Abstract
Research on brain areas involved in experiencing emotion and physical pain is abundant; however, psychological pain has received little attention in studies of the brain. The purpose of this systematic review was to provide an overview of studies on brain function related to psychological pain. The review was limited to studies in which participants experienced actual psychological pain or recalled a significant autobiographical event that may be assumed to have involved psychological pain. Based on results of the studies (N = 18), a tentative neural network for psychological pain is proposed that includes the thalamus, anterior and posterior cingulate cortex, the prefrontal cortex, cerebellum, and parahippocampal gyrus. Results indicated that grief may be a more accurate exemplar of psychological pain than recalled sadness, with indications of greater arousal during psychological pain. The proposed neural network for psychological pain overlaps to some extent with brain regions involved in physical pain, but results suggest a markedly reduced role for the insula, caudate, and putamen during psychological pain. Psychological pain is well known for its association with depression and as a precursor of suicidal behavior. Thus, identification of brain areas involved in psychological pain may help guide development of interventions to decrease mortality and morbidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psychological pain may be as old as human self-awareness, but it remains immature as a concept in that its unique nature and complexities are minimally understood. Unlike physical pain it has received little attention in studies of the brain (Biro 2010). Research on physical pain is abundant and the network of brain areas involved in such pain is well described (Apkarian et al. 2005; Hudson 2000; Schnitzler and Ploner 2000). Similarly, brain areas involved in emotions (e.g. sadness, happiness, anger, fear, disgust) are well studied and comprehensively reviewed (Davidson and Irwin 1999; Phan et al. 2002; Wager et al. 2003). However, none of these reviews have addressed psychological pain.
Mee et al. (2006) were the first to suggest specific brain areas that may play a role in psychological pain. Based on a review of studies on grief, social exclusion, and induced sadness, they concluded that brain areas involved in psychological pain overlap with the physical pain network, particularly the insula, prefrontal cortex (PFC), and anterior cingulate cortex (ACC). Only the somatosensory cortices were uniquely activated in physical pain. While other authors have also suggested that psychological pain and physical pain use similar areas of the brain (Biro 2010; Macdonald and Leary 2005), this assertion has been based on a variety of studies that did not assess actual psychological pain. A further limitation of these studies is the large heterogeneity in methods used to evoke emotions, which included watching sad film clips, displaying sad photos or pictures, reading sad words or sentences, recalling sad events, and playing a game that involved exclusion.
Based upon analysis of the literature to date, psychological pain was defined as “a lasting, unsustainable, and unpleasant feeling resulting from negative appraisal of an inability or deficiency of the self” (Meerwijk and Weiss 2011). This definition is the result of a synthesis of perspectives from existing concepts and theories of psychological pain. Psychological pain is a lasting feeling in that it endures, it does not pass quickly and it takes time to resolve. One does not experience psychological pain for just a few seconds or minutes. Usually, it is hours, days, weeks, or even longer, although the intensity of the pain may vary during that period. Examples of situations that may cause psychological pain include a) the inability to protect oneself or a loved one from injury, disease, or embarrassment, b) feeling deficient because of a lack of love or affiliation, or because of lost autonomy due to a pervasive illness. The needs to love, be loved, to affiliate, and to avoid harm are core psychological needs that were described by Murray in 1938 (2008). In his theory of psychological pain, Shneidman (1998) has noted that frustration of psychological needs may cause unbearable psychological pain. Grief over losing a loved one, for example, represents a frustration of the need to love and be loved, and a frustration of the need to protect someone who is cared about deeply. Psychological pain is the result of negative appraisal of this inability or deficiency of the self.
Although psychological pain has the potential to foster personal growth and an enhanced sense of meaning, it has been associated to a greater extent with decreased mental well-being. In particular, it has been identified as a symptom in major depressive disorder (MDD) and as a precursor of suicidal ideation and behavior (Chavez-Hernandez et al. 2009; Mee et al. 2011; Olié et al. 2010; Orbach et al. 2003a; Pompili et al. 2008; Shneidman 1998; Troister and Holden 2010). In this sense, psychological pain is unsustainable in that it cannot be endured indefinitely without adverse consequences. Growing evidence points to the negative effects of poor mental health on physical health (Prince et al. 2007; Weiss et al. 2009). Thus, identification of brain areas involved in psychological pain may help guide development of interventions to decrease mortality and morbidity.
The purpose of this paper is to provide an up-to-date overview of studies on brain function related to psychological pain. This systematic review was limited to studies in which current psychological pain was assessed and studies in which participants recalled an autobiographical sad event (e.g. loss of a close friend or family member, a relationship break-up), as this was assumed to evoke a mood state closer to actual psychological pain than externally generated methods (e.g. viewing sad pictures or films). Evidence indicates that recall of autobiographical events is an effective method of mood induction (Martin 1990). Specific aims of the review were (a) to describe a potential brain network for psychological pain by identifying brain areas that are activated during the experience of psychological pain, and (b) to identify the extent of overlap between brain areas involved in psychological pain and physical pain.
Methods
Search strategy
Medline was accessed for literature with search terms pertaining to psychological pain and brain function; publications were selected if they matched the combination of these terms. Because various alternatives are used in the literature to refer to psychological pain, a broad search strategy was used including terms like psychological pain, mental pain, emotional pain, psychic pain, and psychache. Publications that mentioned suicide, negative affect, or the combination of pain and emotions were also included as possibly pertaining to psychological pain, as were articles that mentioned suffering, anguish, or torment in the title. Search terms pertaining to brain function included brain, brain function, brain activity, and brain mapping. Combination of the search terms for psychological pain and brain function resulted in 1335 hits up to the publication year 2011, while excluding reviews and limiting to articles on humans published in English.
After assessing the titles for applicability, 128 articles remained of which the abstracts were read. Based on these abstracts, another 73 articles were excluded because they (a) did not report results of a neuroimaging study, (b) reported on simulated, imagined or anticipated nociceptive pain or an affective response to a real nociceptive pain stimulus, (c) reported on an affective response other than psychological pain (e.g. fear, anger, anxiety, aversion) or the affective response was unknown, and (d) did not fit our established definition of psychological pain. The remaining 55 articles were obtained, and another 10 articles were identified via reference lists. As a final limitation, unless they specifically assessed psychological pain, studies were excluded if they did not use a significant autobiographical event to reexperience the sad mood state associated with that event. This left 18 articles that could be used for this review. Use of these criteria aimed to comprise a more homogeneous set of studies so that the analysis would not be confounded by vastly different methods or definitions of psychological pain.
Analysis
If studies reported nonspecific labels to refer to brain areas (e.g. occipital cortex rather than a more specific subregion like cuneus or lingual gyrus), either the reported Brodmann area ([BA], Strotzer 2009) or Talairach coordinates (Talairach and Tournoux 1988) were used to find a more specific label with the Talairach client software developed by the International Consortium for Brain Mapping (Lancaster et al. 1997; Lancaster et al. 2000). If coordinates were reported in the Montreal Neurological Institute reference system (Collins et al. 1994), a conversion to Talairach space was carried out using the BrainMap software developed by the Research Imaging Institute, University of Texas Health Science Center San Antonio (Eickhoff et al. 2009). To facilitate interpretation, all reported labels referring to the PFC (e.g. orbitofrontal cortex, frontal gyri) were relabeled as Brodmann areas 8 – 11 or 44 – 47, using the aforementioned software tools.
A pragmatic stance was taken to focus on brain regions for which at least three studies reported activation, regardless of whether other studies had reported deactivation, or vice versa. A difference of three in observed frequency corresponds to a χ2 > 10.827 (df = 1, p < .001) under a null hypothesis that no activity change would be observed (N = 18). When activation differences were tested between two subsamples, Fisher’s exact test was reported instead of χ2 because Fisher’s exact test is more appropriate when sample sizes and expected cell sizes are small (Munro 2005). For those comparisons that did allow meaningful χ2 analysis, built-in functions in Microsoft Excel® 2008 for Mac (version 12.2.7) were used. Fisher’s exact test was performed in PASW Statistics® for Mac (version 18.0).
Results
Table 1 shows the general characteristics of 18 studies that met the criteria for this review. Indicative of a dearth of research on psychological pain and brain function, only two studies (Reisch et al. 2010; van Heeringen et al. 2010) were found that used actual measures to assess current psychological pain. The remaining studies assessed brain function in participants who experienced grief or who recalled grief (n = 4), or who recalled sadness (n = 12). Many of the studies had subjects recall how they felt after they had lost somebody close to them (e.g. a parent, sibling, spouse, baby). In other studies, subjects recalled break-up of a romantic relationship, and sometimes illness of a loved one was recalled.
Among the results of our search strategy were studies on social pain that resulted from social exclusion (Eisenberger et al. 2003; Onoda et al. 2009). Although, these studies fit our definition of psychological pain (social exclusion represents a frustration of the need to affiliate, which is an inability of the self that is negatively appraised), we did not include them in our review because they employed an experimental manipulation, rather than a real-life autobiographical event that caused the social pain. One more study (Schmahl et al. 2004) that fit our definition of psychological pain (subjects recalled childhood abuse) was not included because the mood state that resulted from recall was not only sadness but could also have involved fear and anger.
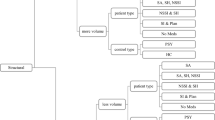
A complete overview of brain regions that showed increased or decreased activity is shown in the Appendix. Figure 1 shows a summary of that Appendix based on brain regions for which three or more studies reported activation or deactivation. Figure 1 distinguishes brain areas within three broad areas: the left and right hemispheres and the medial subcortical area.
Brain areas that showed activity change in response to psychological pain (including the grief and recalled sadness subsamples) for which three or more studies showed activation or deactivation (N = 18). BA: Brodmann area, ACC: anterior cingulate cortex, PCC: posterior cingulate cortex, PFC: prefrontal cortex
Reported brain activity in all studies was based on the subtraction method (Friston et al. 1995), in which values for brain imaging variables (e.g. cerebral blood flow, blood oxygen level dependency) in a reference condition were subtracted from values obtained during an experimental condition or values in a population of interest. The experimental condition involved such tasks as listening to or reading scripts of painful autobiographical events (e.g. illness or death of a loved one, suicidal behavior) and recalling a memory or displaying faces of lost loved ones. In the majority of studies, the reference condition involved a task to evoke a neutral mood that was similarly structured as the task that involved reexperiencing psychological pain, grief, or extreme sadness. Only four studies did not use a specific task as a reference (see Table 1).
Studies involving assessed psychological pain
Van Heeringen et al. (2010) examined the effect of psychological pain severity on resting state activity in patients with major depressive disorder (MDD) by comparing patients who scored high in psychological pain to those who scored low. In addition to effects on brain activity as detailed in the Appendix, psychological pain was positively correlated with hopelessness (r s = .517, p < .001) and suicidal ideation (r s = .425, p < .05). Reisch et al. (2010) tested the hypothesis that negative emotions experienced as psychological pain would exhibit decreased neural activity in the frontal cortex. Their sample included women who had attempted suicide in the two months prior to the study. Both studies found increased activity in the right cuneus, but apart from that no consistent findings were evidenced across the two studies. Reisch et al. reported decreased PFC activity (left BA46, right BA10), whereas van Heeringen et al. reported increased activity in the right PFC (BA9, BA44). It should be noted that different reference conditions were used. Both studies used the Orbach & Mikulincer Mental Pain questionnaire to assess psychological pain, which has been shown to be reliable and have a high degree of validity (Orbach et al. 2003b; Soumani et al. 2011; van Heeringen et al. 2010).
Studies involving grief
All four studies that reported on grief-related brain activity were conducted in otherwise healthy women. Najib et al. (2004) studied women who ruminated over a romantic relationship that ended during the 4 months prior to the study, and O’Connor et al. (2008) studied women who had lost a mother or sister to breast cancer during the past 5 years. Gündel et al. (2003) used a sample of women who had lost a first-degree relative (father, mother, or husband) during the past 12 months. Kersting et al. (2009) compared women who had lost a preterm baby after induced labor during the past 2 months to women who delivered a healthy baby during the past 12 months.
A common finding in three of the four studies was increased activity in the left cuneus and posterior cingulate cortex (PCC) during grief. Two of the four studies found increased activity in the left lingual gyrus, the cerebellum and right middle temporal gyrus (for details see the Appendix). Conflicting results were reported for the left BA10, BA47, inferior temporal gyrus, nucleus accumbens, the thalamus, ACC, and the right BA47 and lingual gyrus. Striking was the relatively large number of deactivated brain areas reported by Najib et al. (2004)
Studies involving recalled sadness
As shown in Table 1, twelve studies reported on changes in brain activity due to recalled sadness, almost half of which focused exclusively on women and two studies included men only. One study had a mixed-gender sample (Pardo et al. 1993), but reported results for male and female participants separately, which were therefore included as separate entries in the Appendix. All studies involved healthy participants and two studies included a comparison with patients with unipolar depression (Liotti et al. 2002) or MDD (Keedwell et al. 2005). The study by Pelletier et al. (2003) was remarkable because they used professional actors for their ability to self-induce and experience powerful emotional states.
While focusing on brain areas that were reported by at least three studies, evidence for increased activity during recalled sadness was most convincing for the caudate (5 studies), the left insula, cerebellum, and putamen (4 studies), and the right insula (3 studies). Conflicting results were reported for the left BA9, BA10, BA47, the ACC, PCC, and thalamus, and the right BA9, BA10, and BA11.
Discussion
Under the null hypothesis that brain activations and deactivations would be equally frequent, this review found a significant difference between the overall numbers of activations versus deactivations associated with psychological pain. Across all studies (N = 18), increased activity was reported 111 times (see Appendix), whereas decreased activity was reported 73 times (χ2 = 7.85, df = 1, p = .005). When comparing increased activity versus decreased activity for the left and right hemispheres, and medial subcortical area separately, only the medial subcortical area showed significantly more activation than deactivation across all studies (47 increases vs. 15 decreases, χ2 = 16.51, df = 1, p < .0001). It should be noted that two studies did not report on brain deactivations (Gündel et al. 2003; Lane et al. 1997). Moreover, decreased brain activity assessed through cerebral blood flow and blood oxygen level dependency should be interpreted with caution. As the amount of blood available to the brain is approximately constant (Sergent 1994), an increase in blood volume in one region is necessarily accompanied by a decrease of blood in another region, and as such decreased brain activity may be unrelated to a specific brain function at that exact moment.
Despite the fact that this review included studies that were relatively homogeneous with respect to the evoked emotion and mood induction method (recall of autobiographical life events), considerable variation exists in study outcomes. Disregarding any variation in study designs and populations, it is clear from Fig. 1 that no one brain region was activated in all studies. Changes in brain activity were not reported by more than 47.4 % of the studies for any brain region, which is not an uncommon outcome: Mee et al. (2006) found that the most frequently implicated brain areas showed activity changes in 47.8 % of the studies, and an extensive meta-analysis of human brain activity studies on emotion (Phan et al. 2002) reported a maximum support in 62 % of the studies. The latter meta-analysis did not distinguish between left and right hemispheres, which may have contributed to the higher percentage of agreement.
Among the limitations of this review is the relatively small number of included studies (N = 18). Therefore, interpretations should be considered tentative, especially where subsamples are compared. Studies involved primarily healthy study populations, but also people who had been diagnosed with depression and people who had attempted suicide. Although a psychological pain experience is what connected all studies, the presence of depression and suicidal thoughts in some subjects may have had a confounding effect. However, suicidality and depression are conditions that are well known for a high level of psychological pain (Mee et al. 2011; Olié et al. 2010; Reisch et al. 2010) so it would seem inappropriate to exclude those studies. In the following discussion, we use the term ‘psychological pain’ to refer to the broader concept and in reference to all studies that were included in the review. In discussions of differences between subsamples, we use ‘assessed psychological pain’ in reference to the subsample of studies that used actual measures of psychological pain to distinguish them from the grief and recalled sadness subsamples.
Brain areas involved in psychological pain
Figure 1 suggests that the evidence for involvement of brain areas in psychological pain is most convincing for the medial subcortical area. Medial brain areas that were most consistently implicated are the ACC, PCC, thalamus, basal ganglia (putamen and caudate), cerebellum and the cerebellar vermis. These areas all predominantly showed activation, with the ACC and cerebellum receiving the greatest support across studies. The involvement of the ACC in negative emotion has been well established (Bush et al. 2000; Davidson and Irwin 1999). A more recent review (Vogt 2005) suggested that the subgenual ACC is involved in the experience of sadness in particular, and recently the anterior midcingulate cortex was identified with a control function for both negative affect and physical pain (Shackman et al. 2011). Although Phan et al. (2002) found the cerebellum to be involved in emotion, it is not typically associated with emotion. However, evidence for a connection between the cerebellum and negative affect is steadily growing (Borsook et al. 2008; Colibazzi et al. 2010; Moulton et al. 2011; Schmahmann 2004; Wolf et al. 2009). Our findings also showed activation of the medial part of the cerebellum, the cerebellar vermis, during grief and recalled sadness. As this part of the cerebellum is believed to be involved in proprioception and perception of self-motion (Yakusheva et al. 2007), it is as yet unclear how this effect relates to psychological pain. Earlier reviews on pain and emotion (Apkarian et al. 2005; Phan et al. 2002) did not distinguish the cerebellar vermis from the lateral parts of the cerebellum.
With respect to the left and right hemispheres, the bilateral insula also showed cogent evidence for activation. The PFC is among the most frequently implicated areas in this review, in particular BA 9, 10, and 47, but the direction of the activity change is not clear. There appears to be support for bilateral increased activity in the lateral PFC (BA 47). In both the left and right dorsomedial PFC (BA 9), however, the number of studies indicating increased versus decreased activity differs by only one. Bidirectional evidence also exists for the left ventromedial PFC (BA 10), whereas the right ventromedial PFC convincingly shows decreased activity. Right-sided decreased activity is opposite of what was previously found (Sackeim et al. 1982; Tucker 1981). An inverse correlation between medial PFC activity and arousal was recently reported (Gerber et al. 2008), which suggests that decreased right ventromedial PFC activity may reflect increased arousal during psychological pain.
Drevets and Raichle (1998) found reciprocal behavior between lateral and medial PFC areas during intense emotion and suggested a more prominent role of the medial PFC in emotion processing (e.g. appraisal of emotion) while neural activity was suppressed in cognitive processing areas (lateral PFC). After collapsing our findings for the PFC into lateral (BA 44 – 47) and medial (BA 8 – 11) areas, this review found no evidence of reciprocal behavior in the left PFC (both lateral and medial PFC showed increased activity), and only weak evidence for reciprocal behavior in the right PFC, most prominently between BA10 and BA47 (see Fig. 1). In fact, the evidence contradicts Drevets and Reichle’s findings (1998) in that it suggests increased activity in the lateral PFC during psychological pain. Our findings are in line with those of Nielen and colleagues (2009) who provide evidence that bilateral activation of the lateral PFC is associated with negative affect. According to Phan et al. (2002), the medial PFC is commonly activated across emotions, and this was found to be true to a much lesser extent for the lateral PFC. It should be noted that the meta-analysis by Phan et al. (2002) did not take decreased brain activity into account. However, even when ignoring decreased brain activity, our findings do not show a clear difference in involvement between lateral and medial PFC areas in psychological pain.
This review also found convincing evidence for increased activity in the cuneus. However, as noted in one of the reviewed studies (Najib et al. 2004), the cuneus is involved in visual processing, and activation of this region could be the result of more vivid imagery during recall of the psychological pain than of the pain per se. Three of the six studies (George et al. 1996; Gündel et al. 2003; Kersting et al. 2009) that reported increased activity in the cuneus had a visual component as part of their experimental manipulation, and two studies included a recall phase (Najib et al. 2004; Reisch et al. 2010). Moreover, Phan et al. (2002) found a significant effect of visual mood induction methods on occipital cortex activation.
Assessed psychological pain and grief versus sadness
Comparison of activity changes between the subsamples (assessed psychological pain, grief, recalled sadness) showed that most affected brain regions were found across the subsamples, providing support for the assumption that grief and extreme sadness are similar emotions or affective states to what would be considered psychological pain in the strictest sense. It is noteworthy, however, that all studies that observed increased activity in the insula, putamen and caudate were in the sadness subsample (see Appendix).
Colibazzi et al. (2010) found evidence for activation of the parahippocampal gyrus (PHCG) during highly arousing emotions. In our review, activation of the PHCG was reported by two studies, neither of which was in the recalled sadness subsample, which suggests that subjects in the recalled sadness subsample may have experienced less arousal than those in the grief and assessed psychological pain subsample. Treating the assessed psychological pain and grief subsample as one group and testing the number of PHCG activations for differences with the recalled sadness subsample approached significance for activation during grief/assessed psychological pain (Fisher’s exact test, p = .058).
Taken together, these comparisons between subsamples show overlap in affected brain areas. However, as evidenced by brain areas that are uniquely activated during recalled sadness or in the grief/assessed psychological pain subsample only, grief may be a more accurate exemplar of psychological pain than recalled sadness.
Psychological pain versus physical pain
Apkarian et al. (2005) described the brain network for physical pain, based on a meta-analysis of studies that used hemodynamic, neuroelectrical, or neurochemical methods. They concluded that the most important components of the pain network in the brain were the insula, ACC, PFC, thalamus, and the primary and secondary somatosensory cortices. These areas were consistently implicated across modalities of pain (various types of induced pain and pain in clinical settings) and modalities of imaging (e.g. PET, fMRI). Areas that were less consistently implicated were the amygdala, cerebellum, basal ganglia, PCC, posterior parietal cortex, and motor cortices.
Comparing the findings of our review, as indicated in Fig. 1, with the brain areas involved in physical pain shows an overlap with the main pain network, in particular the insula, ACC, PFC and thalamus. Overlap is also observed in secondary areas, in particular the cerebellum, basal ganglia (putamen, caudate), and PCC. A striking difference is that our review found the PCC to be involved in 31.6 % of the studies, whereas Apkarian et al. (2005) found it was involved in about 9 % of the physical pain studies. This suggests a more prominent role of the PCC in psychological pain. Activation of the PCC was also found in almost 40 % of studies on emotion (Phan et al. 2002), specifically fear, sadness, and happiness. Hudson (2000) stated that the PCC has an evaluative role, and Vogt (2005) suggested that the PCC, especially the ventral part, serves the purpose of assessing self-relevance of emotional events. These observations provide corroborating evidence for an appraisal function as an essential component of the psychological pain concept (Meerwijk and Weiss 2011). Our findings also indicate that the cerebellum may play a more prominent role in psychological pain than in physical pain. It was one of the brain regions most consistently activated in our review but appeared to be less consistently implicated in Apkarian’s results.
Phan et al. (2002) found that almost 60 % of studies that used recall to induce emotion reported activation of the insula. As all but one (van Heeringen et al. 2010) of the studies included in our review involved recall of autobiographical events, it is interesting that our review showed activation of the insula in sadness studies only. With respect to physical pain, evidence was provided that the insula is activated in acute pain and less so during chronic pain (Apkarian et al. 2009; Apkarian et al. 2005). Taken together, absence of insular activation during psychological pain may indicate an enduring condition that is more similar to chronic pain.
Involvement of the PFC in both psychological pain and physical pain deserves more detailed attention, as Apkarian et al. (2005) presented convincing evidence of PFC activation in physical pain. Although the PFC was implicated by many studies in this review, the results for PFC involvement in psychological pain were not entirely consistent. The PFC area that does appear to be activated in psychological pain is the lateral PFC (BA 47). We found convincing evidence for decreased activity in the right BA 10, whereas results for the left BA 10 and bilateral BA 9 were only slightly in favor of increased activity. The PFC in general is thought to be involved in cognitive, emotional, and memory functions (Apkarian et al. 2005). Specifically, the ventromedial PFC (BA 10) was suggested to be involved in the representation of positive and negative emotional states (Davidson and Irwin 1999). However, others have ascribed it a role in planning and reasoning (Hudson 2000).
The following brain areas that were implicated in physical pain, did not reach our pragmatic threshold of support by at least three studies: amygdala, posterior parietal cortex, motor cortices, and the primary and secondary somatosensory cortices. However, it should be noted that all these areas showed activation or deactivation in one or two studies (see Appendix). A likely explanation for lack of consistent activation of the motor and somatosensory cortices is the absence of a nociceptive stimulus in psychological pain. The posterior parietal cortex is predominantly involved in planned movement. As such it may have a role in avoiding a painful stimulus. Since there is no stimulus in psychological pain that may be avoided by movement, it is likely that the posterior parietal cortex is not involved in psychological pain. An interesting finding is lack of activation of the amygdala in this review. None of the studies reported activity changes in the right amygdala, and only two studies (Najib et al. 2004; Zubieta et al. 2003) reported decreased activity in the left amygdala. The amygdala is thought to be involved in evaluating emotional importance of stimuli and appears to be especially active in response to fear (Phan et al. 2002). About 7 % of studies in the meta-analysis by Apkarian et al. (2005) reported involvement (both activation and deactivation) of the amygdala in physical pain, which may be attributed to some level of fear due to being subjected to a painful stimulus. The lack of amygdala activity in our review could, to some extent, reflect the fact that fear was not a key element of the studies that were included. In addition, the amygdala has been described as a salience and ambiguity detector in response to external stimuli (Gerber et al. 2008). This neural function may not be as relevant to the processing of internal feelings for which salience may already have been established and ambiguity is not an issue.
Conclusions
This systematic review compared changes in brain activity during the experience of psychological pain. Only two studies that included a measure of actual psychological pain were identified for this review, which included 18 studies in total. Four studies involved current or recalled grief, and 12 studies involved recalled sadness. The latter 16 studies were included because they used a significant autobiographical event to induce a sad mood state and fit the definition of psychological pain that was established for the study. Prior to this review it was assumed that brain function in grief and sadness could be used as proxies of psychological pain (Mee et al. 2006). Although the number of studies included in this review was too small to allow statistical testing between subsamples for most comparisons, the evidence suggests that brain regions associated with actual measurement of psychological pain may be more similar to those involved in grief than to those involved in recalled sadness. The PHCG was found to be activated in two studies: one where psychological pain was assessed (Reisch et al. 2010) and the other involving grief (Najib et al. 2004). In contrast, activation of the insula, caudate and putamen was only observed in the sadness studies. As PHCG activation was related to greater arousal (Colibazzi et al. 2010), these findings suggest that psychological pain may be more intense than recalled sadness, and possibly reflect a higher level of arousal. In line with our earlier definition of the concept, grief may be a clear instance of psychological pain.
The central question in this review was about which brain areas are involved in the experience of psychological pain. Taking into account the potential differences between psychological pain and sadness, the following brain areas are most likely activated during the experience of psychological pain: ACC, PCC, thalamus, cerebellum, and PHCG. Implications for the PFC (BA 9, 10, and 47), with the exception of bilateral BA 47 (activated) and right BA 10 (deactivated), are less conclusive because the number of studies that reported activation and deactivation differed by only one. Based on these observations, the following tentative psychological pain network is proposed (see Fig. 2). Initially, the thalamus processes and relays information related to psychological pain from the various sensory systems to corresponding cortical areas (Hudson 2000). The medial thalamus projects to the ACC, which may play a central role in the affective (unpleasant feeling) component of psychological pain. Bidirectional communication exists between the ACC and the PCC (Price 2000), with the PCC possibly involved in evaluating the self-relevance (appraisal component) of psychological pain. In fact, functional connectivity analysis has revealed a significant correlation between cortical activity in the ventral PCC and ACC during evocation of grief (O’Connor et al. 2007). The PFC is also bidirectionally connected to the ACC (Price 2000) and may serve a memory and planning function during acute psychological pain as well as enduring psychological pain. Furthermore, right ventromedial PFC activity was related to the parasympathetic nervous system (Hilz et al. 2006; Tanida et al. 2004), which is generally thought to represent inhibition (Sherwood 2010). As our review found predominant deactivation of the right ventromedial PFC (BA10), this provides additional support for greater arousal during psychological pain. Bidirectional communication exists between the PFC and the cerebellum (Ramnani 2006; Schmahmann and Pandya 1997). The cerebellum has traditionally been associated with motor coordination; however, more recently evidence has emerged regarding its affective role (Wolf et al. 2009), especially in mediating negative emotion (Colibazzi et al. 2010). Moreover, research indicates that the cerebellum modulates the emotional and cognitive experience of physical pain (Borsook et al. 2008), a function it may serve in psychological pain as well. The cerebellum is also connected to paralimbic structures (Schmahmann and Pandya 1997), among which is the PHCG that may have a memory function, especially for negatively valenced and more arousing memories (Kilpatrick and Cahill 2003). As brain areas are involved in multiple functions, we do not suggest that the brain areas implicated in the proposed neural network are solely dedicated to the experience of psychological pain. Additional research (e.g. functional connectivity analysis) is needed to confirm the components of this proposed network for psychological pain and further clarify their individual roles in the experience of psychological pain.
Schematic of tentative neural network involved in psychological pain. ACC: anterior cingulate cortex, PCC: posterior cingulate cortex, PFC: prefrontal cortex, PHCG: parahippocampal gyrus. (Original source http://commons.wikimedia.org/wiki/File:Brain_bulbar_region.svg, modified with permission under the Creative Commons Attribution Generic 2.5 license)
A final question for this review concerned the comparison of brain areas involved in psychological pain and physical pain. The proposed neural network for psychological pain (see Fig. 2) indicates a partial overlap with the pain network identified by Apkarian et al. (2005): ACC, thalamus, PFC, PCC, and cerebellum. Whether the overlap extends to the bilateral insula, as suggested by Mee et al. (2006) and basal ganglia (putamen, caudate) requires further research, as these areas did not show activation in studies of psychological pain proper or grief. Lack of activation in the amygdala, posterior parietal cortex, motor cortices, and primary and secondary somatosensory cortices is most likely due to absence of a nociceptive stimulus in the studies that were included. Additional research is required to confirm the more frequent activation of the PCC, compared to physical pain, as found in this review. Moreover, additional research may find that subregions of the PFC behave differently in psychological pain and physical pain, as the PFC is a relatively large and heterogeneous area (Apkarian et al. 2005).
Millions of people worldwide may experience psychological pain, and enduring psychological pain may result in decreased mental well-being, pathology, and potentially suicide. Knowledge about brain functioning that underlies the experience of psychological pain can guide development of psychosocial and pharmacological therapies to alleviate psychological pain.
References
Apkarian, A. V., Bushnell, M. C., Treede, R. D., & Zubieta, J. K. (2005). Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain, 9(4), 463–484. doi:10.1016/j.ejpain.2004.11.001.
Apkarian, A. V., Baliki, M. N., & Geha, P. Y. (2009). Towards a theory of chronic pain. Progress in Neurobiology, 87(2), 81–97. doi:10.1016/j.pneurobio.2008.09.018.
Biro, D. (2010). Is there such a thing as psychological pain? and why It matters. Culture, Medicine and Psychiatry. doi:10.1007/s11013-010-9190-y.
Borsook, D., Moulton, E. A., Tully, S., Schmahmann, J. D., & Becerra, L. (2008). Human cerebellar responses to brush and heat stimuli in healthy and neuropathic pain subjects. Cerebellum, 7(3), 252–272. doi:10.1007/s12311-008-0011-6.
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222.
Chavez-Hernandez, A. M., Leenaars, A. A., Chavez-de Sanchez, M. I., & Leenaars, L. (2009). Suicide notes from Mexico and the United States: a thematic analysis. Salud Pública de México, 51(4), 314–320.
Colibazzi, T., Posner, J., Wang, Z., Gorman, D., Gerber, A., Yu, S., et al. (2010). Neural systems subserving valence and arousal during the experience of induced emotions. Emotion, 10(3), 377–389. doi:10.1037/a0018484.
Collins, D. L., Neelin, P., Peters, T. M., & Evans, A. C. (1994). Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. Journal of Computer Assisted Tomography, 18(2), 192–205.
Davidson, R. J., & Irwin, W. (1999). The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences, 3(1), 11–21.
Drevets, W. C., & Raichle, M. E. (1998). Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: implications for interactions between emotion and cognition. Cognition and Emotion. Special Issue: Neuropsychological perspectives on affective and anxiety disorders, 12(3), 353–385.
Eickhoff, S. B., Laird, A. R., Grefkes, C., Wang, L. E., Zilles, K., & Fox, P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926. doi:10.1002/hbm.20718.
Eisenberger, N. I., Lieberman, M. D., & Williams, K. D. (2003). Does rejection hurt? An FMRI study of social exclusion. Science, 302(5643), 290–292. doi:10.1126/science.1089134.
Friston, K. J., Holmes, A. P., Worsley, K. J., Poline, J. B., Frith, C. D., & Frackowiak, R. S. J. (1995). Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping, 2(4), 189–210.
George, M. S., Ketter, T. A., Parekh, P. I., Herscovitch, P., & Post, R. M. (1996). Gender differences in regional cerebral blood flow during transient self-induced sadness or happiness. Biological Psychiatry, 40(9), 859–871. doi:10.1016/0006-3223(95)00572-2.
Gerber, A. J., Posner, J., Gorman, D., Colibazzi, T., Yu, S., Wang, Z., et al. (2008). An affective circumplex model of neural systems subserving valence, arousal, and cognitive overlay during the appraisal of emotional faces. Neuropsychologia, 46(8), 2129–2139. doi:10.1016/j.neuropsychologia.2008.02.032.
Gündel, H., O’Connor, M. F., Littrell, L., Fort, C., & Lane, R. D. (2003). Functional neuroanatomy of grief: an FMRI study. The American Journal of Psychiatry, 160(11), 1946–1953.
Hilz, M. J., Devinsky, O., Szczepanska, H., Borod, J. C., Marthol, H., & Tutaj, M. (2006). Right ventromedial prefrontal lesions result in paradoxical cardiovascular activation with emotional stimuli. Brain, 129(Pt 12), 3343–3355. doi:10.1093/brain/awl299.
Hudson, A. J. (2000). Pain perception and response: central nervous system mechanisms. Canadian Journal of Neurological Sciences, 27(1), 2–16.
Keedwell, P. A., Andrew, C., Williams, S. C., Brammer, M. J., & Phillips, M. L. (2005). A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biological Psychiatry, 58(6), 495–503. doi:10.1016/j.biopsych.2005.04.035.
Kersting, A., Ohrmann, P., Pedersen, A., Kroker, K., Samberg, D., Bauer, J., et al. (2009). Neural activation underlying acute grief in women after the loss of an unborn child. The American Journal of Psychiatry, 166(12), 1402–1410. doi:10.1176/appi.ajp.2009.08121875.
Kilpatrick, L., & Cahill, L. (2003). Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. NeuroImage, 20(4), 2091–2099.
Lancaster, J. L., Rainey, L. H., Summerlin, J. L., Freitas, C. S., Fox, P. T., Evans, A. C., et al. (1997). Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapping, 5(4), 238–242. doi:10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4.
Lancaster, J. L., Woldorff, M. G., Parsons, L. M., Liotti, M., Freitas, C. S., Rainey, L., et al. (2000). Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping, 10(3), 120–131. doi:10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8.
Lane, R. D., Reiman, E. M., Ahern, G. L., Schwartz, G. E., & Davidson, R. J. (1997). Neuroanatomical correlates of happiness, sadness, and disgust. The American Journal of Psychiatry, 154(7), 926–933.
Liotti, M., Mayberg, H. S., McGinnis, S., Brannan, S. L., & Jerabek, P. (2002). Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. The American Journal of Psychiatry, 159(11), 1830–1840.
Macdonald, G., & Leary, M. R. (2005). Why does social exclusion hurt? The relationship between social and physical pain. Psychological Bulletin, 131(2), 202–223. doi:10.1037/0033-2909.131.2.202.
Martin, M. (1990). On the induction of mood. Clinical Psychology Review, 10(6), 669–697.
Mee, S., Bunney, B. G., Reist, C., Potkin, S. G., & Bunney, W. E. (2006). Psychological pain: a review of evidence. Journal of Psychiatric Research, 40(8), 680–690. doi:10.1016/j.jpsychires.2006.03.003.
Mee, S., Bunney, B. G., Bunney, W. E., Hetrick, W., Potkin, S. G., & Reist, C. (2011). Assessment of psychological pain in major depressive episodes. Journal of Psychiatric Research, 45(11), 1504–1510. doi:10.1016/j.jpsychires.2011.06.011.
Meerwijk, E. L., & Weiss, S. J. (2011). Toward a unifying definition of psychological pain. Journal of Loss & Trauma, 16(5), 402–412, doi:10.1080/15325024.2011.572044.
Moulton, E. A., Elman, I., Pendse, G., Schmahmann, J., Becerra, L., & Borsook, D. (2011). Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. Journal of Neuroscience, 31(10), 3795–3804. doi:10.1523/JNEUROSCI.6709-10.2011.
Munro, B. H. (2005). Statistical methods for health care research (5th ed.). Philadelphia, PA: Lippincott Williams & Wilkins.
Murray, H. A. (2008). Explorations in personality (70th ed.). New York, NY: Oxford University Press.
Najib, A., Lorberbaum, J. P., Kose, S., Bohning, D. E., & George, M. S. (2004). Regional brain activity in women grieving a romantic relationship breakup. The American Journal of Psychiatry, 161(12), 2245–2256. doi:10.1176/appi.ajp.161.12.2245.
Nielen, M. M., Heslenfeld, D. J., Heinen, K., Van Strien, J. W., Witter, M. P., Jonker, C., et al. (2009). Distinct brain systems underlie the processing of valence and arousal of affective pictures. Brain and Cognition, 71(3), 387–396. doi:10.1016/j.bandc.2009.05.007.
O’Connor, M. F., Gündel, H., McRae, K., & Lane, R. D. (2007). Baseline vagal tone predicts BOLD response during elicitation of grief. Neuropsychopharmacology, 32(10), 2184–2189. doi:10.1038/sj.npp.1301342.
O’Connor, M. F., Wellisch, D. K., Stanton, A. L., Eisenberger, N. I., Irwin, M. R., & Lieberman, M. D. (2008). Craving love? Enduring grief activates brain’s reward center. NeuroImage, 42(2), 969–972. doi:10.1016/j.neuroimage.2008.04.256.
Olié, E., Guillaume, S., Jaussent, I., Courtet, P., & Jollant, F. (2010). Higher psychological pain during a major depressive episode may be a factor of vulnerability to suicidal ideation and act. Journal of Affective Disorders, 120(1–3), 226–230. doi:10.1016/j.jad.2009.03.013.
Onoda, K., Okamoto, Y., Nakashima, K., Nittono, H., Ura, M., & Yamawaki, S. (2009). Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Social Neuroscience, 4(5), 443–454. doi:10.1080/17470910902955884.
Orbach, I., Mikulincer, M., Gilboa-Schechtman, E., & Sirota, P. (2003). Mental pain and its relationship to suicidality and life meaning. Suicide & Life-Threatening Behavior, 33(3), 231–241.
Orbach, I., Mikulincer, M., Sirota, P., & Gilboa-Schechtman, E. (2003). Mental pain: a multidimensional operationalization and definition. Suicide & Life-Threatening Behavior, 33(3), 219–230.
Pardo, J. V., Pardo, P. J., & Raichle, M. E. (1993). Neural correlates of self-induced dysphoria. The American Journal of Psychiatry, 150(5), 713–719.
Pelletier, M., Bouthillier, A., Levesque, J., Carrier, S., Breault, C., Paquette, V., et al. (2003). Separate neural circuits for primary emotions? Brain activity during self-induced sadness and happiness in professional actors. Neuroreport, 14(8), 1111–1116. doi:10.1097/01.wnr.0000075421.59944.69.
Phan, K. L., Wager, T., Taylor, S. F., & Liberzon, I. (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage, 16(2), 331–348. doi:10.1006/nimg.2002.1087.
Pompili, M., Lester, D., Leenaars, A. A., Tatarelli, R., & Girardi, P. (2008). Psychache and suicide: a preliminary investigation. Suicide & Life-Threatening Behavior, 38(1), 116–121.
Price, D. D. (2000). Psychological and neural mechanisms of the affective dimension of pain. Science, 288(5472), 1769–1772.
Prince, M., Patel, V., Saxena, S., Maj, M., Maselko, J., Phillips, M. R., et al. (2007). No health without mental health. Lancet, 370(9590), 859–877. doi:10.1016/S0140-6736(07)61238-0.
Ramnani, N. (2006). The primate cortico-cerebellar system: anatomy and function. Nature Reviews Neuroscience, 7(7), 511–522. doi:10.1038/nrn1953.
Reisch, T., Seifritz, E., Esposito, F., Wiest, R., Valach, L., & Michel, K. (2010). An fMRI study on mental pain and suicidal behavior. Journal of Affective Disorders. doi:10.1016/j.jad.2010.03.005.
Sackeim, H. A., Greenberg, M. S., Weiman, A. L., Gur, R. C., Hungerbuhler, J. P., & Geschwind, N. (1982). Hemispheric asymmetry in the expression of positive and negative emotions. Neurologic evidence. Archives of Neurology, 39(4), 210–218.
Schmahl, C. G., Vermetten, E., Elzinga, B. M., & Bremner, J. D. (2004). A positron emission tomography study of memories of childhood abuse in borderline personality disorder. Biological Psychiatry, 55(7), 759–765. doi:10.1016/j.biopsych.2003.11.007.
Schmahmann, J. D. (2004). Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. The Journal of Neuropsychiatry and Clinical Neurosciences, 16(3), 367–378. doi:10.1176/appi.neuropsych.16.3.367.
Schmahmann, J. D., & Pandya, D. N. (1997). The cerebrocerebellar system. International Review of Neurobiology, 41, 31–60.
Schnitzler, A., & Ploner, M. (2000). Neurophysiology and functional neuroanatomy of pain perception. Journal of Clinical Neurophysiology, 17(6), 592–603.
Sergent, J. (1994). Brain-imaging studies of cognitive functions. Trends in Neurosciences, 17(6), 221–227.
Shackman, A. J., Salomons, T. V., Slagter, H. A., Fox, A. S., Winter, J. J., & Davidson, R. J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12(3), 154–167. doi:10.1038/nrn2994.
Sherwood, L. (2010). Human physiology: from cells to systems (7th ed.). Belmont, CA: Cengage Learning.
Shneidman, E. S. (1998). Perspectives on suicidology: further reflections on suicide and psychache. Suicide & Life-Threatening Behavior, 28(3), 245–250.
Soumani, A., Damigos, D., Oulis, P., Masdrakis, V., Ploumpidis, D., Mavreas, V., et al. (2011). Mental pain and suicide risk: application of the greek version of the Mental Pain and the Tolerance of Mental Pain scale. Psychiatriki, 22(4), 330–340.
Strotzer, M. (2009). One century of brain mapping using Brodmann areas. Klinische Neuroradiologie, 19(3), 179–186. doi:10.1007/s00062-009-9002-3.
Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain. 3-dimensional proportional system: an approach to cerebral imaging. New York: Thieme. Thieme Classics.
Tanida, M., Sakatani, K., Takano, R., & Tagai, K. (2004). Relation between asymmetry of prefrontal cortex activities and the autonomic nervous system during a mental arithmetic task: near infrared spectroscopy study. Neuroscience Letters, 369(1), 69–74. doi:10.1016/j.neulet.2004.07.076.
Troister, T., & Holden, R. R. (2010). Comparing psychache, depression, and hopelessness in their associations with suicidality: a test of Shneidman’s theory of suicide. Personality and Individual Differences, 49(7), 689–693. doi:10.1016/j.paid.2010.06.006.
Tucker, D. M. (1981). Lateral brain function, emotion, and conceptualization. Psychological Bulletin, 89(1), 19–46.
van Heeringen, K., Van den Abbeele, D., Vervaet, M., Soenen, L., & Audenaert, K. (2010). The functional neuroanatomy of mental pain in depression. Psychiatry Research. doi:10.1016/j.pscychresns.2009.07.011.
Vogt, B. A. (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience, 6(7), 533–544. doi:10.1038/nrn1704.
Wager, T. D., Phan, K. L., Liberzon, I., & Taylor, S. F. (2003). Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage, 19(3), 513–531.
Weiss, S. J., Haber, J., Horowitz, J. A., Stuart, G. W., & Wolfe, B. (2009). The inextricable nature of mental and physical health: implications for integrative care. Journal of the American Psychiatric Nurses Association, 15(6), 371–382. doi:10.1177/1078390309352513.
Wolf, U., Rapoport, M. J., & Schweizer, T. A. (2009). Evaluating the affective component of the cerebellar cognitive affective syndrome. The Journal of Neuropsychiatry and Clinical Neurosciences, 21(3), 245–253. doi:10.1176/appi.neuropsych.21.3.245.
Yakusheva, T. A., Shaikh, A. G., Green, A. M., Blazquez, P. M., Dickman, J. D., & Angelaki, D. E. (2007). Purkinje cells in posterior cerebellar vermis encode motion in an inertial reference frame. Neuron, 54(6), 973–985. doi:10.1016/j.neuron.2007.06.003.
Zubieta, J. K., Ketter, T. A., Bueller, J. A., Xu, Y., Kilbourn, M. R., Young, E. A., et al. (2003). Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Archives of General Psychiatry, 60(11), 1145–1153. doi:10.1001/archpsyc.60.11.1145.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The following tables show areas of changed brain activity during the experience of psychological pain (PP), as implicated by 18 studies included in this review. Results for each study are shown for the study condition in which higher intensity of psychological pain was expected (e.g. higher vs. lower psychological pain, complicated grief vs. noncomplicated grief, sadness vs. neutral mood). The brain areas are non-overlapping and organized under the left and right hemispheres and subcortical medial areas. Green cells in the table indicate increased activity and red cells indicate decreased activity. Dotted cells indicate that a study reported a global brain area, whereas other studies may have distinguished subdivisions.





Rights and permissions
About this article
Cite this article
Meerwijk, E.L., Ford, J.M. & Weiss, S.J. Brain regions associated with psychological pain: implications for a neural network and its relationship to physical pain. Brain Imaging and Behavior 7, 1–14 (2013). https://doi.org/10.1007/s11682-012-9179-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-012-9179-y