Abstract
Depressive mood in adolescents with bipolar disorder (BDd) is associated with significant morbidity and mortality, but we have limited information about neural correlates of depression and treatment response in BDd. Ten adolescents with BDd (8 females, mean age = 15.6 ± 0.9) completed two (fearful and happy) face gender labeling fMRI experiments at baseline and after 6-weeks of open treatment. Whole-brain analysis was used at baseline to compare their neural activity with those of 10 age and sex-matched healthy controls (HC). For comparisons of the neural activity at baseline and after treatment of youth with BDd, region of interest analysis for dorsal/ventral prefrontal, anterior cingulate, and amygdala activity, and significant regions identified by wholebrain analysis between BDd and HC were analyzed. There was significant improvement in depression scores (mean percentage change on the Child Depression Rating Scale-Revised 57 % ± 28). Neural activity after treatment was decreased in left occipital cortex in the intense fearful experiment, but increased in left insula, left cerebellum, and right ventrolateral prefrontal cortex in the intense happy experiment. Greater improvement in depression was associated with baseline higher activity in ventral ACC to mild happy faces. Study sample size was relatively small for subgroup analysis and consisted of mainly female adolescents that were predominantly on psychotropic medications during scanning. Our results of reduced negative emotion processing versus increased positive emotion processing after treatment of depression (improvement of cognitive bias to negative and away from positive) are consistent with the improvement of depression according to Beck’s cognitive theory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bipolar Disorder (BD) is a chronic and debilitating illness (Birmaher and Axelson 2006). Depression is the most common manifestation of mood state in BD and is associated with an increased risk for suicide and psychosocial impairment (Chang 2009). Recent research studies have started to identify patterns of neural abnormalities in euthymic and manic adolescents with BD relative to healthy controls (HC) (Leibenluft and Rich 2008). These findings have contributed to elucidate mood state- versus illness-specific neural abnormalities in BD (Passarotti et al. 2011; Pavuluri et al. 2010a, b), yet the neural correlates of depressed mood or treatment response in depressed BD (BDd) adolescents remain largely unexplored (Chang et al. 2008; Diler 2011). Moreover, the few available studies in adults with BDd indicated that there is important variability in the activation patterns of amygdala and prefrontal neural findings (Liu et al. 2012; Townsend and Altshuler 2012). Examining neural circuitry in response to emotional stimuli during depression and after its treatment may help understand neurodevelopmental etiology of BD and identify state neural markers of depression in youth with BD (Delbello and Strakowski 2004). In addition, this may help to identify biomarkers of treatment response with the potential to contribute to more effective and individualized treatments (Blumberg et al. 2003; Ketter and Wang 2002; Pavuluri et al. 2010c; Rich et al. 2006). The main goal of this preliminary study was to explore the neural correlates of depression at baseline and after 6 weeks of open as usual treatment in adolescents with BDd relative to age- and sex-matched 10 HC adolescents. Consistent with Beck’s cognitive theory that suggests attention to negative but away from positive stimuli during depression (Disner et al. 2011), we hypothesized that the neural activity in regions involved in ventral affective circuitry will be higher to negative emotional stimuli but lower to positive emotional stimuli at baseline relative to HC; and the neural activity from baseline to after treatment will be decreased to negative but increased to positive emotional stimuli.
Methods
Study design
Adolescents with BDd were scanned at baseline and after 6 weeks of open as usual treatment while completing a well-validated measure of implicit emotion processing (e.g., gender identification emotional processing task) (Ladouceur et al. 2011; Liu et al. 2012). Healthy control (HC) adolescents were only scanned at baseline. All adolescents and their parents gave informed consent. The University of Pittsburgh Institutional Review Board approved the study and consent forms. Adolescents with BDd met Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) (APA 2004) criteria for BD I/II depressive episode (e.g., duration of at least 2 weeks) as determined by parent and adolescent interviews with the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL) (Kaufman et al. 1997). As described in detail in a prior publication, operationalized criteria were used to diagnose BD-NOS (Axelson et al. 2006). In addition, reviewing the past 2 weeks, a total score on the Children’s Depression Rating Scale-Revised (Poznanski and Mokros 1995) (CDRS-R) ≥ 40 and a total score on the Young Mania Rating Scale (Young et al. 1978) (YMRS) <11 were required for the adolescents with BDd on the day of fMRI. In addition, we measured anxiety with a self-rated anxiety scale (Screen for Child Anxiety Related Emotional Disorders; SCARED) (Birmaher et al. 1997). Urine screening to exclude pregnancy and illicit substance abuse was performed before the scanning. We excluded adolescents with psychotic disorders, pervasive developmental disorders, eating disorders, substance use disorders, learning disorders, and mental retardation. No personal or family psychiatric history was allowed for HC and we excluded adolescents with any contraindications for the fMRI (e.g., braces). Adolescents were reimbursed for their time and participation.
Subjects
Ten right-handed adolescents with BDd (3 BD type I, 4 BD type II, and 3 BD NOS; 8 females, mean age = 15.6 ± 0.9), and 10 right-handed HC adolescents (8 females, mean age = 15.6 ± 1.2) ages 12 to 17 and a Tanner’s Pubertal Development Scale ≥ 3 (Marshall and Tanner 1969) were included. They were not a part of previous research studies and recruited from inpatient (N = 5) and outpatient (N = 5) Child and Adolescent Bipolar Services at University of Pittsburgh. Adolescents with BDd were allowed to be on psychotropic medications (up to 3 non-stimulant medications were allowed: 3 adolescents with BDd were free of medications at baseline and all adolescents were on medications after 6 weeks of treatment). Three adolescents with BDd were on stimulants that were held 24 h before scanning. All adolescents with BDd received individual psychotherapy through their providers plus medication management during the 6 weeks of open as usual treatment: Four adolescents were started on a new medication (two with lamotrigine, one with quetiapine, and one with aripiprazole), five adolescents remained on the same medication combinations (one with lamotrigine + quetiapine, one with lamotrigine + aripiprazole, one with citalopram + aripiprazole, one with lithium + quetiapine, and one with lamotrigine + valproic acid + sertraline combination) but their doses were increased, and one adolescent remained on the same dose of the medication (lamotrigine).
Neuroimaging task
All adolescents performed an emotional facial expression gender labeling task, a well-validated measure of implicit emotion processing (Hassel et al. 2008; Ladouceur et al. 2011; Liu et al. 2012; Surguladze et al. 2006). Two separate 7-min-long event-related functional neuroimaging experiments were used to examine neural activity to positive (happy) and negative (fearful) emotional facial expressions. Here, in each experiment, participants viewed a total of 60 facial expressions comprising either intense (prototypical) happy or fearful (H100% or F100%), mild happy or fearful (H50% or F50%), and neutral (Hn or Fn) facial expressions. Participants were asked to judge whether each face was male or female.
Functional imaging data acquisition and analysis
Neuroimaging data were collected at the Magnetic Resonance Research Center (MRRC), University of Pittsburgh, on a Trio 3.0 Tesla scanner (Siemens, AG). Anatomical images covering the entire brain were acquired using an axial 3D MPRAGE sequence, parallel to the AC–PC line (TE/TI/TR = 3.29 ms/900 ms/2,200 ms, flip angle = 9, isotropic 1 mm3 voxel, 192 axial slices, matrix size = 256 × 192). Blood Oxygen Level Dependent (BOLD) functional images were acquired with a gradient-echo EPI sequence and cover 34 axial slices (3 mm thick, 0 mm gap) encompassing the entire cerebrum and the majority of the cerebellum (TR/TE = 2,000/25 msec, field of view = 205 mm, matrix = 64 × 64). Before the collection of fMRI data for each subject, a reference EPI scan was required and inspected for artifacts (e.g., ghosting), as well as for good signal across the entire volume of acquisition, including the medial temporal lobes.
Using Statistical Parametric Mapping (SPM5; http://www.fil.ion.ucl.ac.uk/spm), data for each participant were first corrected for differences in acquisition time between slices, realigned and unwarped, co-registered, normalized and spatially smoothed. A first-level fixed-effect model was constructed to examine within-subject effects: Three emotion intensities (neutral, mild, intense) for each of the two facial gender labeling experiments (happy and fearful) were entered as separate conditions in an event-related design with fixation cross as baseline in the design matrix. Two second-level random-effects group analyses were conducted on the t-contrast images generated in the previous single-subject analyses: first in a 2 (group: BDd at baseline vs. HC) by 3 (condition: neutral, mild, intense emotion) and second in a 2 (group: BDd at baseline vs. BDd after treatment) by 3 (condition: neutral, mild, intense emotion) repeated-measures analysis of variance (ANCOVA) covarying for age for each experiment (happy and fearful) to avoid any undetected age effects. A cluster-level false-positive detection rate of p ≤ .05 was maintained for whole-brain activity surviving a voxel-wise threshold of p ≤ .05, using a regional anatomic mask from the Wake Forest University (WFU) PickAtlas (Maldjian et al. 2003) for each whole-brain activity cluster ≥10 voxels, and a cluster (k) extent empirically determined by Monte Carlo simulation implemented in AlphaSim, AFNI (Pan et al. 2011; Pavuluri et al. 2010c). For comparisons between BDd baseline and after treatment, we employed region of interest (ROI) analysis using an anatomical mask created with the WFU PickAtlas in the dorsolateral prefrontal cortex (DLPFC, BA 9 and 46), ventrolateral prefrontal cortex (VLPFC, BA 44, 45 and 47), anterior cingulate cortex (BA 24, 25, and 32) and amygdala considering their significance in treatment studies of major depression and BDd in adolescents (Chang et al. 2008; Patel et al. 2008; Yang et al. 2009) and adults (Savitz and Drevets 2009). In ROI analyses between BDd baseline and after treatment, we also included the significant neural regions that we identified during whole-brain analysis of BDd baseline versus HC (Table 1). Peak BOLD signal changes were extracted from regions showing a significant group-by-condition interaction in the 2 × 3 whole-brain or ROI analysis for each experiment. Post hoc tests were performed on these extracted BOLD signal values to examine the extent to which pairwise between-group differences in activity contributed to the significant group-by-condition interactions in these analyses using independent and paired t tests and appropriate statistical thresholds (corrected p ≤ 0.05/6 = 0.008 to control for multiple t-tests in whole-brain analysis and p ≤ 0.05 for ROI analysis).
In exploratory analyses, we used t-test, repeated measures analysis, and Pearson’s correlational analysis (p ≤ .05) appropriately to examine potential relationships among age, gender, severity of depression, mania, and anxiety symptoms, subtype of the BD, Attention Deficit Hyperactivity (ADHD) comorbidity, response to treatment (e.g., Clinical Global Impression-Severity (Spearing et al. 1997) ≤2 and ≥50 % percentage reduction in CDRS-R), and psychotropic medications upon patterns of abnormal neural activity that were identified with whole-brain or ROI analyses. We also explored the correlations of depression severity and changes in depression with the peak BOLD signal changes in the significant regions identified in our analyses.
Results
Treatment
Baseline CDRS-R was 73.2 ± 14 and after 6 weeks of treatment as usual adolescents with BDd showed significant improvement in their depressive symptomatology (mean change: 57 % ± 28, p < .0001). Six out of 10 adolescents were considered as responders (e.g., 50 % decrease in CDRS-R and CGI-Severity ≤2) after treatment: Two adolescents were started on a new medication (one with lamotrigine and one with quetiapine) and four adolescents remained on the same medication combinations (one with lamotrigine + aripiprazole, one with citalopram + aripiprazole, one with lithium + quetiapine, and one with lamotrigine + valproic acid + sertraline combination) but their doses were increased. Baseline and after treatment scores, respectively, were not significant for anxiety (SCARED: 30.9 ± 14 versus 24.3 ± 13.6) and mania (YMRS: 2.9 ± 1.5 versus 2.4 + 1.65).
Adolescents with BDd at baseline versus HC adolescents
In the fearful experiment, there was a significant group-by-condition interaction in bilateral occipital cortex (BA 18) with the whole-brain analysis, and pairwise comparison showed that relative to HC, adolescents with BDd at baseline had higher right occipital cortex activity to neutral faces (Fn) presented in the fearful experiment (Table 1).
In the happy experiment, there was a significant group by condition interaction with whole-brain analysis in several regions (e.g., right parietal cortex (BA 7), right superior temporal cortex (BA 22), right anterior cingulate cortex (ACC, BA 32), right inferior parietal cortex (BA 40), right supplementary motor area (SMA, BA 6), left cerebellum, left occipital cortex (BA 18), left insula, and left middle temporal cortex (BA 39). Pairwise comparisons showed that, relative to HC, adolescents with BDd at baseline had higher activity in right supplementary motor area (SMA, BA 6) to mild happy faces (H50%) and in left cerebellum, left occipital cortex (BA 18), and left insula to neutral faces (Hn) presented in the happy experiment, but lower activity in right ACC (BA 32) to intense happy faces (H100%).
Neural correlates of depressive scores at baseline: Reduced activity to intense happy faces in right VLPFC at baseline (ROI, BA 44, MNI: 51, 18, 6, cluster: 62 voxels, AlphaSim cluster: 10) was correlated with higher baseline depressive scores (r = −.674, p = 0.03). No other correlations were found between neural activity and depression scores in the fearful and happy experiments.
Adolescents with BDd at baseline versus after treatment
In the fearful face experiment, compared to baseline, there was significant activity with ROI analysis in the left occipital cortex (BA 18; MNI:−9, −72, −3, cluster: 3200, AlphaSim cluster: 17, F = 5.81, p = 0.04). Pairwise analysis showed that the difference was due to significantly decreased neural activation to intense fearful faces after treatment in the left occipital cortex (t = 2.85, p = .01). There were no significant differences in other regions or between responders and non-responders. There were no correlations with change in depressive scores.
In the happy face experiment, compared to baseline, there was significant activity with ROI analysis in left insula (MNI:−42, 15, 0; cluster:220, AlphaSim cluster:17, F = 7.73, p = .02), left cerebellum (MNI:−6, −48, −24; cluster:55, AlphaSim cluster:48, F = 21.7, p = .001), right VLPFC (BA 44; MNI: 51, 18, 6; cluster: 62 voxels, AlphaSim cluster: 10, F = 8.06, p = .02), and right ACC (BA 24; ROI analysis; MNI: 9, 36, 0, cluster: 40 voxels, AlphaSim cluster: 20). Pairwise analysis showed that the difference was due to significantly increased neural activation to intense happy faces from baseline to after treatment in left insula (t = 2.77, p = .01), left cerebellum (t = 2.8, p = .01), and right VLPFC (t = 2.66, p = 0.04).
Exploratory analyses
There were no significant group differences for any of the clinical variables or in neural activation in adolescents with BDd: BD I versus II, in BD I/II versus BD NOS, males versus females, in those with medication versus those without any medication and with versus without ADHD. Activity in neural regions was not correlated with age or duration of depressive episode. Depressive, anxiety, and mania scores at baseline and anxiety and mania scores after treatment were not different in responders versus non-responders.
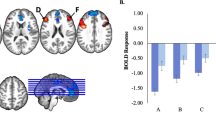
The only significant difference between responders and non-responders was baseline elevated right ventral ACC (BA 24) activity to intense (t = 3.07, p = .02) and mild happy (t = 2.77, p = .02) (Fig. 1)
Right ventral anterior cingulate (ACC) activity to mild happy faces (region of interest analysis). Panel a illustrates positive correlation of baseline neural activity to mild happy faces in ventral anterior cingulate and percentage improvement in depression scores (Child Depression Rating Scale–Revised) from baseline to after treatment (r = .824, p = 0.003). Panel b illustrates ventral anterior cingulate activity to the happy experiment shown in green circles. BA: Brodmann’s area
conditions. In addition, repeated measures analysis resulted in a significant interaction between change in depressive scores and baseline right ventral ACC activation to mild happy condition (F = 33.9, p < 0.0001), and greater improvement in depression scores was correlated with baseline elevated activity to mild happy faces in right ventral ACC (r = .824, p = 0.003). There were no other significant differences in other regions in the fearful and happy experiments.
Discussions
The present study, using an implicit emotion processing experiment, identified significant neural activity in BDd relative to HC in regions involved in affective circuitry (prefrontal cortex, cingulate cortex, and cerebellum) and face-responsive visual processing circuit (e.g., occipital cortex) that are implicated in the pathophysiology of BD (Phillips et al. 2008a; Strakowski et al. 2012). Our results showed increased neural activity to neutral faces in both fearful and happy experiments and to mild happy faces, but decreased neural activity to intense happy faces in BDd relative to HC suggesting increased attention to ambiguous stimuli but decreased attention to positive stimuli during depressive state. Consistent with our hypothesis, there was decreased neural activity to intense fearful faces after treatment (e.g., left occipital cortex) in contrast to increased activity to intense happy faces (e.g., left insula, left cerebellum, and right VLPFC). These patterns of neural activity changes after treatment (e.g., reduced processing of intense fear (negative emotion) and increased processing of intense happy stimuli (positive emotion)) are consistent with improvement of depression according to Beck’s cognitive theory that suggests attention bias towards negative and away from positive emotional stimuli in depression (Disner et al. 2011; Surguladze et al. 2005). In addition, our findings suggested that baseline cortical neural activity to happy faces reflected the severity of depression (e.g., right VLPFC) and was associated with improvement in depression (e.g., right ventral ACC). Similar to the finding that neural activity to happy but not fearful faces was significant in adults with BDd as compared to those with major depressive disorder (Almeida et al. 2009, 2010; Lawrence et al. 2004), our study provided the first results in adolescents that dissociable patterns of neural activity to positive stimuli may help identify neural markers associated with depression and its treatment in BD (Chang et al. 2004).
Studies in BD adults and adolescents have suggested that BD is associated with trait neural abnormalities (Liu et al. 2012; Pavuluri et al. 2010c), but successful treatment may reverse some neural circuitry abnormalities identified during mood episodes (Blumberg et al. 2005; Drevets et al. 2008; Passarotti et al. 2011; Pavuluri et al. 2010b). Although neural activity changes from baseline to after treatment were decreased to fearful but increased to happy faces, significant activation was found only to intense facial emotions in both conditions. Our results were consistent with the reports of impaired attention to emotional faces and the difficulty in labeling emotions correctly when the emotion was not intense in adolescents with BD relative to HC (Leibenluft and Rich 2008; Rich et al. 2008b) and suggested that treatment of depression may help improve the impaired attention to prototypical facial emotion.
Frontal cortex changes are the most common findings in depression and BD studies with normalization of frontal overactivity and underactivity after treatment (Drevets et al. 2008). Studies in adults with BD suggest prefrontal hypoactivity across different mood states including depression (Townsend and Altshuler 2012), but the only published fMRI study in adolescents with BDd (n = 8) reported positive, not negative, correlation of DLPFC activity with baseline depression scores although there was no correlation of DLPFC activity with improvement in depression (Chang et al. 2008). They did not include positive stimuli or a control group and did not analyze the whole brain (Chang et al. 2008). In this study, we did not identify significant prefrontal cortex activity with whole-brain analysis between BDd and HC, but our ROI analysis showed that right VLPFC (BA 44) activity (to intense happy faces), which was positively correlated with reduction of manic symptoms in a recent pediatric BD study (Pavuluri et al. 2010b), was inversely correlated in our study with depressive scores at baseline and significantly increased after treatment. In addition, right ventral cingulate was the only region that was significantly different at baseline in responders versus non-responders and higher baseline right ventral cingulate activity in our study was associated with better symptom resolution. Similar to our findings, higher activity in the affective region of anterior cingulate cortex, a neural hub that has structural and functional connectivity with frontal cortex and subcortical regions and balances neural inputs for emotion regulation, was higher to positively, but not negatively, valanced stimuli in adolescents with BD relative to HC (Chang et al. 2004) and normalized after treatment in adolescents (Yang et al. 2009) and adults (Drevets et al. 2008) with unipolar depression. Thus, ventral ACC is suggested as a target region for predicting treatment response in BDd (Lipsman et al. 2010). Our neural findings during emotion processing suggested that VLPFC activity is a promising region for biological correlates of symptom reduction in BDd and ventral ACC for identifying those depressed adolescents with BDd who may potentially respond to treatment of depression.
In contrast to previous findings (Drevets et al. 2008; Pavuluri et al. 2011), in our study there were no differences in amygdala activation between BDd versus HC and no differences before and after treatment (Chang et al. 2008). On the other hand, recent studies in adults and children also did not find increased amygdala activity to emotional stimuli, suggesting that absence of amygdala activation may be related to habituation of amygdala response after repeated affective stimuli (Breiter et al. 1996), blunted activity during depression (Altshuler et al. 2008; Chen et al. 2006; Townsend and Altshuler 2012), or difference in neurodevelopment of amygdala in adolescents with BD relative to adults (Pfeifer et al. 2008). Future longitudinal studies are needed in BD children and adolescents to better understand the neurodevelopmental effects of depressive state and valence of emotions on neural activity.
The results of this study need to be taken into account with the following limitations. The main limitation is the small sample size. It is important to take into account that many of our adolescents with BDd were on psychotropic medications during scanning at baseline and 9 out of 10 adolescents with BD had new medication or dose changes after 6 weeks of treatment. Our study did not allow us to differentiate possible interactions between changes in medication, depression severity, and neural activation, but available studies in adults and adolescents suggested that medication treatment is not likely to cause new neural abnormalities, but may result in amelioration of neural activity abnormalities found in BD (Almeida et al. 2009, 2010; Hafeman et al. 2012; Hassel et al. 2008; Leibenluft et al. 2007; Nelson et al. 2007; Phillips et al. 2008b; Rich et al. 2008a). On the other hand, including only adolescents with unmedicated BD, who may have a milder form of the illness and therefore can tolerate medication withdrawal, may limit the ability to identify biomarkers of BD (Phillips et al. 2008b). The majority of the treatment studies in youth with depression had 8-week-or-longer trials (Bridge et al. 2005) and some adolescents might need longer time to improve or show neural changes, but our open as usual study for 6 weeks was similar to an open-label treatment study in bipolar depression in youth (Patel et al. 2006), and the decrease in depression scores and the percentage of responders were comparable to previous studies (Bridge et al. 2005; DelBello et al. 2009; Patel et al. 2006) including the only pharmaco-imaging study in depressed youth for 12 weeks (Tao et al. 2012). We didn’t scan HC after 6 weeks to control for practice effects and our small sample size limited us to identify neural activity differences between those who had higher versus lower reduction in depression scores after treatment. We had predominantly female adolescents with relatively low ADHD comorbidity, but our sample characteristics were similar to the only randomized treatment study in adolescents with BDd (DelBello et al. 2009). We didn’t identify any differences in our study between adolescents with BD I and II; however, studies in adults suggest potential clinical and neuroimaging differences between BD I and II (Baek et al. 2011; Summers et al. 2006). The negative findings could be due to our small sample size and potential developmental progression in BD diagnosis in adolescents (e.g., more than 20 % of youth with BD II converted to BD I over 4-year follow up (Birmaher et al. 2009)).
Our results of reduced negative emotion processing versus increased positive emotion processing are consistent with improvement of depression according to Beck’s cognitive theory. Childhood gives a window of opportunity for early identification of markers of risk and potential targets for treatment (Diler 2011). This is particularly important for bipolar depression considering the morbidity and mortality associated with it and limited data about its treatment in adolescents (DelBello et al. 2009; Liu et al. 2011). Because structural and functional connectivity between prefrontal cortex and other regions continue to mature during adolescence, this is a critical period to successfully intervene during depression and possibly halt progression of neural network dysfunction in BD (Luna 2009). These areas should be explored in larger longitudinal studies that investigate valence of emotion stimuli and effects of depressed mood and treatment on development of neural activity and connectivity.
References
Almeida, J. R., Versace, A., Mechelli, A., Hassel, S., Quevedo, K., Kupfer, D. J., Phillips, M. L., Almeida, J. R. C. D., Versace, A., Mechelli, A., Hassel, S., Quevedo, K., Kupfer, D. J., & Phillips, M. L. (2009). Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biological Psychiatry, 66, 451–459.
Almeida, J. R., Versace, A., Hassel, S., Kupfer, D. J., & Phillips, M. L. (2010). Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biological Psychiatry, 67, 414–421.
Altshuler, L., Bookheimer, S., Townsend, J., Proenza, M. A., Sabb, F., Mintz, J., & Cohen, M. S. (2008). Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disorders, 10, 708–717.
APA. (2004). Diagnostic and statistical manual of mental disorders (4th ed.). Washington: American Psychiatric Association.
Axelson, D., Birmaher, B., Strober, M., Gill, M. K., Valeri, S., Chiappetta, L., Ryan, N., Leonard, H., Hunt, J., Iyengar, S., Bridge, J., & Keller, M. (2006). Phenomenology of children and adolescents with bipolar spectrum disorders. Archives of General Psychiatry, 63, 1139–1148.
Baek, J. H., Park, D. Y., Choi, J., Kim, J. S., Choi, J. S., Ha, K., Kwon, J. S., Lee, D., & Hong, K. S. (2011). Differences between bipolar I and bipolar II disorders in clinical features, comorbidity, and family history. Journal of Affective Disorders, 131, 59–67.
Birmaher, B., & Axelson, D. (2006). Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Development and Psychopathology, 18, 1023–1035.
Birmaher, B., Khetarpal, S., Brent, D., Cully, M., Balach, L., Kaufman, J., & Neer, S. M. (1997). The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 545–553.
Birmaher, B., Axelson, D., Goldstein, B., Strober, M., Gill, M. K., Hunt, J., Houck, P., Ha, W., Iyengar, S., Kim, E., Yen, S., Hower, H., Esposito-Smythers, C., Goldstein, T., Ryan, N., & Keller, M. (2009). Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. The American Journal of Psychiatry, 166, 795–804.
Blumberg, H. P., Martin, A., Kaufman, J., Leung, H. C., Skudlarski, P., Lacadie, C., Fulbright, R. K., Gore, J. C., Charney, D. S., Krystal, J. H., & Peterson, B. S. (2003). Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. The American Journal of Psychiatry, 160, 1345–1347.
Blumberg, H. P., Donegan, N. H., Sanislow, C. A., Collins, S., Lacadie, C., Skudlarski, P., Gueorguieva, R., Fulbright, R. K., McGlashan, T. H., Gore, J. C., & Krystal, J. H. (2005). Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology, 183, 308–313.
Breiter, H. C., Etcoff, N. F., Whalen, P. J., Kennedy, W. A., Rauch, S. L., & Buckner, R. L. (1996). Response and habituation of the human amygdala during visual processing of facial expression. Neuron, 17, 875–887.
Bridge, J. A., Salary, C. B., Birmaher, B., Asare, A. G., & Brent, D. A. (2005). The risks and benefits of antidepressant treatment for youth depression. Annals of Medicine, 37, 404–412.
Chang, K. (2009). Challenges in the diagnosis and treatment of pediatric bipolar depression. Dialogues in Clinical Neuroscience, 11, 73–80.
Chang, K., Adleman, N. E., Dienes, K., Simeonova, D. I., Menon, V., & Reiss, A. (2004). Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Archives of General Psychiatry, 61, 781–792.
Chang, K. D., Wagner, C., Garrett, A., Howe, M., & Reiss, A. (2008). A preliminary functional magnetic resonance imaging study of prefrontal-amygdalar activation changes in adolescents with bipolar depression treated with lamotrigine. Bipolar Disorders, 10, 426–431.
Chen, C. H., Lennox, B., Jacob, R., Calder, A., Lupson, V., Bisbrown-Chippendale, R., Suckling, J., & Bullmore, E. (2006). Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biological Psychiatry, 59, 31–39.
Delbello, M. P., & Strakowski, S. M. (2004). Neurochemical predictors of response to pharmacologic treatments for bipolar disorder. Current Psychiatry Reports, 6, 466–472.
DelBello, M. P., Chang, K., Welge, J. A., Adler, C. M., Rana, M., Howe, M., Bryan, H., Vogel, D., Sampang, S., Delgado, S. V., Sorter, M., & Strakowski, S. M. (2009). A double-blind, placebo-controlled pilot study of quetiapine for depressed adolescents with bipolar disorder. Bipolar Disorders, 11, 483–493.
Diler, R. (2011). Neuroimaging can help identify biomarkers of early onset bipolar disorder. Bulletin of Clinical Psychopharmacology, 22, 1–4.
Disner, S. G., Beevers, C. G., Haigh, E. A., & Beck, A. T. (2011). Neural mechanisms of the cognitive model of depression. Nature Review of Neurosciences, 12, 467–477.
Drevets, W. C., Savitz, J., & Trimble, M. (2008). The subgenual anterior cingulate cortex in mood disorders. CNS Spectrum, 13, 663–681.
Hafeman, D. M., Chang, K. D., Garrett, A. S., Sanders, E. M., & Phillips, M. L. (2012). Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disorders, 14, 375–410.
Hassel, S., Almeida, J. R., Kerr, N., Nau, S., Ladouceur, C. D., Fissell, K., Kupfer, D. J., & Phillips, M. L. (2008). Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disorders, 10, 916–927.
Kaufman, J., Birmaher, B., Brent, D., Rao, U., Flynn, C., Moreci, P., Williamson, D., & Ryan, N. (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data [see comments]. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 980–988.
Ketter, T. A., & Wang, P. W. (2002). Predictors of treatment response in bipolar disorders: evidence from clinical and brain imaging studies. The Journal of Clinical Psychiatry, 63(Suppl 3), 21–25.
Ladouceur, C. D., Farchione, T., Diwadkar, V., Pruitt, P., Radwan, J., Axelson, D. A., Birmaher, B., & Phillips, M. L. (2011). Differential patterns of abnormal activity and connectivity in the amygdala-prefrontal circuitry in bipolar-I and bipolar-NOS youth. Journal of the American Academy of Child and Adolescent Psychiatry, 50, 1275–1289.
Lawrence, N. S., Williams, A. M., Surguladze, S., Giampietro, V., Brammer, M. J., Andrew, C., Frangou, S., Ecker, C., & Phillips, M. L. (2004). Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry, 55, 578–587.
Leibenluft, E., & Rich, B. A. (2008). Pediatric bipolar disorder. Annual Review of Clinical Psychology, 4, 163–187.
Leibenluft, E., Rich, B. A., Vinton, D. T., Nelson, E. E., Fromm, S. J., Berghorst, L. H., Joshi, P., Robb, A., Schachar, R. J., Dickstein, D. P., McClure, E. B., & Pine, D. S. (2007). Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. The American Journal of Psychiatry, 164, 52–60.
Lipsman, N., McIntyre, R. S., Giacobbe, P., Torres, C., Kennedy, S. H., & Lozano, A. M. (2010). Neurosurgical treatment of bipolar depression: defining treatment resistance and identifying surgical targets. Bipolar Disorders, 12, 691–701.
Liu, H. Y., Potter, M. P., Woodworth, K. Y., Yorks, D. M., Petty, C. R., Wozniak, J. R., Faraone, S. V., & Biederman, J. (2011). Pharmacologic treatments for pediatric bipolar disorder: a review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 50, 749–762.
Liu, J., Blond, B. N., van Dyck, L. I., Spencer, L., Wang, F., & Blumberg, H. P. (2012). Trait and state corticostriatal dysfunction in bipolar disorder during emotional face processing. Bipolar Disorders, 14, 432–441.
Luna, B. (2009). Developmental changes in cognitive control through adolescence. Advence Child Development Behavaviors, 37, 233–278.
Maldjian, J. A., Laurienti, P. J., Kraft, R. A., & Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19, 1233–1239.
Marshall, W. A., & Tanner, J. M. (1969). Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood, 44, 291–303.
Nelson, E. E., Vinton, D. T., Berghorst, L., Towbin, K. E., Hommer, R. E., Dickstein, D. P., Rich, B. A., Brotman, M. A., Pine, D. S., & Leibenluft, E. (2007). Brain systems underlying response flexibility in healthy and bipolar adolescents: an event-related fMRI study. Bipolar Disorders, 9, 810–819.
Pan, L. A., Batezati-Alves, S. C., Almeida, J. R., Segreti, A., Akkal, D., Hassel, S., Lakdawala, S., Brent, D. A., & Phillips, M. L. (2011). Dissociable patterns of neural activity during response inhibition in depressed adolescents with and without suicidal behavior. Journal of the American Academy of Child and Adolescent Psychiatry, 50, 602–611.
Passarotti, A. M., Sweeney, J. A., Pavuluri, M. N. (2011). Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology (Berl).
Patel, N. C., DelBello, M. P., Bryan, H. S., Adler, C. M., Kowatch, R. A., Stanford, K., & Strakowski, S. M. (2006). Open-label lithium for the treatment of adolescents with bipolar depression. Journal of the American Academy of Child and Adolescent Psychiatry, 45, 289–297.
Patel, N. C., Cecil, K. M., Strakowski, S. M., Adler, C. M., & DelBello, M. P. (2008). Neurochemical alterations in adolescent bipolar depression: a proton magnetic resonance spectroscopy pilot study of the prefrontal cortex. Journal of Child and Adolescent Psychopharmacology, 18, 623–627.
Pavuluri, M. N., Passarotti, A. M., Harral, E. M., & Sweeney, J. A. (2010). Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. The Journal of Clinical Psychiatry, 71, 1526–1534.
Pavuluri, M. N., Passarotti, A. M., Mohammed, T., Carbray, J. A., & Sweeney, J. A. (2010). Enhanced working and verbal memory after lamotrigine treatment in pediatric bipolar disorder. Bipolar Disorders, 12, 213–220.
Pavuluri, M. N., Passarotti, A. M., Parnes, S. A., Fitzgerald, J. M., & Sweeney, J. A. (2010). A pharmacological functional magnetic resonance imaging study probing the interface of cognitive and emotional brain systems in pediatric bipolar disorder. Journal of Child and Adolescent Psychopharmacology, 20, 395–406.
Pavuluri, M. N., Passarotti, A. M., Lu, L. H., Carbray, J. A., & Sweeney, J. A. (2011). Double-blind randomized trial of risperidone versus divalproex in pediatric bipolar disorder: fMRI outcomes. Psychiatry Research, 193, 28–37.
Pfeifer, J. C., Welge, J., Strakowski, S. M., Adler, C. M., & DelBello, M. P. (2008). Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 1289–1298.
Phillips, M. L., Ladouceur, C. D., & Drevets, W. C. (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13, 833–857.
Phillips, M. L., Travis, M. J., Fagiolini, A., & Kupfer, D. J. (2008). Medication effects in neuroimaging studies of bipolar disorder. The American Journal of Psychiatry, 165, 313–320.
Poznanski, E., & Mokros, H. (1995). Children’s depression rating scale-revised. Los Angeles: Western Psychological Services.
Rich, B. A., Vinton, D. T., Roberson-Nay, R., Hommer, R. E., Berghorst, L. H., McClure, E. B., Fromm, S. J., Pine, D. S., & Leibenluft, E. (2006). Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceedings of the National Academy of Sciences of the United States of America, 103, 8900–8905.
Rich, B. A., Fromm, S. J., Berghorst, L. H., Dickstein, D. P., Brotman, M. A., Pine, D. S., & Leibenluft, E. (2008). Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 49, 88–96.
Rich, B. A., Grimley, M. E., Schmajuk, M., Blair, K. S., Blair, R. J., & Leibenluft, E. (2008). Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Developmental Psychopathology, 20, 529–546.
Savitz, J., & Drevets, W. C. (2009). Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neuroscience and Biobehavioral Reviews, 33, 699–771.
Spearing, M. K., Post, R. M., Leverich, G. S., Brandt, D., & Nolen, W. (1997). Modification of the Clinical Global Impressions (CGI) scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Research, 73, 159–171.
Strakowski, S. M., Adler, C. M., Almeida, J., Altshuler, L. L., Blumberg, H. P., Chang, K. D., Delbello, M. P., Frangou, S., McIntosh, A., Phillips, M. L., Sussman, J. E., & Townsend, J. D. (2012). The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disorders, 14, 313–325.
Summers, M., Papadopoulou, K., Bruno, S., Cipolotti, L., & Ron, M. A. (2006). Bipolar I and bipolar II disorder: cognition and emotion processing. Psychological Medicine, 36, 1799–1809.
Surguladze, S., Brammer, M. J., Keedwell, P., Giampietro, V., Young, A. W., Travis, M. J., Williams, S. C., & Phillips, M. L. (2005). A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry, 57, 201–209.
Surguladze, S., Russell, T., Kucharska-Pietura, K., Travis, M. J., Giampietro, V., David, A. S., & Phillips, M. L. (2006). A reversal of the normal pattern of parahippocampal response to neutral and fearful faces is associated with reality distortion in schizophrenia. Biological Psychiatry, 60, 423–431.
Tao, R., Calley, C. S., Hart, J., Mayes, T. L., Nakonezny, P. A., Lu, H., Kennard, B. D., Tamminga, C. A., & Emslie, G. J. (2012). Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. The American Journal of Psychiatry, 169, 381–388.
Townsend, J., & Altshuler, L. L. (2012). Emotion processing and regulation in bipolar disorder: a review. Bipolar Disorders, 14, 326–339.
Yang, T. T., Simmons, A. N., Matthews, S. C., Tapert, S. F., Frank, G. K., Bischoff-Grethe, A., Lansing, A. E., Wu, J., Brown, G. G., & Paulus, M. P. (2009). Depressed adolescents demonstrate greater subgenual anterior cingulate activity. Neuroreport, 20, 440–444.
Young, R. C., Biggs, J. T., Ziegler, V. E., & Meyer, D. A. (1978). A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry, 133, 429–435.
Acknowledgment
This study is supported by the University of Pittsburgh Western Psychiatric Institute Pilot Neuroimaging Grant (WPC-9414).
Dr. Ladouceur (K01 MH083001) and Dr. Pan (K23 MH082884) received funding from NIMH.
Conflicts of interest
Dr. Birmaher receives royalties from Random House Inc and Lippincott Williams and Wilkins. Other authors reported no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diler, R.S., Ladouceur, C.D., Segreti, A. et al. Neural correlates of treatment response in depressed bipolar adolescents during emotion processing. Brain Imaging and Behavior 7, 227–235 (2013). https://doi.org/10.1007/s11682-012-9219-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-012-9219-7