Slobounov et al. 2011), and altered task-evoked activity in the visual cortex has been documented in mTBI patients at the semi-acute (i.e., 3 weeks) and early chronic (i.e., 4 months) phases during a multisensory cognitive control task (Mayer et al. 2015b). The abnormalities observed at rest within sensory networks in the current work may reflect the functional reorganization or possibly altered metabolism of these networks. Such abnormalities could potentially be associated with sensorimotor deficits present in some athletes following SRC (McCrory et al. 2013) as well as in military populations following mild traumatic brain injury (Pogoda et al. 2012; Lew et al. 2011). However, exploratory analyses showed no significant relationships between post-concussion symptoms and ReHo in the sensory networks or frontal cortices. Future studies relating clusters of concussion symptoms with connectivity measures in a priori selected anatomical regions are needed.
It is possible that the longitudinal changes in ReHo observed in the current study reflect recovery following SRC. However, cross-sectional comparisons to healthy athletes suggest a potentially more nuanced explanation. For regions that showed ReHo changes (both increases and decreases), significant differences relative to HA were primarily observed only at one month post-concussion rather than at the more acute visits. One potential explanation is that changes in local connectivity have a delayed onset and are driven by secondary effects of SRC, such as inflammation (Patterson and Holahan 2012). An alternative explanation is that changes in local connectivity increase beyond normal levels as a compensatory mechanism following concussion. In either case, the limited follow-up period in the current study does not permit full characterization of the subsequent recovery of ReHo, and additional studies with more extended follow-up scans (e.g., six months post-injury) are required. In addition, the physiological mechanism behind simultaneous observations of post-concussion increases and decreases in ReHo in separate regions is unknown. However, a recent study found that association areas (including frontoparietal and DMN regions) and primary sensory areas (visual, motor, and auditory) showed distinct network boundaries based on the functional covariance of surface-based ReHo (Jiang et al. 2015), indicating that these regions could conceivably show opposite patterns of ReHo changes in response to SRC.
From a neurophysiological perspective, changes in local functional connectivity may reflect changes in short-range neuronal synchronicity as a result of SRC. However, the BOLD signal is made up of a combination of physiological signals that are likely affected by SRC, including the cerebral metabolic rates of oxygen and glucose, cerebral blood volume, and cerebral blood flow (Ogawa et al. 1990). For example, we have previously demonstrated acute hypo-perfusion in the right insular cortex and superior temporal cortex with recovery toward normal levels by one month post-concussion (Meier et al. 2015b), and increased cerebrovascular reactivity has also been observed within the first week following SRC (Militana et al. 2015). Thus, longitudinal, studies with multiple neuroimaging metrics, including susceptibility-weighted or T2*-weighted images, are needed to pinpoint the exact physiological consequences of mild head trauma, which have been shown to multi-faceted and complex in animal models (Giza and Hovda 2014). The simultaneous assessment of arterial spin labeling perfusion and BOLD-dependent fMRI would be particularly informative (S. Zhu et al. 2013).
We also hypothesized that GBC would be affected by SRC due to the diffuse nature of white matter abnormalities previously observed following TBI (V. E. Johnson et al. 2013), which could conceivably affect local and global functional connectivity. However, this hypothesis was not supported by the current data. Several previous studies that have relied on methods requiring a priori selection of seed-regions or resting state networks have reported difference in long-range connectivity following SRC and mTBI (reviewed in (Mayer et al. 2015a)). The fact that GBC represents the average connectivity of a voxel with all other voxels of the brain could potentially result in averaging out of any differences that might exist between specific seed-regions or networks. Accordingly, supplementary analyses found that analyses of local connectivity with defined neighborhoods up to 5000 voxels (i.e., a closer approximation to GBC analyses; see supplement) had a reduction in the number of significant clusters, which supports this hypothesis. Thus, this could explain why no GBC differences in the DMN were observed despite previous reports of DMN abnormalities using seed-based and ICA methods. Nevertheless, the current results suggest that local connectivity is potentially a more sensitive marker of SRC than global connectivity.
The current study has caveats and limitations that merit discussion. The primary goal of the current study was to elucidate the effects of a recent SRC within the time period typically associated with symptom recovery. We therefore included contact-sport athletes as our control group to control for the potential effects of previous concussions, sub-concussive head injuries, and other demands associated with being a collegiate student-athlete. An alternative strategy would have been to include only athletes that had no self-reported history of concussion as healthy controls. However, that approach would not allow the separation of the effects of previous concussion from the current concussion, as concussed athletes in the current study had a varying history of concussions prior to the current injury. Nevertheless, one primary limitation of the current study is that concussed athletes had more prior concussions than HA, which is consistent with previous work demonstrating that a history of prior concussions increases the likelihood of additional incidents (Guskiewicz et al. 2003). Furthermore, not every participant was able to complete every visit, though statistical approaches capable of dealing with missing data (i.e., linear mixed-effects models) were used for longitudinal analyses. In addition, our analyses extended across the time window of return-to-play decisions, and different levels of exercise between injured athletes and those cleared for return-to-play may be a confounding factor on analyses. Although we did not exclude for Attention Deficit Hyperactivity Disorder, supplementary analyses indicated that local connectivity differences remained largely unchanged when excluding these subjects (see supplement). Finally, although we observed longitudinal changes across multiple time points following concussion, pre-injury connectivity levels were not available in the current sample. Instead, cross-sectional comparisons to healthy, non-concussed athletes were used as a proxy for pre-injury levels. Additional longitudinal studies with true pre-injury baselines are needed to map out recovery curves for resting state connectivity metrics following SRC.
Conclusion
These results demonstrate both cross-sectional and longitudinal evidence of changes in local functional connectivity at the acute and sub-acute time frame following SRC in a large sample of collegiate athletes. Local connectivity differences emerged in prefrontal cortex, sensory, and motor networks one month following SRC, highlighting these regions as potential targets for future MRI biomarker development.
References
Abbas, K., Shenk, T. E., Poole, V. N., Breedlove, E. L., Leverenz, L. J., Nauman, E. A., et al. (2015). Alteration of default mode network in high school football athletes due to repetitive subconcussive mild traumatic brain injury: a resting-state functional magnetic resonance imaging study. Brain Connectivity, 5(2), 91–101. doi:10.1089/brain.2014.0279.
Avants, B. B., Tustison, N. J., Song, G., Cook, P. A., Klein, A., & Gee, J. C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage, 54(3), 2033–2044. doi:10.1016/j.neuroimage.2010.09.025.
Borich, M., Babul, A. N., Yuan, P. H., Boyd, L., & Virji-Babul, N. (2015). Alterations in resting-state brain networks in concussed adolescent athletes. Journal of Neurotrauma, 32(4), 265–271. doi:10.1089/neu.2013.3269.
Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. doi:10.1196/annals.1440.011.
Buckner, R. L., Sepulcre, J., Talukdar, T., Krienen, F. M., Liu, H., Hedden, T., et al. (2009). Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. The Journal of Neuroscience, 29(6), 1860–1873. doi:10.1523/JNEUROSCI.5062-08.2009.
Cernich, A., Reeves, D., Sun, W., & Bleiberg, J. (2007). Automated neuropsychological assessment metrics sports medicine battery. Arch Clin Neuropsychol, 22 Suppl 1, S101-114. doi:10.1016/j.acn.2006.10.008.
Chen, G., Saad, Z. S., Britton, J. C., Pine, D. S., & Cox, R. W. (2013). Linear mixed-effects modeling approach to FMRI group analysis. NeuroImage, 73, 176–190. doi:10.1016/j.neuroimage.2013.01.047.
Cole, M. W., Pathak, S., & Schneider, W. (2010). Identifying the brain's most globally connected regions. NeuroImage, 49(4), 3132–3148. doi:10.1016/j.neuroimage.2009.11.001.
Cox, R. W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173.
Czerniak, S.M., Sikoglu, E.M., Liso Navarro, A.A., McCafferty, J., Eisenstock, J., Stevenson, J. H., et al. (2015). A resting state functional magnetic resonance imaging study of concussion in collegiate athletes. Brain Imaging and Behavior, 9(2), 323–332. doi:10.1007/s11682-014-9312-1.
Dettwiler, A., Murugavel, M., Putukian, M., Cubon, V., Furtado, J., & Osherson, D. (2014). Persistent differences in patterns of brain activation after sports-related concussion: a longitudinal functional magnetic resonance imaging study. Journal of Neurotrauma, 31(2), 180–188. doi:10.1089/neu.2013.2983.
Eickhoff, S. B., Stephan, K. E., Mohlberg, H., Grefkes, C., Fink, G. R., Amunts, K., et al. (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage, 25(4), 1325–1335. doi:10.1016/j.neuroimage.2004.12.034.
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355.
Giza, C. C., & Hovda, D. A. (2014). The new neurometabolic cascade of concussion. Neurosurgery, 75 Suppl 4, S24-33. doi:10.1227/NEU.0000000000000505.
Gotts, S. J., Simmons, W. K., Milbury, L. A., Wallace, G. L., Cox, R. W., & Martin, A. (2012). Fractionation of social brain circuits in autism spectrum disorders. Brain, 135(Pt 9), 2711–2725. doi:10.1093/brain/aws160.
Guskiewicz, K. M., McCrea, M., Marshall, S. W., Cantu, R. C., Randolph, C., Barr, W., et al. (2003). Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA concussion study. JAMA, 290(19), 2549–2555. doi:10.1001/jama.290.19.2549.
Henry, L. C., Tremblay, S., Boulanger, Y., Ellemberg, D., & Lassonde, M. (2010). Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. Journal of Neurotrauma, 27(1), 65–76. doi:10.1089/neu.2009.0962.
Jiang, L., Xu, T., He, Y., Hou, X. H., Wang, J., Cao, X. Y., et al. (2015). Toward neurobiological characterization of functional homogeneity in the human cortex: regional variation, morphological association and functional covariance network organization. Brain Structure & Function, 220(5), 2485–2507. doi:10.1007/s00429-014-0795-8.
Jo, H. J., Saad, Z. S., Simmons, W. K., Milbury, L. A., & Cox, R. W. (2010). Mapping sources of correlation in resting state FMRI, with artifact detection and removal. NeuroImage, 52(2), 571–582. doi:10.1016/j.neuroimage.2010.04.246.
Johnson, B., Zhang, K., Gay, M., Horovitz, S., Hallett, M., Sebastianelli, W., et al. (2012). Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. NeuroImage, 59(1), 511–518. doi:10.1016/j.neuroimage.2011.07.081.
Johnson, V. E., Stewart, W., & Smith, D. H. (2013). Axonal pathology in traumatic brain injury. Experimental Neurology, 246, 35–43. doi:10.1016/j.expneurol.2012.01.013.
Johnson, B., Neuberger, T., Gay, M., Hallett, M., & Slobounov, S. (2014). Effects of subconcussive head trauma on the default mode network of the brain. Journal of Neurotrauma, 31(23), 1907–1913. doi:10.1089/neu.2014.3415.
Jones, T. B., Bandettini, P. A., Kenworthy, L., Case, L. K., Milleville, S. C., Martin, A., et al. (2010). Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. NeuroImage, 49(1), 401–414. doi:10.1016/j.neuroimage.2009.07.051.
Karr, J. E., Areshenkoff, C. N., & Garcia-Barrera, M. A. (2014). The neuropsychological outcomes of concussion: a systematic review of meta-analyses on the cognitive sequelae of mild traumatic brain injury. Neuropsychology, 28(3), 321–336. doi:10.1037/neu0000037.
Kasahara, M., Menon, D. K., Salmond, C. H., Outtrim, J. G., Taylor Tavares, J. V., Carpenter, T. A., et al. (2010). Altered functional connectivity in the motor network after traumatic brain injury. Neurology, 75(2), 168–176. doi:10.1212/WNL.0b013e3181e7ca58.
Lew, H. L., Pogoda, T. K., Baker, E., Stolzmann, K. L., Meterko, M., Cifu, D. X., et al. (2011). Prevalence of dual sensory impairment and its association with traumatic brain injury and blast exposure in OEF/OIF veterans. The Journal of Head Trauma Rehabilitation, 26(6), 489–496. doi:10.1097/HTR.0b013e318204e54b.
Mayer, A. R., Bellgowan, P. S., & Hanlon, F. M. (2015a). Functional magnetic resonance imaging of mild traumatic brain injury. Neuroscience and Biobehavioral Reviews, 49, 8–18. doi:10.1016/j.neubiorev.2014.11.016.
Mayer, A. R., Hanlon, F. M., Dodd, A. B., Ling, J. M., Klimaj, S. D., & Meier, T. B. (2015b). A functional magnetic resonance imaging study of cognitive control and neurosensory deficits in mild traumatic brain injury. Human Brain Mapping, 36(11), 4394–4406. doi:10.1002/hbm.22930.
McCrea, M., Guskiewicz, K. M., Marshall, S. W., Barr, W., Randolph, C., Cantu, R. C., et al. (2003). Acute effects and recovery time following concussion in collegiate football players: the NCAA concussion study. JAMA, 290(19), 2556–2563. doi:10.1001/jama.290.19.2556.
McCrory, P., Meeuwisse, W. H., Aubry, M., Cantu, B., Dvorak, J., Echemendia, R. J., et al. (2013). Consensus statement on concussion in sport: the 4th international conference on concussion in sport held in Zurich, November 2012. British Journal of Sports Medicine, 47(5), 250–258. doi:10.1136/bjsports-2013-092313.
Meier, T. B., Bellgowan, P. S., Bergamino, M., Ling, J. M., & Mayer, A. R. (2015a). Thinner cortex in collegiate football players with, but not without, a self-reported history of concussion. Journal of Neurotrauma. doi:10.1089/neu.2015.3919.
Meier, T. B., Bellgowan, P. S., Singh, R., Kuplicki, R., Polanski, D. W., & Mayer, A. R. (2015b). Recovery of cerebral blood flow following sports-related concussion. JAMA Neurol, 72(5), 530–538. doi:10.1001/jamaneurol.2014.4778.
Meier, T. B., Bergamino, M., Bellgowan, P. S., Teague, T. K., Ling, J. M., Jeromin, A., et al. (2015c). Longitudinal assessment of white matter abnormalities following sports-related concussion. Human Brain Mapping. doi:10.1002/hbm.23072.
Militana, A. R., Donahue, M. J., Sills, A. K., Solomon, G. S., Gregory, A. J., Strother, M. K., et al. (2015). Alterations in default-mode network connectivity may be influenced by cerebrovascular changes within 1 week of sports related concussion in college varsity athletes: a pilot study. Brain Imaging and Behavior. doi:10.1007/s11682-015-9407-3.
Ogawa, S., Lee, T. M., Kay, A. R., & Tank, D. W. (1990). Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America, 87(24), 9868–9872.
Park, E., Bell, J. D., Siddiq, I. P., & Baker, A. J. (2009). An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: a role for hypoxia-inducible factors in traumatic brain injury. Journal of Cerebral Blood Flow and Metabolism, 29(3), 575–584. doi:10.1038/jcbfm.2008.151.
Patterson, Z. R., & Holahan, M. R. (2012). Understanding the neuroinflammatory response following concussion to develop treatment strategies. Frontiers in Cellular Neuroscience, 6, 58. doi:10.3389/fncel.2012.00058.
Pogoda, T. K., Hendricks, A. M., Iverson, K. M., Stolzmann, K. L., Krengel, M. H., Baker, E., et al. (2012). Multisensory impairment reported by veterans with and without mild traumatic brain injury history. Journal of Rehabilitation Research and Development, 49(7), 971–984.
Roebuck-Spencer, T. M., Vincent, A. S., Gilliland, K., Johnson, D. R., & Cooper, D. B. (2013). Initial clinical validation of an embedded performance validity measure within the automated neuropsychological metrics (ANAM). Archives of Clinical Neuropsychology, 28(7), 700–710. doi:10.1093/arclin/act055.
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27(9), 2349–2356. doi:10.1523/JNEUROSCI.5587-06.2007.
Seichepine, D. R., Stamm, J. M., Daneshvar, D. H., Riley, D. O., Baugh, C. M., Gavett, B. E., et al. (2013). Profile of self-reported problems with executive functioning in college and professional football players. Journal of Neurotrauma, 30(14), 1299–1304. doi:10.1089/neu.2012.2690.
Sepulcre, J., Liu, H., Talukdar, T., Martincorena, I., Yeo, B. T., & Buckner, R. L. (2010). The organization of local and distant functional connectivity in the human brain. PLoS Computational Biology, 6(6), e1000808. doi:10.1371/journal.pcbi.1000808.
Sheline, Y. I., Barch, D. M., Price, J. L., Rundle, M. M., Vaishnavi, S. N., Snyder, A. Z., et al. (2009). The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 1942–1947. doi:10.1073/pnas.0812686106.
Singh, R., Savitz, J., Teague, T. K., Polanski, D. W., Mayer, A. R., Bellgowan, P. S., et al. (2015). Mood symptoms correlate with kynurenine pathway metabolites following sports-related concussion. Journal of Neurology, Neurosurgery, and Psychiatry. doi:10.1136/jnnp-2015-311369.
Slobounov, S. M., Gay, M., Zhang, K., Johnson, B., Pennell, D., Sebastianelli, W., et al. (2011). Alteration of brain functional network at rest and in response to YMCA physical stress test in concussed athletes: RsFMRI study. NeuroImage, 55(4), 1716–1727. doi:10.1016/j.neuroimage.2011.01.024.
Taylor, P. A., & Saad, Z. S. (2013). FATCAT: (an efficient) functional and tractographic connectivity analysis toolbox. Brain Connectivity, 3(5), 523–535. doi:10.1089/brain.2013.0154.
Thorne, D. R. (2006). Throughput: a simple performance index with desirable characteristics. Behavior Research Methods, 38(4), 569–573.
Urban, K. J., Barlow, K. M., Jimenez, J. J., Goodyear, B. G., & Dunn, J. F. (2015). Functional near-infrared spectroscopy reveals reduced interhemispheric cortical communication after pediatric concussion. Journal of Neurotrauma, 32(11), 833–840. doi:10.1089/neu.2014.3577.
Vincent, J. L., Kahn, I., Snyder, A. Z., Raichle, M. E., & Buckner, R. L. (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100(6), 3328–3342. doi:10.1152/jn.90355.2008.
Yeo, B. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M., et al. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. doi:10.1152/jn.00338.2011.
Zang, Y., Jiang, T., Lu, Y., He, Y., & Tian, L. (2004). Regional homogeneity approach to fMRI data analysis. NeuroImage, 22(1), 394–400. doi:10.1016/j.neuroimage.2003.12.030.
Zhan, J., Gao, L., Zhou, F., Kuang, H., Zhao, J., Wang, S., et al. (2015). Decreased regional homogeneity in patients with acute mild traumatic brain injury: a resting-state fMRI study. The Journal of Nervous and Mental Disease, 203(10), 786–791. doi:10.1097/NMD.0000000000000368.
Zhang, L., Yang, K. H., & King, A. I. (2004). A proposed injury threshold for mild traumatic brain injury. Journal of Biomechanical Engineering, 126(2), 226–236.
Zhang, Z., Liu, Y., Jiang, T., Zhou, B., An, N., Dai, H., et al. (2012). Altered spontaneous activity in Alzheimer's disease and mild cognitive impairment revealed by regional homogeneity. NeuroImage, 59(2), 1429–1440. doi:10.1016/j.neuroimage.2011.08.049.
Zhu, S., Fang, Z., Hu, S., Wang, Z., & Rao, H. (2013). Resting state brain function analysis using concurrent BOLD in ASL perfusion fMRI. PloS One, 8(6), e65884. doi:10.1371/journal.pone.0065884.
Zhu, D. C., Covassin, T., Nogle, S., Doyle, S., Russell, D., Pearson, R. L., et al. (2015). A potential biomarker in sports-related concussion: brain functional connectivity alteration of the default-mode network measured with longitudinal resting-state fMRI over thirty days. Journal of Neurotrauma, 32(5), 327–341. doi:10.1089/neu.2014.3413.
Zuo, X. N., Xu, T., Jiang, L., Yang, Z., Cao, X. Y., He, Y., et al. (2013). Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. NeuroImage, 65, 374–386. doi:10.1016/j.neuroimage.2012.10.017.
Acknowledgments
This research was conducted using internal funds from the Laureate Institute for Brain Research, which is supported by The William K. Warren Foundation. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Meier had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors thank the psychiatric assessment team, the MRI technicians, and Dr. Rashmi Singh at the Laureate Institute for Brain Research for their assistance in data collection and Christopher Nerio, David Polanski, Dr. Thomas Allen, and Dr. Lamont Cavanagh for athlete referral and injury oversight.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures and informed consent
The authors declare that they have no conflict of interest. This article was prepared while Dr. Bellgowan was employed at The National Institute of Neurological Disorders and Stroke. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government. All procedures performed were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic Supplementary Material
ESM 1
(DOCX 6761 kb)
Rights and permissions
About this article
Cite this article
Meier, T.B., Bellgowan, P.S.F. & Mayer, A.R. Longitudinal assessment of local and global functional connectivity following sports-related concussion. Brain Imaging and Behavior 11, 129–140 (2017). https://doi.org/10.1007/s11682-016-9520-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-016-9520-y