Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by progressive muscular weakness and atrophy. Several morphometric studies have been conducted to investigate the gray matter volume or thickness changes in ALS, whereas the cortical folding pattern remains poorly understood. In the present study, we applied a surface-based local gyrification index (LGI) from high resolution MRI data to quantify the cortical folding in matched samples of 25 ALS patients versus 25 healthy controls. Using resting-state fMRI data, we further conducted seed-based functional connectivity analysis to explore the functional correlate of the cortical folding changes. We found that ALS patients had significantly reduced LGI in right occipital cortex and that abnormality in this region associated with decreased functional connectivity in the bilateral precuneus. This set of findings was speculated to result from disturbed white matter connectivity in ALS. In the patient group, we revealed significant negative correlations between disease duration and the LGIs of a cluster in the left superior frontal gyrus, which may reflect the cognitive deterioration in ALS. In summary, our results suggest that LGI may provide a useful means to assess ALS-related neurodegeneration and to study the pathophysiology of ALS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that is characterized by progressive muscular weakness and atrophy (Witiuk et al. 2014; Stoppel et al. 2014). Although ALS has long been deemed as a disease of the motor system (Mitchell and Borasio 2007), increasing evidence suggests that it is a multisystem disorder that involves widespread extra-motor brain regions (Stoppel et al. 2014; Verma et al. 2013). Indeed, both neuroimaging and neuropathological studies have documented alternations in non-motor areas especially in the brain regions that subserve cognition and behavior in ALS. For instance, Dalakas et al. showed decreased cortical glucose consumption in patients with ALS throughout the cortex (Dalakas et al. 1987), and Kushner et al. found evidences for astrogliosis in several cortical areas including the occipital cortex (Kushner et al. 1991). Moreover, symptoms associated with cognitive decline and behavioral impairment involving non-motor systems were also found in ALS patients (Phukan et al. 2007; Zago et al. 2011). Hence, investigation of the neural mechanisms underlying the non-motor symptom in ALS may provide valuable insights into the pathophysiology of this disorder.
Since the advent of magnetic resonance imaging (MRI), a number of morphometric analytical approaches have been developed to identify macroscopic changes in the human brain in vivo. The most popular ones are those whole-brain based methods, for example, voxel-based morphometry (VBM) (Ashburner and Friston 2000) and surface-based morphometry (SBM)(Palaniyappan and Liddle 2012), allowing for the identification of the structural abnormalities that are not confined to certain focal regions. Whole-brain based methods provide promising ways of characterizing the neural basis of the non-motor symptoms in ALS. Notably, the majority of existing morphometric studies on ALS was conducted through VBM, reporting focal loss of grey and white matter in some specific brain regions (Grosskreutz et al. 2006; Bede et al. 2013). The interpretation of VBM results can be difficult given that an actual physical characteristic is not measured directly (Singh et al. 2006). Moreover, the specific contribution of the anatomical properties of the cortical mantle to these results remains unknown since VBM provides a mixed measure of cortical gray matter including cortical thickness, cortical surface area and/or cortical folding (Palaniyappan and Liddle 2012). In contrast, SBM allows us to fractionate the specific contributions of such physical properties using cortical thickness, surface area, and local gyrification index (LGI) (Schaer et al. 2008). Using SBM, previous studies have documented decreased cortical thickness but comparable surface area in patients with ALS (Rajagopalan and Pioro 2015; Mezzapesa et al. 2013; Schuster et al. 2014a). However, the cortical folding pattern, a morphometric characteristic that is thought to be both genetically and phenotypically independent of cortical thickness(Hogstrom et al. 2013; Panizzon et al. 2009), remains unexplored in patients with ALS. In fact, given the frequently reported white matter abnormalities in ALS(Zhang et al. 2014a; Ash et al. 2015; Evans et al. 2015) and the hypothesis that cortical folding was mainly caused by the tension of the underlying white matter connectivity (Van Essen 1997), cortical folding changes are expected in patients with ALS.
Using a surface-based LGI, the present study aimed to investigate the cortical folding pattern in patients with ALS compared with matched healthy controls. Specifically, we contrasted the LGI between healthy controls and ALS patients using a surface-based general linear model (GLM) tool to map group contrasts on a vertex-by-vertex basis. By choosing the cortical regions with significant difference in the LGI analysis as region of interest (ROI), we further performed seed-based functional connectivity analysis to examine the functional correlate of such LGI changes.
Materials and methods
Subjects
Twenty-five patients (17 men) with the diagnosis of sporadic probable or definite ALS, according to the revised El Escorial criteria of the World Federation of Neurology (Brooks et al. 2000), were consecutively recruited from the Department of Neurology at Southwest Hospital in Chongqing. Exclusion criteria were the following: (1) family history of motor neuron diseases; (2) clinical diagnosis of frontotemporal dementia (Neary et al. 1998); (3) presence of other major systemic, psychiatric, or neurological illnesses; and (4) cognitive impairment as determined by Montreal Cognitive Assessment (MoCA) score < 26 (Nasreddine et al. 2005). Clinical status of the patients was assessed by the ALS Functional Rating Scale-Revised (ALSFRS-R) (Cedarbaum et al. 1999) within 12 h from MRI. Twenty-five healthy controls with no previous history of neurological or psychiatric diseases and with normal brain MRI were recruited from the local community. All the participants were right-handed determined by Edinburgh inventory (Oldfield 1971). Detailed demographic and clinical data are shown in Table 1. The study was approved by the Medical Research Ethics Committee of Southwest Hospital, and written informed consent was obtained from all the participants.
Data acquisition
Three-dimensional structural MRI scans were obtained on a 3-T Siemens Tim Trio (Siemens heathcare, Erlangen, Germany) with an eight-channel head coil. Detailed scan parameters were as follows: TR = 1900 ms, TE = 2.52 ms, inversion time = 900 ms, flip angle =9o, matrix =256 × 256, thickness = 1.0 mm, no gap, 176 slices, and voxel size =1× 1 × 1 mm3.
Functional imaging data were collected transversely with an echo-planar imaging (EPI) sequence. During scanning, the subjects were instructed to rest with their eyes closed, not think of anything in particular, and not fall asleep. Detailed scan parameters were as follows: TR = 2000 ms, TE = 30 ms, flip angle =90°, FOV = 192 mm × 192 mm, slices =36, in-plane matrix =64 × 64, thickness = 3 mm, and voxel size =3.0 mm × 3.0 mm × 3.0 mm. For each subject, a total of 240 volumes were acquired, resulting in a total scan time of 480 s.
LGI analysis
Each structural scan was processed using the FreeSurfer5.3.0 image analysis suite, which is documented and freely available online (http://surfer.nmr.mgh.harvard.edu/), to obtain the LGI (Schaer et al. 2008). Briefly, the LGI map is obtained in four steps: First, the pial surface is reconstructed in 3-dimensional space. Second, the outer surface is obtained from the outer hull, which tightly wraps the pial surface. Third, the LGI is calculated for each vertex on the outer surface as a ratio of areas of circular region centered on this vertex and the area of the corresponding region of the pial surface. Thus, the LGI can be used to quantify the amount of cortical surface invaginated in the sulci and to measure the spatial frequency of the cortical gyrification and the depth of the sulci. Fourth, the LGI map is obtained by propagating the LGI values from the outer surface to the pial surface. For comparison, all of the individual reconstructed cortical surfaces were aligned to an average template using a surface-based registration algorithm. Then, the LGI maps were resampled and smoothed with a heat kernel with a width of 10 mm.
Functional connectivity analysis
All the functional MRI (fMRI) data were preprocessed using DPARSFA (Data Processing Assistant for Resting-State fMRI Advanced Edition, http://www.restfmri.net/forum/DPARSF). The first 10 volumes of each scan were discarded to allow for magnetization equilibrium. Subsequent preprocessing included slice timing, head motion correction, spatially normalizing to the MNI template, resampling to 3 × 3 × 3 mm3, smoothing with a 4 mm Gaussian kernel to decrease spatial noise, temporal band-pass filtering, and regressing out nuisance signals including head motion parameters, white matter and cerebrospinal fluid. The region with significant between-group differences in the LGI analysis was extracted as an ROI. We calculated the average time series of the seed ROI for each subject. Then we computed Pearson correlation coefficients between the mean time series of the ROI and those of all the voxels throughout the whole brain and converted the correlation coefficient to z values using Fisher’s r-to-z transformation to improve normality.
Statistical analyses
Vertex-by-vertex contrasts of the LGI were performed to compare the controls and ALS patients using the SurfStat package (http://www.math.mcgill.ca/keith/surfstat/). Specifically, each contrast was entered into a vertex-by-vertex GLM with diagnosis, gender and exact age as covariates. A threshold of P < 0.001 was then used to define clusters of difference. Subsequently, a corrected cluster-wise P value was obtained for each cluster using random field theory (RFT) (Hayasaka et al. 2004). The level of significance for vertices was set at P < 0.05 after correction for multiple comparisons.
In the patient group, vertex-wise correlation analyses were performed to determine the associations between LGI and the clinical data, such as ALSFRS-R and disease duration, while adjusting for age and gender. Then, the same procedures as the above between-group analysis on LGI were repeated to correct for multiple comparisons and to report the results of the correlation analysis.
In the functional connectivity analysis, the GLMs used in the between-group LGI analysis were fitted to test the functional connectivity difference between the two groups. A threshold of P < 0.005 was then used to define clusters of difference. Subsequently, a corrected cluster-wise P value was obtained for each cluster using Gaussian random field theory. The level of significance was set at P < 0.05 after correction for multiple comparisons. In the patient group, partial correlation analyses were performed between the clinical data and the average z values of regions with significant functional connectivity difference while controlling for the effect of age and gender.
Results
LGI analysis
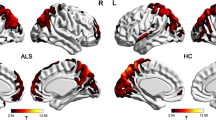
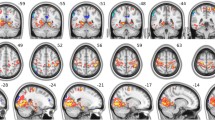
Compared with healthy controls, patients with ALS showed significant LGI reductions in the right occipital cortex (Fig. 1). No region with significantly higher LGI was observed in patients with ALS, relative to healthy controls. In the patient group, vertex-wise correlation analysis revealed a cluster in the left superior frontal gyrus where significant negative correlation was found between the LGI and disease duration (Fig. 2). No significant correlation was observed between LGI and ALSFRS-R in patients with ALS.
Statistical maps of the LGI differences between healthy controls and ALS patients. a Brain regions with cortical gyrification reductions in ALS. The results were corrected for multiple comparisons (P < 0.05, RFT-corrected). The color bar indicates the corrected P-values. b T-statistic of the corresponding between-group contrast (healthy controls-ALS patients)
Negative correlation between the LGI of the left superior frontal gyrus and the duration of illness in ALS. On bottom, we report the results of the vertex-wise correlation analysis. On top, we show the scatterplot of the negative correlation by using the disease duration and average LGI of the significant region in the vertex-wise correlation analysis
Functional connectivity analysis
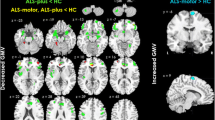
Compared with healthy controls, patients with ALS showed significantly decreased functional connectivity between the right occipital cortex and bilateral precuneus (Fig. 3). In the patient group, no significant correlation was observed between the functional connectivity and clinical data including disease duration and ALSFRS-R.
Discussion
In the present study, a surface-based LGI was applied to quantify the cortical folding in matched samples of ALS patients versus healthy controls. Compared with healthy controls, ALS patients showed significantly reduced LGI in right occipital cortex. In the patient group, significant negative correlation was found between disease duration and the LGI of a cluster in the left superior frontal gyrus. Furthermore, seed-based functional connectivity analysis showed significantly decreased functional connectivity between the right occipital cortex and bilateral precuneus in patients with ALS.
Compared with healthy controls, the present study found significant LGI reductions in the right occipital cortex in patients with ALS. The LGI reductions observed in the visual cortex is in line with previous structural studies, which reported significant reductions of gray matter volume (Bede et al. 2013) and cortical thickness (Agosta et al. 2012; Mezzapesa et al. 2013; Thorns et al. 2013) in patients with ALS. In addition, functional alterations have also been documented in the occipital areas in ALS. For instance, a MR spectroscopy imaging study demonstrated significantly lower NAA/Cho ratio as well as NAA/Cr ratio in multiple brain regions of ALS patients, including several occipital areas (Verma et al. 2013). Using the positron emission tomography (PET) technique, significant cortical glucose hypometabolism was observed in the occipital areas in ALS patients (Pagani et al. 2014; Dalakas et al. 1987). More importantly, a task-based fMRI study found that ALS patients showed reduced BOLD response in secondary visual cortex during visual stimulation, which was suggested to reflect a reduced velocity of information transport in the visual cortex (Lule et al. 2010). In the current study, the observed cortical folding abnormalities measured by LGI provided further structural evidence for the involvement of visual cortex in ALS.
To explore the functional correlate of the gyrification abnormality, seed-based functional connectivity analysis was conducted for the two groups. We found significantly decreased functional connectivity between the right occipital cortex and bilateral precuneus in ALS patients. This finding is partially supported by a previous PET study showing significant hypometabolism in the precuneus, lingual gyrus and right primary visual cortex in ALS patients (Cistaro et al. 2012; Pagani et al. 2014). Additionally, morphological studies reported significant cortical thinning in the precuneus (Schuster et al. 2014b) as well as some occipital areas in ALS patients (Schuster et al. 2014a; Agosta et al. 2012), which might be the anatomical substrate underlying the functional deficits observed in ALS.
In the present study, the cortical gyrification reductions in the visual cortex of the ALS patients appeared to associate closely with the decreased functional connectivity between the visual cortex and precuneus. The exact mechanism underlying the cortical gyrification changes however remains elusive. According to van Essen’s neuromechanical hypothesis (Van Essen 1997), cortical gyrification is mainly driven by the tension of the underlying white matter connectivity (both cortico-cortical and cortico-subcortical). The observed abnormal functional connectivity may reflect lesioned white matter fiber tract in ALS patients since functional connectivity is largely constrained by white matter connectivity (Hagmann et al. 2010). In fact, white matter abnormalities have been frequently reported in ALS. For instance, a neuropathological study reported widespread astrogliosis in the subcortical white matter including the occipital area (Kushner et al. 1991). Diffusion tensor imaging studies demonstrated significantly reduced fractional anisotrophy (FA) in several major fiber tracts in ALS patients (Evans et al. 2015; Ash et al. 2015; Sarro et al. 2011; Zhang et al. 2014a), such as the superior longitudinal fasciculus, inferior frontal-occipital fasciculus. Using voxel-based intensitometry, Hartung et al. revealed widespread white matter intensity increases including the frontal and occipital white matter tracts (Hartung et al. 2014). Hence, although we cannot rule out the involvement of other theories, it is tempting to speculate that the neuromechanical hypothesis (Van Essen 1997) may play a leading role and that the cortical gyrification reductions may be closely related to the functional changes of the underlying fiber tracts.
In the patient group, vertex-wise correlational analysis revealed significant negative correlation between disease duration and the LGI of a cluster in the left superior frontal gyrus, indicating the longer the disease is, the lower LGI is observed in this region. The superior frontal gyrus is involved in higher-order cognitive functions, which were frequently shown to be impaired in ALS (Phukan et al. 2007; Evans et al. 2015). Given previous studies indicated that established patterns of cognitive processes can affect cortical gyrification (Luders et al. 2012; Zhang et al. 2014b), the negative correlation between disease duration and LGI of this region was speculate to reflect the cognitive deterioration in ALS.
There are several limitations that should be addressed. Firstly, as a preliminary study, our results are limited to a small patient cohort, which may have an effect on the power of the statistical analysis of this study. Further studies with more patients are needed to replicate the current findings. Secondly, given the nature of cross-sectional study, the present study does not allow for the investigation of the dynamic development of the structural abnormalities in these patients. Studies with a longitudinal design is needed to unveil the dynamic pattern of the structural abnormalities as the disease progresses. Finally, the absence of detailed non-motor symptoms evaluation, such as visual deficits, weakens the interpretability of our findings.
In conclusion, using a surface-based LGI, the present study investigated the cortical folding pattern in ALS patients as compared matched healthy controls. We found that ALS patients showed significant LGI reductions in the visual cortex. Such structural abnormalities were shown to associate closely with weakened functional connectivity with bilateral precuneus. In the patients, we also observed a significant negative correlation between disease duration and the LGI of a cluster in the left superior frontal gyrus. Taken together, our results suggest that LGI may provide a useful means to assess ALS-related neurodegeneration and to study the pathophysiology of ALS.
References
Agosta, F., Valsasina, P., Riva, N., Copetti, M., Messina, M. J., Prelle, A., Comi, G., & Filippi, M. (2012). The cortical signature of amyotrophic lateral sclerosis. PloS One, 7(8), e42816.
Ash, S., Olm, C., McMillan, C. T., Boller, A., Irwin, D. J., McCluskey, L., Elman, L., & Grossman, M. (2015). Deficits in sentence expression in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis & Frontotemporal Degeneration, 16(1–2), 31–39. doi:10.3109/21678421.2014.974617.
Ashburner, J., & Friston, K. J. (2000). Voxel-based morphometry—the methods. NeuroImage, 11(6), 805–821.
Bede, P., Bokde, A., Elamin, M., Byrne, S., McLaughlin, R. L., Jordan, N., Hampel, H., Gallagher, L., Lynch, C., Fagan, A. J., Pender, N., & Hardiman, O. (2013). Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): a neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. Journal of Neurology, Neurosurgery, and Psychiatry, 84(7), 766–773. doi:10.1136/jnnp-2012-302674.
Brooks, B. R., Miller, R. G., Swash, M., & Munsat, T. L. (2000). El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders: Official Publication of the World Federation of Neurology, Research Group on Motor Neuron Diseases, 1(5), 293–299.
Cedarbaum, J. M., Stambler, N., Malta, E., Fuller, C., Hilt, D., Thurmond, B., & Nakanishi, A. (1999). The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (phase III). Journal of the Neurological Sciences, 169(1–2), 13–21.
Cistaro, A., Valentini, M. C., Chio, A., Nobili, F., Calvo, A., Moglia, C., Montuschi, A., Morbelli, S., Salmaso, D., Fania, P., Carrara, G., & Pagani, M. (2012). Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. European Journal of Nuclear Medicine and Molecular Imaging, 39(2), 251–259. doi:10.1007/s00259-011-1979-6.
Dalakas, M. C., Hatazawa, J., Brooks, R. A., & Di Chiro, G. (1987). Lowered cerebral glucose utilization in amyotrophic lateral sclerosis. Annals of Neurology, 22(5), 580–586. doi:10.1002/ana.410220504.
Evans, J., Olm, C., McCluskey, L., Elman, L., Boller, A., Moran, E., Rascovsky, K., Bisbing, T., McMillan, C. T., & Grossman, M. (2015). Impaired cognitive flexibility in amyotrophic lateral sclerosis. Cognitive and Behavioral neurology: Official Journal of the Society for Behavioral and Cognitive Neurology, 28(1), 17–26. doi:10.1097/WNN.0000000000000049.
Grosskreutz, J., Kaufmann, J., Fradrich, J., Dengler, R., Heinze, H. J., & Peschel, T. (2006). Widespread sensorimotor and frontal cortical atrophy in amyotrophic lateral sclerosis. BMC Neurology, 6, 17. doi:10.1186/1471-2377-6-17.
Hagmann, P., Sporns, O., Madan, N., Cammoun, L., Pienaar, R., Wedeen, V. J., Meuli, R., Thiran, J. P., & Grant, P. E. (2010). White matter maturation reshapes structural connectivity in the late developing human brain. Proceedings of the National Academy of Sciences of the United States of America, 107(44), 19067–19072. doi:10.1073/pnas.1009073107.
Hartung, V., Prell, T., Gaser, C., Turner, M. R., Tietz, F., Ilse, B., Bokemeyer, M., Witte, O. W., & Grosskreutz, J. (2014). Voxel-based MRI intensitometry reveals extent of cerebral white matter pathology in amyotrophic lateral sclerosis. PloS One, 9(8), e104894. doi:10.1371/journal.pone.0104894.
Hayasaka, S., Phan, K. L., Liberzon, I., Worsley, K. J., & Nichols, T. E. (2004). Nonstationary cluster-size inference with random field and permutation methods. NeuroImage, 22(2), 676–687. doi:10.1016/j.neuroimage.2004.01.041.
Hogstrom, L. J., Westlye, L. T., Walhovd, K. B., & Fjell, A. M. (2013). The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cerebral Cortex, 23(11), 2521–2530. doi:10.1093/cercor/bhs231.
Kushner, P. D., Stephenson, D. T., & Wright, S. (1991). Reactive astrogliosis is widespread in the subcortical white matter of amyotrophic lateral sclerosis brain. Journal of Neuropathology and Experimental Neurology, 50(3), 263–277.
Luders, E., Kurth, F., Mayer, E. A., Toga, A. W., Narr, K. L., & Gaser, C. (2012). The unique brain anatomy of meditation practitioners: alterations in cortical gyrification. Frontiers in Human Neuroscience, 6, 34. doi:10.3389/fnhum.2012.00034.
Lule, D., Diekmann, V., Muller, H. P., Kassubek, J., Ludolph, A. C., & Birbaumer, N. (2010). Neuroimaging of multimodal sensory stimulation in amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry, 81(8), 899–906. doi:10.1136/jnnp.2009.192260.
Mezzapesa, D. M., D'Errico, E., Tortelli, R., Distaso, E., Cortese, R., Tursi, M., Federico, F., Zoccolella, S., Logroscino, G., Dicuonzo, F., & Simone, I. L. (2013). Cortical thinning and clinical heterogeneity in amyotrophic lateral sclerosis. PloS One, 8(11), e80748. doi:10.1371/journal.pone.0080748.
Mitchell, J. D., & Borasio, G. D. (2007). Amyotrophic lateral sclerosis. Lancet, 369(9578), 2031–2041. doi:10.1016/S0140-6736(07)60944-1.
Nasreddine, Z. S., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., Cummings, J. L., & Chertkow, H. (2005). The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. doi:10.1111/j.1532-5415.2005.53221.x.
Neary, D., Snowden, J. S., Gustafson, L., Passant, U., Stuss, D., Black, S., Freedman, M., Kertesz, A., Robert, P. H., Albert, M., Boone, K., Miller, B. L., Cummings, J., & Benson, D. F. (1998). Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology, 51(6), 1546–1554.
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113.
Pagani, M., Chio, A., Valentini, M. C., Oberg, J., Nobili, F., Calvo, A., Moglia, C., Bertuzzo, D., Morbelli, S., De Carli, F., Fania, P., & Cistaro, A. (2014). Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology, 83(12), 1067–1074. doi:10.1212/WNL.0000000000000792.
Palaniyappan, L., & Liddle, P. F. (2012). Differential effects of surface area, gyrification and cortical thickness on voxel based morphometric deficits in schizophrenia. NeuroImage, 60(1), 693–699. doi:10.1016/j.neuroimage.2011.12.058.
Panizzon, M. S., Fennema-Notestine, C., Eyler, L. T., Jernigan, T. L., Prom-Wormley, E., Neale, M., Jacobson, K., Lyons, M. J., Grant, M. D., Franz, C. E., Xian, H., Tsuang, M., Fischl, B., Seidman, L., Dale, A., & Kremen, W. S. (2009). Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex, 19(11), 2728–2735. doi:10.1093/cercor/bhp026.
Phukan, J., Pender, N. P., & Hardiman, O. (2007). Cognitive impairment in amyotrophic lateral sclerosis. The Lancet Neurology, 6(11), 994–1003. doi:10.1016/S1474-4422(07)70265-X.
Rajagopalan, V., & Pioro, E. P. (2015). Comparing brain structural MRI and metabolic FDG-PET changes in patients with ALS-FTD: 'the chicken or the egg?' question. Journal of Neurology, Neurosurgery, and Psychiatry, 86(9), 952–958. doi:10.1136/jnnp-2014-308239.
Sarro, L., Agosta, F., Canu, E., Riva, N., Prelle, A., Copetti, M., Riccitelli, G., Comi, G., & Filippi, M. (2011). Cognitive functions and white matter tract damage in amyotrophic lateral sclerosis: a diffusion tensor tractography study. AJNR American Journal of Neuroradiology, 32(10), 1866–1872. doi:10.3174/ajnr.A2658.
Schaer, M., Cuadra, M. B., Tamarit, L., Lazeyras, F., Eliez, S., & Thiran, J. P. (2008). A surface-based approach to quantify local cortical gyrification. IEEE Transactions on Medical Imaging, 27(2), 161–170. doi:10.1109/TMI.2007.903576.
Schuster, C., Kasper, E., Dyrba, M., Machts, J., Bittner, D., Kaufmann, J., Mitchell, A. J., Benecke, R., Teipel, S., Vielhaber, S., & Prudlo, J. (2014a). Cortical thinning and its relation to cognition in amyotrophic lateral sclerosis. Neurobiology of Aging, 35(1), 240–246. doi:10.1016/j.neurobiolaging.2013.07.020.
Schuster, C., Kasper, E., Machts, J., Bittner, D., Kaufmann, J., Benecke, R., Teipel, S., Vielhaber, S., & Prudlo, J. (2014b). Longitudinal course of cortical thickness decline in amyotrophic lateral sclerosis. Journal of Neurology, 261(10), 1871–1880. doi:10.1007/s00415-014-7426-4.
Singh, V., Chertkow, H., Lerch, J. P., Evans, A. C., Dorr, A. E., & Kabani, N. J. (2006). Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer's disease. Brain, 129(Pt 11), 2885–2893. doi:10.1093/brain/awl256.
Stoppel, C. M., Vielhaber, S., Eckart, C., Machts, J., Kaufmann, J., Heinze, H. J., Kollewe, K., Petri, S., Dengler, R., Hopf, J. M., & Schoenfeld, M. A. (2014). Structural and functional hallmarks of amyotrophic lateral sclerosis progression in motor- and memory-related brain regions. NeuroImage Clinical, 5, 277–290. doi:10.1016/j.nicl.2014.07.007.
Thorns, J., Jansma, H., Peschel, T., Grosskreutz, J., Mohammadi, B., Dengler, R., & Munte, T. F. (2013). Extent of cortical involvement in amyotrophic lateral sclerosis–an analysis based on cortical thickness. BMC Neurology, 13, 148. doi:10.1186/1471-2377-13-148.
Van Essen, D. C. (1997). A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature, 385(6614), 313–318. doi:10.1038/385313a0.
Verma, G., Woo, J. H., Chawla, S., Wang, S., Sheriff, S., Elman, L. B., McCluskey, L. F., Grossman, M., Melhem, E. R., Maudsley, A. A., & Poptani, H. (2013). Whole-brain analysis of amyotrophic lateral sclerosis by using echo-planar spectroscopic imaging. Radiology, 267(3), 851–857. doi:10.1148/radiol.13121148.
Witiuk, K., Fernandez-Ruiz, J., McKee, R., Alahyane, N., Coe, B. C., Melanson, M., & Munoz, D. P. (2014). Cognitive deterioration and functional compensation in ALS measured with fMRI using an inhibitory task. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34(43), 14260–14271. doi:10.1523/JNEUROSCI.1111-14.2014.
Zago, S., Poletti, B., Morelli, C., Doretti, A., & Silani, V. (2011). Amyotrophic lateral sclerosis and frontotemporal dementia (ALS-FTD). Archives Italiennes de Biologie, 149(1), 39–56. doi:10.4449/aib.v149i1.1263.
Zhang, J., Yin, X., Zhao, L., Evans, A. C., Song, L., Xie, B., Li, H., Luo, C., & Wang, J. (2014a). Regional alterations in cortical thickness and white matter integrity in amyotrophic lateral sclerosis. Journal of Neurology, 261(2), 412–421. doi:10.1007/s00415-013-7215-5.
Zhang, Y., Zhang, J., Xu, J., Wu, X., Zhang, Y., Feng, H., Wang, J., & Jiang, T. (2014b). Cortical gyrification reductions and subcortical atrophy in Parkinson's disease. Movement Disorders: official Journal of the Movement Disorder Society, 29(1), 122–126. doi:10.1002/mds.25680.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures performed in this study of human participants were in accordance with the ethical standards of the institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all the participants. The study was approved by the Medical Research Ethics Committee of Southwest Hospital, The Third Military Medical University, People’s Republic of China.
Conflict of interest
All authors declare that they have no conflict of interest.
Funding
This work was funded by the National Basic Research Program of China (973 program, Grant No. 2011CB707800), the Natural Science Foundation of China (Grant Nos. 81101000), and Plan A of Science and Technology Support Program from Science and Technology Department of Sichuan Province (Grant No. 2014SZ0014).
Additional information
Yuanchao Zhang and Tao Fang contributed equally to this work and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Zhang, Y., Fang, T., Wang, Y. et al. Occipital cortical gyrification reductions associate with decreased functional connectivity in amyotrophic lateral sclerosis. Brain Imaging and Behavior 11, 1–7 (2017). https://doi.org/10.1007/s11682-015-9499-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-015-9499-9