Abstract
The purpose of this study was to identify areas of abnormal white matter microstructure in adolescents with Major Depressive Disorder (MDD) using diffusion tensor imaging (DTI). Fractional anisotropy (FA) values representing preferential diffusivity along major tracts were examined using tract-based spatial statistics across the whole brain in adolescents ages 13–19 with MDD (n = 31) compared with demographically-matched healthy controls (n = 31). We not only examined frontal lobe tracts that have been most frequently identified as abnormal in previous DTI studies of older depressed patients, but also tested for FA group differences across the whole brain to determine if adolescent depression was related to any other regional white matter abnormality. MDD-diagnosed adolescents had significantly lower FA in many regions concentrated predominantly in the frontal lobe. There also was strong evidence for lower FA in bilateral anterior/posterior limbs of the internal capsules, as well as tracts through the midbrain, left external capsule, right thalamic radiation and left inferior longitudinal fasciculus. Consistent with previous findings in depressed young and elderly adults, the current study found evidence for abnormal microstructure in white matter connections of the frontal lobe in MDD adolescents. There also was strong evidence for FA abnormalities in corpus callosum genu, internal and external capsule tracts, thalamus and midbrain, notable for both the relative magnitude of these effects and absence from most previous white matter studies of depression. These abnormalities might represent important markers of early life-onset depression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Major Depressive Disorder (MDD) is a chronic and severe disorder estimated to affect more than 1 in 30 adolescents at any given time (Costello et al. 2003). Over 8 million people each year develop MDD, while approximately 15 % suffer from MDD during their lifetime (Kessler and Walters 1998). Current theories recognize that both environmental and biological factors contribute to the onset and persistence of depressive illness. Adverse experiences such as early life trauma are thought to potentiate the risk for developing depression by acting through a poorly-understood pre-existing neurobiological liability (Savitz and Drevets 2009). Studies estimate that between 40 % and 50 % of the risk for MDD is genetic in origin (Shih et al. 2004), conferring a higher likelihood of depression when at least one first-degree relative is affected. Epidemiological studies have confirmed across cultures that female, but not male, prevalence rates increase two-fold at puberty, suggesting that biological sex processes which begin at puberty confer higher risk for depression by sex hormones acting on neurobiology (Basser and Pierpaoli 1996; Cullen et al. 2010; Huang et al. 2012). These studies have led researchers to seek to identify brain abnormalities in early life-onset MDD that could represent types of biological liability.
Diffusion tensor imaging (DTI) is a technique to localize white matter abnormalities that could be useful in identifying etiological mechanisms for depression. DTI quantifies microstructural integrity using indices of preferential diffusion such as fractional anisotropy (FA; Basser and Pierpaoli 1996). There has been only one DTI study of 14 adolescents diagnosed with MDD to date (Cullen et al. 2010). FA was examined in two tracts originating from subgenual anterior cingulate, as localized by probabilistic tractography. There was reduced mean FA in one tract projecting to the amygdala. However, evidence for other MDD FA deficits from supplemental voxelwise analysis failed to survive statistical corrections. Another study examined FA deficits in 19 adolescents exposed to childhood maltreatment, but without psychiatric illness, compared to 13 healthy control adolescents (Huang et al. 2012). Those exposed to maltreatment in childhood showed FA deficits in the splenium of the corpus callosum (CC), bilateral superior longitudinal fasciculi, left inferior fronto-occipital fasciculus, and right cingulum bundle down to the hippocampus. Furthermore, the six adolescents who developed MDD and the five who developed a substance use disorder at follow-up (approximately 3.5 years) exhibited FA deficits in the superior longitudinal fasciculi and right cingulum-hippocampal projection compared to those without psychopathology. A similar study examined alterations in white matter integrity in 18 adolescents at high risk (parental depression) for development of MDD to determine whether neural abnormalities preceded the onset of illness (Huang et al. 2011). Lower FA was found in high-risk adolescents in the left cingulum, splenium of the CC, superior longitudinal fasciculi, uncinate and inferior fronto-occipital fasciculi. The majority of other published MDD DTI studies were conducted in elderly depressed patients. These found fairly reliable evidence for white matter abnormalities in the frontal lobe (typically within or proximal to superior and middle frontal gyrus) and temporal lobe regions (Yang et al. 2007; Nobuhara et al. 2006; Bae et al. 2006; Taylor et al. 2004). However, late life-onset depression often results from degenerative processes that affect white matter integrity (Savitz and Drevets 2009). Thus, while certain white matter pathways might be linked to depressive disorder in both aging and youthful samples, pediatric white matter studies are needed to identify any unique neurodevelopmental factors. Another handful of DTI studies have examined depressed young and middle-aged adults. Despite inconsistencies, these also typically find reduced FA in various frontal lobe regions, and mixed evidence for deficits in parietal, occipital, temporal, and subcortical regions (Kieseppa et al. 2010; Li et al. 2007b; Ma et al. 2007; Zou et al. 2008; Zhu et al. 2010; Fig. 1).
The purpose of this study was to use DTI to identify white matter microstructure abnormalities in MDD-diagnosed adolescents, with the hope that better understanding of structural brain abnormalities might clarify specific etiological factors for early life-onset depression. There has not yet been a whole brain DTI study of depressed adolescence, so this effort is needed to ensure understanding of the full scope of possible white matter microstructural abnormalities in this population. Additionally, early-onset MDD might be linked to unique neurobiological risk factors that cannot be inferred from studies of older patient groups. Not only is there a putatively stronger link between etiology and symptoms due to early life-onset, but any early onset-linked abnormalities might be more prominent because younger patients do not possess the same confounds of varying illness course, intervention history, or ongoing neural development that could serve to conceal specific neural correlates if assessed by DTI at older ages. Numerous MDD functional neuroimaging reports support a model of ventral prefrontal hyperfunction along with hypofunction of lateral and dorsomedial prefrontal regions (Mayberg 2009). These observations support a reasonable prediction that disruption of any major connection among these structures might contribute to abnormal neural function, and ultimately to MDD symptom expression. We employed tract-based spatial statistics (TBSS; Smith et al. 2006) to determine if adolescents diagnosed with MDD had abnormalities in any of the brain’s major white matter tracts. Our broad hypothesis was that we would detect abnormalities in adolescent MDD not seen in previous studies of older samples. Plausible candidates for pediatric MDD FA abnormalities included the genu of the CC (connecting cingulate and lateral prefrontal cortex regions across hemispheres), and superior longitudinal fasciculi connecting frontal and parietal brain regions, as these are major pathways connecting brain regions known to function abnormally in MDD. Because no previous DTI study of depression found regions of increased FA, we hypothesized only MDD deficits in these tracts. We also expected to confirm that adolescents with MDD would have reduced FA in other frontal lobe tracts previously reported, especially those which continue to show development into adulthood (Lenroot et al. 2007), reflecting disruption of typical maturation that might worsen long-term prognosis in a way described for early-onset MDD. For instance, the sole previous adolescent DTI MDD study found reduced FA in uncinate fasciculus (connecting amygdala with ventromedial prefrontal cortex), and cingulum bundle (association fibers connecting cingulate to other prefrontal structures, striatal tracts to the caudate, and fibers projecting to the thalamus and brainstem; Cullen et al. 2010). A secondary goal was to begin to explore the relationship of any MDD FA deficits to gender to further understand this important putative etiological factor.
Method
Participants
Thirty-one healthy adolescents (19 females, 12 males) and 31 depressed adolescents (24 females, 7 males) ranging from age 13 to 19 were recruited to participate in this study. Participants with MDD were recruited from inpatient and outpatient clinical services at the Institute of Living at Hartford Hospital, as well as community flyers and internet postings. Healthy control participants were recruited from the community. Potential depressed participants were excluded if they had a current diagnosis of schizophrenia, bipolar disorder, depression with psychotic features, AD/HD, pervasive developmental disorder, or substance dependence. Other psychiatric comorbidities were permitted (e.g., anxiety disorders), provided that MDD appeared to be the primary diagnosis. Healthy controls did not meet criteria for any DSM-IV psychiatric or substance disorder. The study was approved by Hartford Hospital’s Institutional Review Board. For participants ages 18+, signed informed consent was obtained following a meeting that detailed all study procedures and risks. Those under age 18 provided assent, and a parent provided signed permission.
Clinical assessment
DSM-IV Axis I diagnoses were established using the Schedule of Affective Disorders and Schizophrenia for Children-Present and Lifetime Version (K-SADS-PL; Kaufman et al. 1997) for participants under age 18, and the Structured Clinical Interview for DSM-IV Disorders (SCID-IV) for participants aged 18 and over. All psychiatric interviews were conducted by trained research assistants under the supervision of a licensed psychologist (MCS) with over 8 years of experience supervising these instruments’ use. Diagnoses were established following detailed case discussion in weekly diagnostic consensus meetings. The Beck Depression Inventory-II (BDI-II) further quantified the severity of depressive symptoms (Beck et al. 1961). For all participants, health status was assessed to rule out possible CNS abnormalities through interview, parent-completed questionnaire, and available patient collateral file review.
Demographic and major clinical characteristics of the two study groups are shown in Table 1, including medication information in the MDD sample. The groups did not differ on age, proportion of gender, or race. Consistent with the primary study grouping criteria, the groups differed on DSM-IV MDD symptom count, BDI-II scores and number of suicide attempts (Table 1). Not unexpectedly, the proportion of comorbid alcohol abuse and anxiety diagnoses in MDD participants also was significantly greater than controls. Fifteen of the MDD participants (48.4 %) were taking antidepressant medications at the time of the study, mostly one of a handful of commonly-prescribed SSRIs.
Neuroimaging
Magnetic resonance imaging (MRI) data were obtained on a Siemens 3T Allegra MRI machine located at the Olin Neuropsychiatry Research Center at The Institute of Living/Hartford Hospital. The pulse sequence was single-shot spin echo EPI sequence, TR/TE = 6,300/82 ms, FOV = 200 mm, matrix = 128 × 128, 8 averages, diffusion sensitizing orientations = 12 with b = 1,000 s/mm2, and one with b = 0 s/mm2, 45 contiguous axial slices with 1.6 × 1.6 × 3.0 mm resolution. To minimize blood flow and CSF pulsation effects, scanning was gated with peripheral arterial pulse. Total scan time was 11:02 min.
MRI DTI images were prepared for analysis using several steps within the FMRIB Software Library v5.0 (FSL; Jenkinson et al. 2012). An initial data quality check ensured that gradient direction measurements with excessive motion artifacts or noise were identified and removed. Next, motion and eddy current correction was done by registering diffusion weighted images to a common non-diffusion weighted image by FLIRT/FSL with a mutual information cost function. We then calculated diffusion tensor and scalar FA measures for voxels within a mask created from the B0 image. These data were spatially normalized to a common Montreal Neurological Institute (MNI) space template using FNIRT/FSL. For TBSS, we calculated a subject specific mean FA image from the 41 participants, then applied tract based spatial statistics (TBSS/FSL) method (Smith et al. 2006) to calculate subject specific skeletons. The TBSS algorithm searches for the maximum FA in the direction perpendicular to the skeleton within a limited range and projects it onto the skeleton. Thus, we created subject-specific FA values defined on one common skeleton for the whole group. These skeletonized FA images were used in subsequent random effects analyses to test study hypotheses.
Study analyses
Study hypotheses were tested using two-sample t test on participants’ individual FA skeleton images using FSL’s ‘randomise’ program (Jenkinson et al. 2012). Relevant t contrasts were Controls > Depressed and Controls < Depressed. Statistical significance was evaluated using non-parametric, permutation-based (5,000 permutations) threshold-free cluster enhancement (TFCE; Smith and Nichols 2009), using options that optimally evaluated the 2D connectivity structure found in skeletonized images. Primary group contrast results are presented if they survived corrections for searching the entire skeleton, p < .05 TFCE. Findings were illustrated using relevant 3 mm axial slices after “thickening” the comparison results for display purposes by smoothing the statistical image and multiplying by the mean FA image to constrain the width of the enlarged clusters. We also computed Cohen’s d effect sizes (Cohen 1988) based upon the uncorrected peak t statistic for reported clusters (\( d=2t/\sqrt{ df} \)) for all significant findings to help differentiate weaker findings from more robust effects that might best guide future research efforts. We also performed an exploratory post hoc analysis to examine FA gender differences within the sample of depressed adolescents to determine whether any MDD abnormalities were more severe in either subgroup. Because of the exploratory nature of this analysis, we examined only white matter regions on the FA skeleton where MDD < controls in the overall study analysis, using liberal thresholds (i.e., p < .05 uncorrected, extent 10 contiguous voxels). Finally, because there is little guidance from published reports as to whether a history of antidepressant medication might affect DTI-measured white matter microstructure, we contrasted MDD-diagnosed participants who were or were not undergoing antidepressant treatment (see Table 1) at the time of the study to ensure medication status did not influence study findings. This comparison was evaluated using both TFCE and uncorrected (p < .05, extent 10 contiguous voxels) levels of significance.
Results
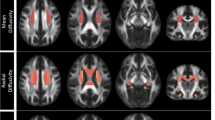
FSL analyses on the skeletonized FA maps found multiple clusters where FA was significantly reduced in MDD-diagnosed adolescents compared to controls. Corticospinal tracts with abnormal FA in depressed teens included bilateral anterior and posterior limbs of the internal capsule, right anterior and left posterior thalamic radiations, left external capsule, bilateral corticospinal and pontine crossing tracts through the brainstem, bilateral tracts in the middle cerebellar peduncle and crus, and bilateral superior and anterior corona radiata. Abnormal inter-hemispheric connections in MDD included reduced FA in the body and genu of the CC, extending down into left posterior cingulum, bilateral medial frontal and bilateral pregenual cingulate. There also were significant clusters of reduced FA in the left inferior longitudinal fasciculus, bilateral inferior fronto-occipital fasciculi, and left uncinate fasciculus. There were no significant findings of greater FA in MDD relative to controls.
Clusters labels, peak t scores from group comparison, TFCE-corrected significance levels, and Cohen’s d effect sizes for all findings are reported in Table 2. Tract labels were taken from the JHU atlas within FSL combined with visual inspection of results with reference to published stereotactic atlases (Mori et al. 2005). However, given that there was no probabilistic tractography used to confirm projections, some labels should be viewed as preliminary.
Post hoc analysis identified numerous areas where MDD abnormality differed between males and females (Table 2). To summarize, females showed lower FA in three clusters in the region of the right thalamus, three clusters in the left cingulum, right CC, bilateral orbitofrontal, left inferior frontal gyrus, and left anterior portion of the uncinate fasciculus. Males had decreased white matter FA compared to females in the left thalamus, left inferior frontal pole, left inferior longitudinal fasciculus, and right inferior fronto-occipital fasciculus.
We found no regions where MDD-diagnosed antidepressant-treated adolescents and unmedicated patients had different FA after TFCE-corrections for searching whole white matter skeleton. Only one region was found at uncorrected significance levels (p < .05, extent 10 voxels), where unmedicated MDD had greater FA than medicated; x,y,z = 10, 4, 50, t = 3.63 proximal to the superior corona radiata. In the absence of any strong evidence, we concluded that medication status was unrelated to our findings.
Discussion
The primary purpose of this first-ever whole brain DTI study of major white matter tracts was to characterize the extent of white matter microstructure abnormalities in the brains of adolescents diagnosed with MDD. As expected, we found evidence for decreased FA in several tracts connecting the frontal lobe to other brain regions. Our primary study hypothesis was that we would detect abnormally low MDD FA in tracts not consistently implicated in previous DTI studies of older depressed persons that could reflect unique neurobiological correlates of early-onset MDD. Consistent with this, we found significantly decreased adolescent MDD FA in CC genu and body, midbrain white matter tracts, and corticospinal tracts, as well as the middle frontal gyrus.
MDD FA was reduced bilaterally in multiple clusters near the thalamus, the corticospinal tracts and anterior and posterior limbs of the internal capsule. The internal capsule contains numerous segregated pathways that interconnect diverse cortical regions with the thalamus, of which some ultimately project to the brainstem (Mori et al. 2005; Schmahmann and Pandya 2006). Given that disrupted tract integrity is thought to hinder neurotransmission among widespread, distributed networks, these abnormalities could compromise various fronto-thalamic circuits. Interestingly, Zou et al. (2008) linked depression severity directly to the degree of FA reduction in the internal capsule, further emphasizing the likely importance of this abnormality.
The CC is the largest fiber bundle in the human brain, whose commissural fibers interconnect the frontal lobes. From detailed analysis of rhesus monkey white matter architecture, genu fibers have been found to specifically connect homotypic lateral prefrontal, caudate cingulate (BA 24) and supplemental motor area structures between the two hemispheres (Schmahmann and Pandya 2006). We detected genu and body MDD deficits, likely innervating ventromedial prefrontal cortex regions. Two studies to date have found deficits throughout the CC, although these studies were with older recurrently depressed adults and bipolar adolescents (Cole et al. 2012; Barnea-Goraly et al. 2009). Specifically in MDD, two studies have found reduced FA in the body of the CC with middle-aged adults (Kieseppa et al. 2010) and younger treatment-resistant adults (Guo et al. 2012a). Another treatment-resistant MDD young adult study also found callosal deficits (Li et al. 2007a). Deficits in the body of the CC raise interesting questions about shared biological liability across putatively different affective disorders for chronicity, recurrence or treatment response. Young adults with treatment-responsive MDD only showed deficits in the genu (Guo et al. 2012b). These findings also suggest that genu deficits could be related to an earlier onset of affective disorders.
Midbrain or brainstem white matter microstructure abnormalities also have not typically been reported in DTI studies of older depressed adults. Some previous studies specifically seeking reduced FA in MDD brainstem regions have reported null results (Steele et al. 2005). We found evidence for MDD adolescent FA reductions in the medial cerebellar peduncle within the midbrain, along with other midbrain and brainstem corticospinal tract structures. The medial cerebellar peduncle forms the major connection from the pons to the cerebellum, relaying information from the forebrain. Future studies will need to determine whether these midbrain/brainstem abnormalities impact ascending dopamine and noradrenergic innervation of ventral striatal reward, basal forebrain, and cortical control systems of the brain. Finally, we also found relatively novel evidence for adolescent MDD FA deficits in the corticospinal projections proximal to the thalamus, and in left inferior longitudinal fasciculus connecting temporal and occipital lobe regions. The functional significance of these findings remains to be determined.
Collectively, these results suggest a greater involvement of brainstem, midbrain, and CC white matter pathology in early life-onset MDD compared to that reported in previous studies of older depressed patients. Because this was not a prospective study, we could not determine whether these deficits represent developmental delays, i.e., those which resolve in early adulthood following the normal late adolescent peak of white matter development (Schmithorst and Yuan 2010), or represent static deficits marking persistent risk for MDD across the lifespan. For instance, previous DTI MDD studies have frequently implicated uncinate and cingulum bundle abnormality regardless of patient age, suggesting these might be reliable disorder risk markers. In contrast, depression in the elderly appears most often associated with white matter microstructural deficits in lobar white matter and other cingulate connections (Bae et al. 2006). Those specific frontal lobe abnormalities have been observed only in the most severely affected or youngest adult age range patients (Li et al. 2007a, b; Ma et al. 2007). These age-discrepancies probably in part reflect different causal mechanisms, but this must be ascertained in future longitudinal designs. Neurodegenerative factors (e.g., periventricular white matter disease, ischemic injury, etc.) are believed to more commonly compromise white matter in late-onset MDD (Santos et al. 2009), whereas neurodevelopmental abnormalities yet to be fully described likely underlie the white matter abnormalities observed in early-onset MDD.
In addition, our post hoc analyses found that gender was linked to the severity of MDD deficits throughout the brain. For example, we found greater FA deficits in MDD females in the anterior corona radiata bilaterally, whereas MDD males had greater FA deficits in the anterior and posterior limbs of the internal capsule bilaterally. White matter development curves predict that adolescent males will continue to have higher white matter FA than females (Schmithorst and Yuan 2010), thus this study’s findings of regionally-specific higher FA in males most likely corresponds to normal developmental differences. However, this study’s finding that MDD females show regionally-specific higher FA than males may point to white matter developmental abnormalities, as some of these regions (e.g., inferior fronto-occipital fasciculus, posterior limbs of the internal capsule, and corticospinal tracts) have shown continued development into adulthood in males (Bava et al. 2010; Perrin et al. 2009; Lenroot et al. 2007). Many of these regions have also been previously implicated in depression (Cullen et al. 2010; Zou et al. 2008; Zhu et al. 2010), and emotion regulation (Kieseppa et al. 2010), and thus suggest regions of biological liability to depression that might be more important for boys at risk for depression. Future research to confirm whether these specific white matter structures are targets of gender-specific interactions with environmental stressors or other forms of biological liability due to developmental abnormalities or familial history are needed to carefully untangle these complex effects. These results provide a starting place for such studies.
It also is hoped this study can help further develop neurobiological theories of early life-onset depression. For instance, there has been increasing interest in the study of psychiatric illness endophenotypes. Endophenotypes are measurable abnormalities presumably closer to the genetic causes of complex neuropsychiatric illness than are variable clinical phenotypes (Cannon and Keller 2006). It might be productive to link specific white matter tract abnormalities to proposed cognitive endophenoptyes in the context of distributed networks linked to affective processing bias or compromised emotional regulation ability. Though highly important, this was outside the scope of our available dataset. If such links could be verified, it would provide frameworks to test models of neural circuit dysfunction related to different biological factors, and in turn specific genetic profiles. As noted by many others, some of the tracts found to be abnormal in depression are major pathways between abnormally-functioning brain regions in MDD-diagnosed samples (Mayberg 2009). Future functional connectivity (e.g., Cullen et al. 2009) and multimodal neuroimaging studies that link anatomical and functional measures of network connectivity could determine whether the degree of network disruption among brain regions related to a specific endophenotype is directly related to DTI-indexed abnormality among two network nodes. The current results also might help direct the ongoing development of novel therapeutic techniques, e.g., suggest novel targets for deep brain stimulation (Mayberg 2009).
To our knowledge, this is only the second study to examine white matter microstructure in depressed adolescents. Importantly, we replicated the findings from that study (Cullen et al. 2010), including FA deficits in left middle frontal gyrus, left uncinate fasciculus, and left cingulum bundle (albeit in slightly different parts of these structures than found in that study). The uncinate fasciculus connects parts of the limbic system (e.g., the hippocampus and amygdala) with the frontal lobe, while the cingulum projects from the cingulate gyrus to medial temporal lobe regions of the brain. The consistency not only is important in understanding the neural correlates of adolescent MDD, but also bolsters confidence in our novel findings. In contrast to Cullen et al. (2010), we detected numerous FA reductions at whole brain significance thresholds, probably due to increased power from our larger sample size. TBSS allowed us to examine major tracts across the whole brain, including smaller tracts like the fornix that can be missed with other voxelwise approaches that require spatial smoothing. Future tractography studies are still needed to identify the origin and termination of abnormal pathways, which would support arguments for the functional relevance of these abnormalities.
The key study limitation is that DTI is not able to conclusively determine the mechanism underlying reduced FA. Although reduced FA is often taken as evidence for lower myelination, it can reflect loss of axons, gliosis, edema/hydration, cell-packing density, or even fiber-orientation (Barkovich 2000; Madler et al. 2008; Shimony et al. 1999; Virta et al. 1999). Understanding the nature of microstructural abnormality is crucial if we are to clarify the complex interplay of biological liability for depression and neurodevelopment. For instance, DTI studies of white matter development suggest that normative FA increases in adolescence might reflect axonal diameter growth not just myelination (Giorgio et al. 2010; Paus 2010). If some of the MDD FA deficits found here reflect disruption of axonal growth, while others involve disordered myelination, only careful study within a developmental framework can effectively tease such factors apart. Recent insights into the genetic control of white matter development (Emery 2010; Feng 2008) also might represent a research avenue that can clarify complex relationships among abnormality, neurodevelopment, and disorder expression. Finally, although some evidence has pointed towards a differential sensitivity of other DTI indices to specific types of microstructural abnormality (e.g., axial diffusivity, radial diffusivity, mean diffusivity), we chose not to examine these indices in this study due to an absence of a priori hypotheses, and instead pursued our primary goal of providing a more extensive characterization of FA abnormalities that has yet been made available.
Other study limitations were that MDD illness duration was not assessed and the sample had a highly variable antidepressant treatment history. Some studies have shown grey matter volume deficits normalize in MDD successfully treated with antidepressants (Smith et al. 2013) or antidepressant-resistant MDD (Phillips et al. 2012). Because there also is emerging evidence that abnormal MDD white matter volume normalizes with antidepressant treatment (Zeng et al. 2012), the influence of medication usage on white matter microstructure in MDD must be carefully teased apart. A rigorous evaluation of this was not possible in our sample—half the MDD-diagnosed participants were currently on antidepressants, none had yet achieved remission of symptoms, and their previous MDD medication histories were not systematically ascertained. However, we did not observe any meaningful difference in DTI-measured FA between currently medicated and unmedicated MDD patients. A final noteworthy issue is that roughly a third of the sample was comorbid for anxiety disorders. This raises important questions about etiological specificity of these findings in the context of ongoing research into mixed anxiety and depression presentations (Hettema 2008).
In conclusion, this study provides important new information to help researchers understand the contribution of white matter microstructural abnormalities to early-onset mood disorder. We have linked several novel white matter abnormalities to risk for early-onset MDD, providing specific targets for future theory development and empirical study of mechanisms that might produce the white matter structural deficits observed here.
References
Bae, J. N., MacFall, J. R., Krishnan, K. R., Payne, M. E., Steffens, D. C., & Taylor, W. D. (2006). Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biological Psychiatry, 60(12), 1356–1363. doi:10.1016/j.biopsych.2006.03.052.
Barkovich, A. J. (2000). Concepts of myelin and myelination in neuroradiology. American Journal of Neuroradiology, 21(6), 1099–1109.
Barnea-Goraly, N., Chang, K. D., Karchemskiy, A., Howe, M. E., & Reiss, A. L. (2009). Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biological Psychiatry, 66(3), 238–244. doi:10.1016/j.biopsych.2009.02.025.
Basser, P. J., & Pierpaoli, C. (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. Series B, 111(3), 209–219.
Bava, S., Thayer, R., Jacobus, J., Ward, M., Jernigan, T. L., & Tapert, S. F. (2010). Longitudinal characterization of white matter maturation during adolescence. Brain Research, 1327, 38–46. doi:10.1016/j.brainres.2010.02.066.
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J., & Erbaugh, J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571.
Cannon, T. D., & Keller, M. C. (2006). Endophenotypes in the genetic analyses of mental disorders. Annual Review of Clinical Psychology, 2, 267–290. doi:10.1146/annurev.clinpsy.2.022305.095232.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale: L. Erlbaum Associates.
Cole, J., Chaddock, C. A., Farmer, A. E., Aitchison, K. J., Simmons, A., McGuffin, P., et al. (2012). White matter abnormalities and illness severity in major depressive disorder. British Journal of Psychiatry, 201(1), 33–39. doi:10.1192/bjp.bp.111.100594.
Costello, E. J., Mustillo, S., Erkanli, A., Keeler, G., & Angold, A. (2003). Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry, 60(8), 837–844. doi:10.1001/archpsyc.60.8.837.
Cullen, K. R., Gee, D. G., Klimes-Dougan, B., Gabbay, V., Hulvershorn, L., Mueller, B. A., et al. (2009). A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Letters, 460(3), 227–231. doi:10.1016/j.neulet.2009.05.022.
Cullen, K. R., Klimes-Dougan, B., Muetzel, R., Mueller, B. A., Camchong, J., Houri, A., et al. (2010). Altered white matter microstructure in adolescents with major depression: a preliminary study. Journal of the American Academy of Child and Adolescent Psychiatry, 49(2), 173–183. e1. doi:10.1016/j.jaac.2009.11.005.
Emery, B. (2010). Regulation of oligodendrocyte differentiation and myelination. Science, 330(6005), 779–782. doi:10.1126/science.1190927.
Feng, Y. (2008). Convergence and divergence in the etiology of myelin impairment in psychiatric disorders and drug addiction. Neurochemical Research, 33(10), 1940–1949. doi:10.1007/s11064-008-9693-x.
Giorgio, A., Watkins, K. E., Chadwick, M., James, S., Winmill, L., Douaud, G., et al. (2010). Longitudinal changes in grey and white matter during adolescence. NeuroImage, 49(1), 94–103. doi:10.1016/j.neuroimage.2009.08.003.
Guo, W. B., Liu, F., Chen, J. D., Xu, X. J., Wu, R. R., Ma, C. Q., et al. (2012a). Altered white matter integrity of forebrain in treatment-resistant depression: a diffusion tensor imaging study with tract-based spatial statistics. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 38(2), 201–206. doi:10.1016/j.pnpbp.2012.03.012.
Guo, W. B., Liu, F., Xue, Z. M., Gao, K., Wu, R. R., Ma, C. Q., et al. (2012b). Altered white matter integrity in young adults with first-episode, treatment-naive, and treatment-responsive depression. Neuroscience Letters, 522(2), 139–144. doi:10.1016/j.neulet.2012.06.027.
Hettema, J. M. (2008). The nosologic relationship between generalized anxiety disorder and major depression. Depression and Anxiety, 25(4), 300–316. doi:10.1002/da.20491.
Huang, H., Fan, X., Williamson, D. E., & Rao, U. (2011). White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Neuropsychopharmacology, 36(3), 684–691. doi:10.1038/npp.2010.199.
Huang, H., Gundapuneedi, T., & Rao, U. (2012). White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Neuropsychopharmacology, 37(12), 2693–2701. doi:10.1038/npp.2012.133.
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., & Smith, S. M. (2012). Fsl. [Historical article review]. NeuroImage, 62(2), 782–790. doi:10.1016/j.neuroimage.2011.09.015.
Kaufman, J., Birmaher, B., Brent, D., Rao, U., Flynn, C., Moreci, P., et al. (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988. doi:10.1097/00004583-199707000-00021.
Kessler, R. C., & Walters, E. E. (1998). Epidemiology of DSM-III-R major depression and minor depression among adolescents and young adults in the National Comorbidity Survey. Depression and Anxiety, 7(1), 3–14. doi:10.1002/(SICI)1520-6394(1998)7:1<3::AID-DA2>3.0.CO;2-F.
Kieseppa, T., Eerola, M., Mantyla, R., Neuvonen, T., Poutanen, V. P., Luoma, K., et al. (2010). Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. Journal of Affective Disorders, 120(1–3), 240–244. doi:10.1016/j.jad.2009.04.023.
Lenroot, R. K., Gogtay, N., Greenstein, D. K., Wells, E. M., Wallace, G. L., Clasen, L. S., et al. (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage, 36(4), 1065–1073. doi:10.1016/j.neuroimage.2007.03.053.
Li, C., Sun, X., Zou, K., Yang, H., Huang, X., Wang, Y., et al. (2007a). Voxel based analysis of DTI in depression patients. International Journal of Magnetic Resonance Imaging, 1(1), 43–48.
Li, L., Ma, N., Li, Z., Tan, L., Liu, J., Gong, G., et al. (2007b). Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Research, 1168, 124–128. doi:10.1016/j.brainres.2007.06.094.
Ma, N., Li, L., Shu, N., Liu, J., Gong, G., He, Z., et al. (2007). White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. The American Journal of Psychiatry, 164(5), 823–826. doi:10.1176/appi.ajp.164.5.823.
Madler, B., Drabycz, S. A., Kolind, S. H., Whittall, K. P., & MacKay, A. L. (2008). Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magnetic Resonance Imaging, 26(7), 874–888. doi:10.1016/j.mri.2008.01.047.
Mayberg, H. S. (2009). Targeted electrode-based modulation of neural circuits for depression. Journal of Clinical Investigation, 119(4), 717–725. doi:10.1172/JCI38454.
Mori, S., Wakana, S., Nagae-Poetscher, L. M., & van Zijl, P. C. M. (2005). MRI atlas of human white matter. Amsterdam: Elsevier, B.V.
Nobuhara, K., Okugawa, G., Sugimoto, T., Minami, T., Tamagaki, C., Takase, K., et al. (2006). Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. Journal of Neurology, Neurosurgery and Psychiatry, 77(1), 120–122. doi:10.1136/jnnp.2004.055129.
Paus, T. (2010). Growth of white matter in the adolescent brain: myelin or axon? Brain and Cognition, 72(1), 26–35. doi:10.1016/j.bandc.2009.06.002.
Perrin, J. S., Leonard, G., Perron, M., Pike, G. B., Pitiot, A., Richer, L., et al. (2009). Sex differences in the growth of white matter during adolescence. NeuroImage, 45(4), 1055–1066. doi:10.1016/j.neuroimage.2009.01.023.
Phillips, J. L., Batten, L. A., Aldosary, F., Tremblay, P., & Blier, P. (2012). Brain-volume increase with sustained remission in patients with treatment-resistant unipolar depression. [Research Support, Non-U.S. Gov’t]. Journal of Clinical Psychiatry, 73(5), 625–631. doi:10.4088/JCP.11m06865.
Santos, M., Kovari, E., Hof, P. R., Gold, G., Bouras, C., & Giannakopoulos, P. (2009). The impact of vascular burden on late-life depression. Brain Research Reviews, 62(1), 19–32. doi:10.1016/j.brainresrev.2009.08.003.
Savitz, J., & Drevets, W. C. (2009). Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neuroscience and Biobehavioral Reviews, 33(5), 699–771. doi:10.1016/j.neubiorev.2009.01.004.
Schmahmann, J., & Pandya, D. (2006). Fiber pathways of the brain. New York: Oxford University Press, Inc.
Schmithorst, V. J., & Yuan, W. (2010). White matter development during adolescence as shown by diffusion MRI. Brain and Cognition, 72(1), 16–25. doi:10.1016/j.bandc.2009.06.005.
Shih, R. A., Belmonte, P. L., & Zandi, P. P. (2004). A review of the evidence from family, twin and adoption studies for a genetic contribution to adult psychiatric disorders. International Review of Psychiatry, 16(4), 260–283. doi:10.1080/09540260400014401.
Shimony, J. S., McKinstry, R. C., Akbudak, E., Aronovitz, J. A., Snyder, A. Z., Lori, N. F., et al. (1999). Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology, 212(3), 770–784. doi:10.1148/radiology.212.3.r99au51770.
Smith, S. M., & Nichols, T. E. (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. doi:10.1016/j.neuroimage.2008.03.061.
Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., et al. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage, 31(4), 1487–1505. doi:10.1016/j.neuroimage.2006.02.024.
Smith, R., Chen, K., Baxter, L., Fort, C., & Lane, R. D. (2013). Antidepressant effects of sertraline associated with volume increases in dorsolateral prefrontal cortex. [Research Support, Non-U.S. Gov’t]. Journal of Affective Disorders, 146(3), 414–419. doi:10.1016/j.jad.2012.07.029.
Steele, J. D., Bastin, M. E., Wardlaw, J. M., & Ebmeier, K. P. (2005). Possible structural abnormality of the brainstem in unipolar depressive illness: a transcranial ultrasound and diffusion tensor magnetic resonance imaging study. Journal of Neurology, Neurosurgery and Psychiatry, 76(11), 1510–1515. doi:10.1136/jnnp.2004.057612.
Taylor, W. D., MacFall, J. R., Payne, M. E., McQuoid, D. R., Provenzale, J. M., Steffens, D. C., et al. (2004). Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. The American Journal of Psychiatry, 161(7), 1293–1296. doi:10.1176/appi.ajp.161.7.1293.
Virta, A., Barnett, A., & Pierpaoli, C. (1999). Visualizing and characterizing white matter fiber structure and architecture in the human pyramidal tract using diffusion tensor MRI. Magnetic Resonance Imaging, 17(8), 1121–1133. doi:10.1016/S0730-725X(99)00048-X.
Yang, Q., Huang, X., Hong, N., & Yu, X. (2007). White matter microstructural abnormalities in late-life depression. International Psychogeriatrics, 19(4), 757–766. doi:10.1017/S1041610207004875.
Zeng, L. L., Liu, L., Liu, Y., Shen, H., Li, Y., & Hu, D. (2012). Antidepressant treatment normalizes white matter volume in patients with major depression. [Research Support, Non-U.S. Gov’t]. PLoS One, 7(8), e44248. doi:10.1371/journal.pone.0044248.
Zhu, X., Wang, X., Xiao, J., Zhong, M., Liao, J., & Yao, S. (2010). Altered white matter integrity in first-episode, treatment-naive young adults with major depressive disorder: a tract-based spatial statistics study. Brain Research, 1369, 223–229. doi:10.1016/j.brainres.2010.10.104.
Zou, K., Huang, X., Li, T., Gong, Q., Li, Z., Ou-yang, L., et al. (2008). Alterations of white matter integrity in adults with major depressive disorder: a magnetic resonance imaging study. Journal of Psychiatry and Neuroscience, 33(6), 525–530.
Acknowledgments
This study was funded by K23 MH070036 (PI Stevens) and funding from the American Foundation for Suicide Prevention.
Disclosures
Dr. Stevens, Dr. Caprihan, Ms. Nave and Ms. Bessette have no competing interests
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bessette, K.L., Nave, A.M., Caprihan, A. et al. White matter abnormalities in adolescents with major depressive disorder. Brain Imaging and Behavior 8, 531–541 (2014). https://doi.org/10.1007/s11682-013-9274-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-013-9274-8