Abstract
Chronic Traumatic Encephalopathy (CTE) is a neurodegenerative disease thought to be caused, at least in part, by repetitive brain trauma, including concussive and subconcussive injuries. It is thought to result in executive dysfunction, memory impairment, depression and suicidality, apathy, poor impulse control, and eventually dementia. Beyond repetitive brain trauma, the risk factors for CTE remain unknown. CTE is neuropathologically characterized by aggregation and accumulation of hyperphosphorylated tau and TDP-43. Recent postmortem findings indicate that CTE may affect a broader population than was initially conceptualized, particularly contact sport athletes and those with a history of military combat. Given the large population that could potentially be affected, CTE may represent an important issue in public health. Although there has been greater public awareness brought to the condition in recent years, there are still many research questions that remain. Thus far, CTE can only be diagnosed post-mortem. Current research efforts are focused on the creation of clinical diagnostic criteria, finding objective biomarkers for CTE, and understanding the additional risk factors and underlying mechanism that causes the disease. This review examines research to date and suggests future directions worthy of exploration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
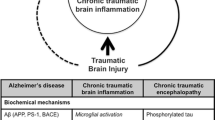
Chronic Traumatic Encephalopathy (CTE) is a neurodegenerative disease thought to be caused, at least in part, by repetitive brain trauma that can occur during contact sports and military participation (McKee et al. 2009). This trauma can include mild traumatic brain injury (mTBI), or concussions, as well as subconcussive injuries, that is, mild brain trauma that does not result in the readily observable signs and symptoms of a concussion (Gavett et al. 2011a; McKee et al. 2009; Spiotta et al. 2011; Stern et al. 2011a). CTE is distinct from the acute sequelae of concussion or traumatic brain injury (TBI), and is not merely prolonged post-concussive syndrome (PCS) (Gavett et al. 2011b). While post-concussive syndrome symptoms endure following an acute concussion without complete relief of symptoms of the initial injury, the symptoms of CTE typically do not present until years after the trauma-producing activity, and the symptoms of initial injury, if any, have ended. CTE is pathologically distinct from other neurodegenerative diseases, including Alzheimer’s disease and Frontotemporal Lobar Degeneration (Corsellis et al. 1973; McKee et al. 2009).
For almost a century, it has been known that repeated blows to the head are associated with cognitive and behavioral impairments later in life. One of the first publications on the topic was a 1928 paper by Martland who called the condition he observed in boxers, “punch drunk.” Martland hypothesized that the symptoms he observed resulted from the repeated blows to the head that these fighters took during their careers (1928). In 1937, Millspaugh outlined the disease marked by motor deficits and cognitive dysfunction under the name “dementia pugilistica,” as he too observed the disorder primarily in boxers. Corsellis and colleagues presented a 15 case series in 1973 that neuropathologically distinguished dementia pugilistica from other neurodegenerative disorders.
Although the term Chronic Traumatic Encephalopathy (CTE) was first used in the literature in the 1960’s, the disease’s ability to affect a broader population beyond boxers was not fully recognized until more recently (McKee et al. 2009; Omalu et al. 2005; Omalu et al. 2006). Since that time, CTE has been found in others with a history of repetitive concussions from sports (e.g., American football players, professional wrestlers, professional hockey players) and from other activities (e.g., a victim of physical abuse, an epileptic, a self-injurer, a circus clown who was repeatedly shot out of a cannon) (Gavett et al. 2011b; Geddes et al. 1999; Hof et al. 1991; McKee et al. 2009; Omalu et al. 2005; Omalu et al. 2006; Omalu et al. 2010; Roberts et al. 1990; Stern et al. 2011a). Also, in recent years, our group at the Boston University Center for the Study of Traumatic Encephalopathy (CSTE) has found neuropathologically confirmed CTE in football players with no history of diagnosed or reported concussions (but who played positions, such as lineman, with the greatest exposure to repetitive hits to the head [Greenwald et al. 2008]), suggesting that repetitive subconcussive trauma, not just symptomatic concussions, may also lead to the development of this neurodegenerative disease (Gavett et al. 2011a; McKee et al. 2009). This paper will review research on CTE to date including its risk factors, clinical presentation, and neuropathology. In addition it will explore future directions for CTE research with a specific focus on methods that may be useful for in vivo diagnosis, including neuroimaging techniques.
Clinical presentation and course
To date, more than 70 retrospective clinical examinations have been conducted by the CSTE with the family members of deceased athletes and military personnel whose brains have been donated for study. The information obtained from the semistructured interview is combined with a review of patient medical records and analyzed by the neuropsychologist [RAS] to gain an understanding of the clinical presentation and progression of the deceased brain donors whose ages range from teens to 80s. During this process the neuropsychologist remains blind to the neuropathological diagnosis, helping to eliminate potential bias; similarly, the neuropathologist [ACM] remains blind to the clinical history and medical records until the neuropathological examination and diagnosis is complete. From these interviews we have been able to gain a greater understanding of the clinical presentation and course of CTE. Although a clinical “picture” of CTE has been created using these retrospective measures, there are currently no consensus-based or prospective neuropathologically validated clinical diagnostic criteria.
Neuropsychological and neuropsychiatric changes
The cognitive and behavioral symptoms associated with CTE are reflective of the regions that have been pathologically determined to be most affected by CTE. As will be explained in further detail in the neuropathology section of this paper, the regions of the brain most severely damaged by CTE include the cerebral cortex and the medial structures of the limbic system (amygdala, mammillary bodies, hippocampus, etc.) (Gavett et al. 2011a; Stern et al. 2011a). The severity of the clinical manifestation progresses through the course of the disease as the neurodegeneration increases (Stern et al. 2011a).
The neuropsychological and neuropsychiatric changes associated with CTE can be classified into the categories of cognition, mood, and behavior (Table 1). CTE presents with changes in each branch of this symptom triad and the severity of the symptoms appears to progress with the course of the disease. These symptoms generally begin years or decades after repeated brain trauma, when the neurodegeneration is severe enough to manifest clinical symptoms (Stern et al. 2011a). The earliest neuropathological stages of CTE may present without clinical symptoms (Stern et al. 2011a). Early cognitive symptoms primarily include learning and memory impairment as well as executive dysfunction. Mood changes typically include depression, apathy, and irritability, as well as suicidality. The behavioral changes primarily include poor impulse control, with individuals described as having a “short fuse” or being “out of control.” Aggression and increased violence are often experienced. Disinhibition and problems with substance and other forms of abuse also occur. Later in the disease course, these cognitive, mood, and behavioral impairments worsen, with dementia evident in all older cases (i.e., 65 years or greater) with advanced stage CTE.
As with most neurodegenerative causes of dementia, the later in the course a patient with CTE is seen, the more difficult it is to differentiate the specific underlying disease based on clinical presentation. That is, once an adequate amount of neural tissue is destroyed, differential diagnosis of most cases of moderate-severe dementia is difficult just based on current presentation. However, the early presentation and course of CTE can distinguish it from most other causes of dementia. The closest symptom profile to CTE is that caused by FTLD, behavioral variant. The symptoms of FTLD typically begin between the ages of 45–65, there is a somewhat rapid symptom progression, and there is a positive family history in approximately 40 % of cases. In contrast, the early symptoms of CTE (Table 1) typically present between the ages of 30 and 50, there is a slow, prolonged course of progression, and there does not appear to be a familial risk. Although not a completely definitive method of distinguishing between CTE and FTLD behavioral variant, all cases of CTE will have had a history of exposure to repetitive brain trauma, whereas FTLD will not typically have such a history.
It is important to note that although CTE is thought to result from repeated mTBI, it is separate from the acute PCS, and it is not the accumulation of immediate symptoms from multiple concussive or subconcussive events. PCS is not thought to directly cause CTE pathology. Given the noticeable overlap in symptomology between PCS and CTE and the fact that, in some cases, there may be overlap in the onset and expression of the two disorders, differentiating between the two can sometimes be difficult (Stern et al. 2011a).
Clinicopathological associations
In a review of the world’s published case studies of neuropathologically confirmed CTE (the vast majority being boxers), McKee et al. noted that 63 % (32 of 51) had memory loss (2009). Like AD, those with CTE appear to have anterograde amnesia, or difficulty remembering newly learned information (Sperling et al. 2010). This is consistent with the deterioration of the hippocampus and other medial temporal structures seen in cases of CTE. Further, individuals with CTE commonly have executive dysfunction (Omalu et al. 2011). Executive functions refer to a group of cognitive abilities responsible for goal-directed behaviors (Stern et al. 2011b); individuals with CTE often have impaired judgment, poor insight, and disinhibition (Gavett et al. 2011a). This symptomology seems to reflect the neuropathologic changes and atrophy of the frontal lobes described by McKee et al. in almost all CTE cases (2009).
Mood and behavior changes are hallmark features of CTE (McKee et al. 2009; Omalu et al. 2011). As with changes from other neurodegenerative diseases, the mood and behavioral changes associated with CTE are often the most concerning to family members and caregivers (Stern et al. 2011b). These clinical manifestations are consistent with the neuropathologic changes in the medial temporal lobe (especially the amygdala) and orbitofrontal regions. The combination of altered emotional responses (including rage) from amygdala involvement and disinhibition and reduced impulse control from frontal involvement appears to lead to many of the more significant clinical manifestations of the disease, including suicidality (Gavett et al. 2011b).
Neurological and motor changes
CTE often results in neurologic dysfunction, especially alterations in movement and motor coordination. These signs include difficulty with balance and gait (parkinsonism) and speech changes (including slowed, slurred, and dysarthric speech) (McKee et al. 2009). In a smaller portion of cases, there appears to be abnormalities in gaze (McKee et al. 2009). A small subset of individuals with CTE have a variant referred to as chronic traumatic encephalomyelopathy (CTEM) that also affects the spinal cord and is associated with motor neuron disease, clinically mimicking Amyotrophic Lateral Sclerosis (ALS), or Lou Gehrig’s disease (McKee et al. 2010). These individuals have a different and more severe neurologic profile including clinical evidence of motor neuron disease as marked by progressive muscle weakness and atrophy, fasciculations, balance and gait problems, dysphagia, and hyperactive deep tendon reflexes (McKee et al. 2010).
Neuropathological characteristics
Neuropathological findings of CTE were first described by Corsellis et al. (1973). McKee and colleagues at the CSTE reviewed the world’s literature of neuropathologically confirmed CTE and found 49 cases at the time (2009). These 49 cases, along with three new cases from the CSTE were described in 2009 by McKee et al. Since that time, the VA CSTE Brain Bank has grown from the original three to over 100 brains with over 60 cases of neuropathologically diagnosed CTE thus far (i.e., not all of the remaining 40 brains have had completed examinations to date), making it, by far, the largest CTE tissue repository in the world. The gross and microscopic neuropathology of CTE described below is based on the combination of the previous literature review and the findings from the VA CSTE Brain Bank.
Gross pathological characteristics
Advanced stages of CTE are accompanied by generalized atrophy of the brain with reduced brain weight, as well as atrophy of the frontal and temporal cortices and medial temporal lobe (McKee et al. 2009). There is often pronounced atrophy of the thalamus, hypothalamus, and mammillary bodies. Thinning of the corpus callosum and generalized atrophy of the cerebral subcortical white matter is common. Pallor of the substantia nigra and locus coeruleus is also a typical feature of advanced CTE. Dilation of the lateral and third ventricles, anterior cavum septum pellucidum, and posterior septal fenestrations are frequent findings (McKee et al. 2009).
A cavum septum pellucidum occurs when the leaflets of the septum pellucidum are separated and the space is filled with cerebrospinal fluid (Tubbs et al. 2011). Repetitive concussive and subconcussive brain trauma likely produces a fluid wave within the ventricles that damages the septum pellucidum (Gavett et al. 2011a; McKee et al. 2009). Cavum septum pellucidum was found in 12 of 13 boxers studied by Corsellis et al. (1973).
Microscopic neuropathological characteristics
Microscopically, CTE is characterized by accumulation of phosphorylated tau protein as neurofibrillary tangles (NFTs), neurites, and glial tangles (GTs) throughout the frontal, insular, and temporal cortices; diencephalon; brainstem; cerebellar dentate nucleus and spinal cord. Figure 1 demonstrates phosphorylated tau deposition in CTE brains as compared to normal control. Accumulations of TAR DNA-Binding Protein 43 (TDP-43) as neuronal and glial inclusions, neurites and intranuclear inclusions are also found in CTE and are usually most prominent in cases with severe tau pathology. Prominent neuronal loss is seen in the hippocampus, entorhinal cortex, and amygdala as well as less severe degrees of neuronal loss in the subcallosal and insular cortex, olfactory bulbs, mammillary bodies, locus coeruleus, substantia nigra, medial thalamus and cerebral cortex (McKee et al. 2009).
Neuropathological analysis section. Coronal sections of a brain immunostained for hyperphosphorylated tau protein and counterstained with cresyl violet. The normal brain on the left shows no deposits of hyperphosphorylated tau protein. The brain on the right with CTE shows irregular tau deposits (dark brown discoloration) in the cerebral cortex. There are also dense tau NFTs in the amygdala (asterisk), entorhinal cortex and medial temporal lobe
The tau-immunoreactive neurofibrillary pathology is characteristically irregular and affects primarily the superficial cortical layers with focal epicenters at the depths of the sulci and surrounding small blood vessels. Tau-immunoreactive NFTs may be particularly dense in the hippocampus, amygdala, entorhinal cortex and olfactory bulbs in advanced stages of the disease (Gavett et al. 2011a; McKee et al. 2009).
Although the specific tau isoforms found in CTE are indistinguishable from AD (Schmidt et al. 2001), the irregular nature of tau deposition and the perivascular clustering of tau-immunoreactive abnormalities at the depth of the sulci are unique to CTE and distinguish it from other tauopathies, including AD (McKee et al. 2009). In addition, the density of the NFTs and GTs is often far greater in CTE than in other tauopathies (Gavett et al. 2011a).
TDP-43 immunoreactivity is most commonly seen in the frontal and medial temporal cortices, brainstem, diencephalon, insula, subcortical white matter, substantia nigra pars compacta, amygdala, hippocampus, caudate, putamen, thalamus, and hypothalamus (McKee et al. 2010; Stern et al. 2011a). TDP-43 immunoreactive inclusions have been found throughout the anterior horn of the spinal cord and motor cortex in a subset of individuals with CTEM (McKee et al. 2010; Stern et al. 2011a).
Aβ deposition is an inconsistent finding in CTE. While neuritic Aβ plaques are an essential feature of AD, Aß is found in only 40–45 % of CTE cases (McKee et al. 2009). When Aß is present in CTE, it generally consists of primarily diffuse plaques with relatively few neuritic plaques (McKee et al. 2009). The presence of tau proteinopathy has been shown to enhance Aβ neurotoxicity (Mann et al. 1990; Roberson et al. 2007).
Brain trauma and other risk factors
To date, all pathologically diagnosed cases of CTE have come from individuals with a history of repetitive brain trauma (McKee et al. 2009). As such, it seems that repetitive trauma is necessary for incurring CTE; however, there are numerous individuals with a history of repeated brain trauma who do not have CTE upon neuropathological examination. Therefore, concussions and other brain trauma alone are not sufficient to cause the disease. Importantly, it is also possible that this repetitive trauma does not necessarily have to be at the concussive (mTBI) or more structural (e.g., TBI) level (Gavett et al. 2011b; McKee et al. 2009; Stern et al. 2011a). Subconcussive brain injury (Spiotta et al. 2011), or a blow to the head with adequate g force to produce a non-structural brain injury (though with the neuronal changes of concussion) that does not result in apparent clinical symptoms, may be a sufficient trauma load to initiate the neurodegenerative cascade (Gavett et al. 2011b; McKee et al. 2009; Stern et al. 2011a). Given that repetitive brain trauma is necessary, but not sufficient, it is evident that other risk factors may be involved in initiating or mediating CTE.
Although all individuals with neuropathologically confirmed CTE have had repetitive brain trauma, the nature of this trauma is a crucial factor that requires further scientific investigation. The age at which the brain starts being exposed to trauma may be a critical factor in determining whether or not an individual develops CTE. It is possible that assaulting a young brain, which is still developing and more vulnerable to injury, may have more catastrophic consequences later in life (Schneider 1979). This theory has yet to be validated, but it has been shown that concussions and brain injuries in youth result in more severe and longer lasting cognitive deficits (Field et al. 2003; Pullela et al. 2006). Additionally, it is not understood whether or not the severity and frequency of the brain trauma influence the development of CTE. Within a given sport, position could play a significant role. A recent study utilizing accelerometers placed inside the helmets of college football players found that there were significant differences in the exposure to brain trauma based on position (Crisco et al. 2010). Further, a study by Talavage and colleagues examined a cohort of high school football players and showed measurable neurocognitive and neurophysiologic deficits after hits to the head that did not cause any reported symptoms of concussion (2010). Importantly, individuals who received subconcussive blows were unlikely to undergo clinical assessment and thus continued playing in the game, exposing their brains to further injury (Talavage et al. 2010).
Some individuals may have a genetic predisposition to developing CTE. Initial studies indicate that the apolipoprotein E (APOE) gene’s ε4 allele may be a useful area of investigation. APOE is the strongest susceptibility gene for Alzheimer’s disease (AD). APOE ε4 has also been associated with longer recovery time and more severe cognitive deficits following single TBI in boxers and professional football players (Jordan et al. 1997; Kutner et al. 2000; Teasdale et al. 1997). APOE ε4 carriers have also been shown to have worse outcomes both in the short-term and in the long-term following head injury (Friedman et al. 1999; Jordan et al. 1997; Katzman et al. 1996; Teasdale et al. 1997). In contrast to its role in AD, it is thought that APOE ε4 may decrease the capacity to repair damage following brain injury (Crawford et al. 2009). Further, older retired football players who were ε4 carriers were shown to have lower cognitive performance (Kutner et al. 2000). Additionally, in a sample of 12 neuropathologically confirmed cases of CTE, 5 (42 %) cases were ε4 carriers with 2 (17 %) of those cases being ε4 homozygous (McKee et al. 2010). This is in contrast to population prevalence studies of the APOE ε4 allele that have shown that at least one ε4 allele is carried by 27–29 % of the population and that ε4 homozygosity only occurs in 1–2 % of the population (Hill et al. 2007). Much more research is required to substantiate the possible link between APOE ε4 and CTE.
There are numerous other risk factors that require further investigation. While it has been shown that females are diagnosed with more concussions and tend to have prolonged recoveries, it is unknown whether this is due to differential symptom reporting between males and females, possibly weaker neck and upper body strength in women, the potential role in sex hormones in concussion, or other variables. It is also unknown whether females have a different CTE-risk profile than their male counterparts (Covassin and Elbin 2011; Dick 2009). To date, the large majority of brains studied with CTE have been male due to the bias of brain donations to date being made by families of deceased football and other collision sport athletes. Furthermore, an individual’s “cognitive reserve” or “brain reserve” may affect the timing of the clinical manifestation of CTE. More specifically, given approximately the same level of neurodegeneration, an individual with a greater cognitive or brain reserve may be less likely to display clinical signs and symptoms of CTE than an individual with a lesser reserve (Schneider 1979). This finding has been shown in several studies of AD and other neurodegenerative diseases (Stern 2007).
Possible diagnostic tools and biomarkers
As with other neurodegenerative diseases, such as AD, FTLD, and Lewy-Body Disease, CTE can only be diagnosed neuropathologically. However, in recent years progress has been made in improving in vivo diagnostic accuracy, especially for AD, through an integration of clinical diagnostic features (e.g., neurological and neuropsychological evaluations, history, course) with more objective biomarkers of disease (Dubois et al. 2007; De Meyer et al. 2010). These biomarker methods include: measurement of proteins through CSF and blood (including beta amyloid and tau), as well as through PET neuroimaging techniques with ligands for beta amyloid; structural neuroimaging (e.g., measurement of hippocampal volume); biochemical neuroimaging (e.g., magnetic resonance spectroscopy; MRS); and genetic susceptibility markers (e.g., APOE genotyping). These approaches have now led to significant changes to research diagnostic criteria for AD, incorporating both clinical and biomarker criteria (Jack et al. 2011; McKhann et al. 2011). A major goal of this new diagnostic approach is to be able to detect disease prior to dementia and even prior to symptom onset in order to intervene more successfully with disease modifying agents when they become available (Sperling et al. 2011).
Similar approaches to accurate in vivo diagnosis of CTE seem quite plausible, utilizing the knowledge already gained in AD biomarker research. In addition to CSF and blood measurements of proteins and genotyping of potential susceptibility genes, there appears to be a large array of neuroimaging techniques that would be appropriate for CTE diagnostic purposes. Of course, as with AD clinical diagnostic criteria, these techniques—if found to be sensitive and specific to CTE—would be part of a multifactorial diagnostic approach, including clinical evaluations and history, as well as CSF and blood measures of proteins. The potential neuroimaging approaches are explored below.
Structural and volumetric magnetic resonance imaging
Gross neuropathological changes attributed to CTE indicate that Magnetic Resonance Imaging (MRI) may be a useful diagnostic technique. Volumetric MRI can detect whole brain atrophy as well as atrophy of specific areas of interest (e.g., amygdala) present in CTE, as well as cavum septum pellucidum with or without fenestrations (McKee et al. 2009). The ability of volumetric MRI to grossly detect CTE-related atrophy was demonstrated in an initial pilot study examining 5 former professional contact sport athletes with CTE-like symptoms (Gavett et al. 2011b).
Susceptibility weighted imaging
A proposed mechanism of CTE pathogenesis begins with a disruption in the blood brain barrier and changes in the cerebral vasculature (Gavett et al. 2011b; McKee et al. 2009). This, along with the hallmark findings of perivascular tau deposition in CTE, indicates that Susceptibility Weighted Imaging (SWI) (a method of detecting microhemorrhages) could be useful for differential diagnosis of CTE. SWI has been found to detect microhemorrhages resulting from neurotrauma (Ashwal et al. 2006). SWI’s current predictive validity has been limited in adults, but there has been some success in utilizing SWI to determine long-term outcome following TBI in children (Chastain et al. 2009; Colbert et al. 2010). More research is required to determine SWI’s clinical utility for understanding the long-term effects of repeated brain trauma, including CTE, in adults (Gavett et al. 2011b).
Diffusion tensor imaging
Diffusion Tensor Imaging (DTI) is sensitive to diffuse axonal injury (see Shenton et al. for a review of structural neuroimaging findings, including DTI, in mTBI in this issue.). This is thought to be one of the causal mechanisms involved in CTE, but is also known to be an injury indicative of acute and chronic TBI (Liu et al. 1999; Prabhu 2011). DTI has been used in both animals and humans to examine the effects of brain trauma (Immonen et al. 2009; Lipton et al. 2009). However, knowledge of DTI’s usefulness in CTE research is limited. In experimental models in rats, DTI has been shown to be predictive of long-term outcomes following TBI (Immonen et al. 2009). DTI has supported the link between executive dysfunction and axonal injuries in humans (Lipton et al. 2009). Further, in our pilot study of 5 former professional athletes, there was an association between overall exposure to repetitive brain trauma and degradation of callosal white matter nerve fiber bundles (Shenton et al. unpublished; Fig. 2). Figure 2 demonstrates the callosal nerve fiber degradation in the contact sport athlete brain as compared to normal control brain.
Diffusion tensor imaging section. Diffusion tensor images captured on a 3 T magnet analyzed with streamline tractography using Slicer 3. Control brain on the left and the brain of a former professional boxer in his 40’s on the right. The top two images are sagittal views with the callosal fiber tracts delineated; it is notable that the boxer’s fiber tracts are markedly shorter than the control. The bottom two images are a coronal view of the same two individuals and it can be seen that the athlete’s corpus callosum (red structure in the middle of the brain) is noticeably thinner than the control
Functional magnetic resonance imaging
Functional Magnetic Resonance Imaging (fMRI) has been useful in understanding brain-behavior relationships in numerous neurologic diseases (Seeley et al. 2009). Additionally, blood oxygen level dependent (BOLD) fMRI has been able to differentiate between various types of neurodegeneration including AD, FTD, and dementia with Lewy bodies (Galvin et al. 2011; Zhou et al. 2010). Recent studies of high school football players have utilized fMRI and found significant changes (from pre-season to post-season) in fMRI results in those athletes with repetitive subconcussive hits (as determined by helmet accelerometer data) (Talavage et al. 2010). Given its current uses, it is possible that fMRI will be helpful in determining brain-behavior associations in CTE as well as differentiating CTE from other neurodegenerative disorders (Gavett et al. 2011b).
Magnetic resonance spectroscopy
Magnetic Resonance Spectroscopy (MRS) utilizes the same clinical MR scanners utilized for MRI to non-invasively measure in vivo brain biochemical metabolites (see Lin et al. for a review of MRS in TBI in this issue). MRS studies have found significant chemical changes in the brains of individuals with various levels of brain trauma; however, most of these studies have been conducted in the acute, rather than the long-term, time frame (Ashwal et al. 2000; Brooks et al. 2001; Cimatti 2006; Henry et al. 2010; Holshouser et al. 2005; Ross et al. 1998; Shutter et al. 2004; Vagnozzi et al. 2008).
However, there has been one pilot study examining the utility of MRS for determining the long-term effects of repetitive brain trauma and possible CTE. In this small-scale study, Lin and colleagues found significant increases in Cho and Glx in former athletes with a history of repetitive brain trauma as compared to age-matched controls (Lin et al. 2010).
Positron emission tomography
Current Positron Emission Tomography (PET) ligands are useful for AD. However these ligands selectively bind to beta-amyloid (e.g., Pittsburgh Compound B or PiB, florbetapir) or bind to both beta-amyloid and tau (2-(1-{6-[(2-[F-18]fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile; FDDNP). However, CTE is primarily a tauopathy with only infrequent beta-amyloid. In cases when it is present, the beta amyloid is aggregated into more diffuse plaques rather than neuritic plaques (McKee et al. 2009). Therefore, tau-selective ligands are likely to play a critical role in in vivo biomarker detection of CTE. Unfortunately, these ligands are not yet available for human imaging.
Single photon emission computed tomography
Single Photon Emission Computed Tomography (SPECT) scans measure regional cerebral blood flow. As such, SPECT is often a sensitive tool for detecting regional abnormalities in brain function. However, the specificity of SPECT is poor (e.g., Masterman et al. 1997). In a recent study comparing a group of retired NFL players and a control group, Amen reported significant differences on SPECT (2011). However, there were many methodological problems with the study, making it difficult to appropriately interpret their results.
Discussion
Although public awareness and media attention surrounding the long-term effects of repetitive concussions and other brain trauma have increased in recent years, scientific knowledge regarding CTE has progressed more slowly, i.e., at a typical speed of scientific discovery. Research related to CTE has been limited thus far, and there are still many questions about this disorder. In fact, some aspects of CTE remain controversial, including the relationship between CTE and other neurodegenerative diseases, such as AD and FTD. Recent research suggests that this disease, previously only known to affect boxers, may be problematic for a much broader population, including other athletes and military personnel. As such, there is an even greater need to understand the mechanism behind this disease (e.g., Blaylock and Maroon 2011), its incidence and prevalence, and how to diagnose, treat, and prevent the disease during life. Knowledge of risk factors could allow for interventions that would help prevent the disease in the future. For example, if an age threshold is found (e.g., individuals who do not experience any brain trauma exposure before a certain age, X, do not go on to develop CTE), then appropriate recommendations and policy changes could follow, (e.g., limiting or restricting activities with brain trauma exposure in children under the age of X). In addition, it is critical to improve understanding of the severity and number of hits necessary to initiate the neurodegenerative cascade leading to CTE. It may be the case that fewer, more severe TBIs result in a different outcome than more frequent, but less severe concussions or subconcussive blows. The different types of forces incurred by the brain during various activities could prove important; however, further research is necessary.
CTE research should utilize the advances made in research of other neurodegenerative diseases such as AD in order to progress most rapidly. For example, Similarities between CTE and other neurodegenerative diseases provide insight into other genes that may be involved in CTE and are therefore worth investigating. Based on studies of other neurodegenerative diseases, additional genetic factors worthy of consideration may be the TARDBP gene involved with TDP-43 protein production in frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS), the GRN gene involved with the production of granulin and associated with FTLD, and the MAPT gene associated with tau protein and FTLD, as well as other genes associated with causes of dementia and/or motor neuron disease.
Examining the applicability of the neuroimaging modalities outlined in this paper as well as clinical measures, basic translational research, animal modeling, and epidemiologic studies will all help advance knowledge of CTE. Increased scientific knowledge about CTE will assist policy makers (e.g., league officials, legislators, military leadership) in creating appropriate guidelines for prevention and treatment of brain trauma whether in the sports arena or on the military battlefield.
Ongoing and future research
Research related to CTE is in its infancy. Although the neuropathology of CTE has been elucidated in recent years, important areas of research remain, including investigations of CTE’s epidemiology, specific risk factors (in addition to repetitive brain trauma exposure), underlying disease mechanism, and the ability to diagnose CTE during life. The development of biomarkers for the purpose of early detection, differential diagnosis, treatment, and prevention has been an important goal of research in other neurodegenerative diseases, such as AD, FTLD, lewy body dementia, and others, and it is also the goal of future CTE research at the BU CSTE (Stern et al. 2011a). With funding from the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, and the National institute of Child Health and Human Development, our group is in the early stages of research investigating potential biomarkers for CTE. This research will includes a number of the diagnostic tools described above, including volumetric MRI, DTI, SWI, MRS, CSF protein determination, and genetic testing, as well as neuropsychological, psychiatric, and neurological examinations. The goal of this research is to create valid diagnostic criteria though the combination of clinical symptoms, history, and objective biomarkers. This research could lead to the ability to diagnose CTE in the early stages of the disease, possibly before the symptoms of the disease present themselves. Neuroimaging strategies could lead to non-invasive methods of diagnosing CTE in the living. Additionally, genetic testing may indicate specific predisposing factors, such as APOE ε4, that could assist in identifying at-risk individuals. In theory, the earlier we are able to diagnose CTE, the better effect interventions may have on the symptoms and disease progression.
Conclusion
CTE is a progressive neurodegenerative disease linked to repetitive brain trauma from contact sports and other activities. The disease is distinct from post-concussive syndrome or the additive symptomatic effect of multiple concussions. Rather, symptoms begin years or decades after brain trauma exposure and include a triad of cognitive, mood, and behavioral impairments. Neuropathologically distinct from other neurodegenerative diseases, CTE is characterized by hyperphosphorylated tau and TDP-43 deposition. As with other neurodegenerative diseases, such as AD, CTE can only be diagnosed postmortem at this time. However, unlike AD, CTE research is in its infancy, and there are neither published and validated clinical diagnostic criteria nor biomarkers for the disease. As such, there are many unanswered questions about the development of CTE. Although it is believed that repetitive brain trauma is associated with the neuropathogenesis of the disease (Stern et al. 2011a), whether CTE can occur following a single traumatic brain injury in at-risk individuals is not yet known. The type, number, and severity of concussive and/or subconcussive hits necessary to trigger the neurodegenerative cascade leading to CTE has yet to be determined (Gavett et al. 2010). Moreover, other factors, including duration of exposure to head trauma, age at first exposure, gender, age, race, and genetic predisposition, may play a role in the development of CTE, although further research is needed in these areas (Gavett et al. 2010; McKee et al. 2009; Stern et al. 2011a). Given its potential to impact a broad population of those who have experienced repetitive brain trauma, CTE is an important public health issue. A critical first step is the ability to diagnose CTE during life. Several neuroimaging techniques have the potential to serve as biomarkers for the disease.

Abbreviations
- Aβ:
-
Beta amyloid
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- APOE:
-
Apolipoprotein E
- APP:
-
Amyloid precursor protein
- BOLD:
-
Blood oxygen level dependent
- Cho:
-
Choline
- CSF:
-
Cerebrospinal fluid
- CTE:
-
Chronic traumatic encephalopathy
- CTEM:
-
Chronic traumatic enceohalomyelopathy
- DTI:
-
Diffusion tensor imaging
- ERP:
-
Event-related potential
- fMRI:
-
Functional magnetic resonance imaging
- FDDNP:
-
2-(1-{6-[(2-[F-18]fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile
- FTD:
-
Frontotemporal dementia
- GT:
-
Glial tangle
- GRN:
-
Granulin
- MAPT:
-
Microtubule-associated protein tau
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Magnetic Resonance Spectroscopy
- NAA:
-
N-acetyl asparate
- NFT:
-
Neurofibrilary tangle
- NT:
-
Neurophil thread
- PCS:
-
Post-concussion syndrome
- PET:
-
Positron emission tomography
- SPECT:
-
Single photon emission computed tomography
- SWI:
-
Susceptibility weighted imaging
- TDP-43:
-
TAR DNA-binding protein 43
- TBI:
-
Traumatic brain injury
References
Amen, D. G., Newberg, A., Thatcher, R., Jin, Y., Wu, J., Keator, D., et al. (2011). Impact of playing American professional football on long-term brain function. Journal of Neuropsychiatry and Clinical Neuroscience, 23(1), 98–106.
Ashwal, S., Holshouser, B. A., Shu, S. K., Simmons, P. L., Perkin, R. M., Tomasi, L. G., et al. (2000). Predictive value of proton magnetic resonance spectroscopy in pediatric closed head injury. Pediatric Neurology, 23(2), 114–125.
Ashwal, S., Babikian, T., Gardner-Nichols, J., Freier, M., Tong, K. A., & Holshouser, B. A. (2006). Susceptibility-weighted imaging and proton magnetic resonance spectroscopy in assessment of outcome after pediatric traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 87(12, Supplement), 50–58.
Blaylock, R. L., & Maroon, J. (2011). Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy-A unifying hypothesis. Surgical Neurology International, 2, 107.
Brooks, W. M., Friedman, S. D., & Gasparovic, C. (2001). Magnetic resonance spectroscopy in traumatic brain injury. Journal of Head Trauma Rehabilitation, 16(2), 149–164.
Chastain, C. A., Oyoyo, U. E., Zipperman, M., Joo, E., Ashwal, S., Shutter, L. A., et al. (2009). Predicting outcomes of traumatic brain injury by imaging modality and injury distribution. Journal of Neurotrauma, 26(8), 1183–1196.
Cimatti, M. (2006). Assessment of metabolic cerebral damage using proton magnetic resonance spectroscopy in mild traumatic brain injury. Journal of Neurosurgical Sciences, 50(4), 83–88.
Colbert, C. A., Holshouser, B. A., Aaen, G. S., Sheridan, C., Oyoyo, U., Kido, D., et al. (2010). Value of cerebral microhemorrhages detected with susceptibility-weighted MR Imaging for prediction of long-term outcome in children with nonaccidental trauma. Radiology, 256(3), 898–905.
Corsellis, J. A., Bruton, C. J., & Freeman-Browne, D. (1973). The aftermath of boxing. Psychological Medicine, 3(3), 270–303.
Covassin, T., & Elbin, R. J. (2011). The female athlete: the role of gender in the assessment and management of sport-related concussion. Clinics in Sports Medicine, 30, 125–131, x.
Crawford, F., Wood, M., Ferguson, S., Mathura, V., Gupta, P., Humphrey, J., et al. (2009). Apolipoprotein E-genotype dependent hippocampal and cortical responses to traumatic brain injury. Neuroscience, 159(4), 1349–1362.
Crisco, J. J., Fiore, R., Beckwith, J. G., Chu, J. J., Bronlinson, P. G., Duma, S., et al. (2010). Frequency and location of head impact exposures in individual collegiate football players. Journal of Athletic Training, 45, 549–559.
De Meyer, G., Shapiro, F., Vanderstichele, H., et al. (2010). Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Archives of Neurology, 67(8), 949–956.
Dick, R. W. (2009). Is there a gender difference in concussion incidence and outcomes? British Journal of Sports Medicine, 43(Suppl 1), i46–i50.
Dubois, B., Feldman, H. H., Jacova, C., Cummings, J. L., Dekosky, S. T., Barberger-Gateau, P., et al. (2007). Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurology, 6(8), 734–746.
Field, M., Collins, M. W., Lovell, M. R., & Maroon, J. (2003). Does age play a role in recovery from sports related concussion? A comparison of high school and collegiate athletes. Journal of Pediatrics, 142, 546–553.
Friedman, G., Froom, P., Sazbon, L., Grinblatt, I., Shochina, M., Tsenter, J., et al. (1999). Apolipoprotein E-epsilon4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology, 52(2), 244–248.
Galvin, J. E., Price, J. L., Yan, Z., Morris, J. C., & Sheline, Y. I. (2011). Resting bold fMRI differentiates dementia with Lewy bodies vs Alzheimer disease. Neurology, 76(21), 1797–1803.
Gavett, B. E., Stern, R. A., Cantu, R. C., Nowinski, C. J., & McKee, A. C. (2010). Mild traumatic brain injury: a risk factor for neurodegeneration. Alzheimer’s Research and Therapy, 2, 18.
Gavett, B. E., Stern, R. A., & McKee, A. C. (2011a). Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clinic in Sports Medicine, 30(1), 179–188.
Gavett, B. E., Cantu, R. C., Shenton, M., Lin, A. P., Nowinski, C. J., McKee, A. C., Stern, R. A. (2011b). Clinical appraisal of chronic traumatic encephalopathy: current perspectives and future directions. Current Opinion in Neurology, 24(6), 525–531.
Geddes, J. F., Vowles, G. H., Nicoll, J. A., & Revesz, T. (1999). Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathologica, 98, 171–178.
Greenwald, R. M., Gwin, J. T., Chu, J. J., & Crisco, J. J. (2008). Head impact severity measures for evaluating mild traumatic brain injury risk exposure. Neurosurgery, 62, 789–798. discussion, 798.
Henry, L. C., Tremblay, S., & Boulanger, Y. (2010). Neurometabolic changes in the acute phase following sports concussions correlate with symptom severity. Journal of Neurotrauma, 27(1), 65–76.
Hill, J. M., Bhattacharjee, P. S., & Neumann, D. M. (2007). Apolipoprotein E alleles can contribute to the pathogenesis of numerous clinical conditions including HSV-1 corneal disease. Experimental Eye Research, 84(5), 801–811.
Hof, P. R., Knabe, R., Bovier, P., & Bouras, C. (1991). Neuropathological observations in a case of autism presenting with self-injury behavior. Acta Neuropathologica, 82, 321–326.
Holshouser, B. A., Tong, K. A., Ashwal, S., & Proton, M. R. (2005). Spectroscopic imaging depicts diffuse axonal injury in children with traumatic brain injury. AJNR American Journal of Neuroradiology, 26(5), 1276–1285.
Immonen, R. J., Kharatishvili, I., Gröhn, H., Pitkänen, A., & Gröhn, O. H. (2009). Quantitative MRI predicts long-term structural and functional outcome after experimental traumatic brain injury. Neuroimage, 45(1), 1–9.
Jack, C. R., Jr., Albert, M. S., Knopman, D. S., McKhann, G. M., Sperling, R. A., Carrillo, M. C., et al. (2011). Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia, 7(3), 257–262.
Jordan, B. D., Relkin, N. R., Ravdin, L. D., Jacobs, A. R., Bennett, A., & Gandy, S. (1997). Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA, 278, 136–140.
Katzman, R., Galasko, D. R., Saitoh, T., Chen, X., Pay, M. M., Booth, A., et al. (1996). Apolipoprotein-epsilon4 and head trauma: synergistic or additive risks? Neurology, 46(3), 889–891.
Kutner, K. C., Erlanger, D. M., Tsai, J., Jordan, B., & Relkin, N. R. (2000). Lower cognitive performance of older football players possessing apolipoprotein E epsilon4. Neurosurgery, 47, 651–657. discussion 657–658.
Lin, A., Ramadan, S., Box, H., et al. (2010). Neurochemical changes in athletes with chronic traumatic encephalopathy. Chicago: Radiological Society of North America.
Lipton, M. L., Gulko, E., Zimmerman, M. E., Friedman, B. W., Kim, W., Kim, M., Gellella, E., et al. (2009). Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology, 252(3), 816–824.
Liu, A. Y., Maldjian, J. A., Bagley, L. J., Sinson, G. P., & Grossman, R. I. (1999). Traumatic brain injury: diffusion-weighted MR imaging findings. American Journal of Neuroradiology, 20, 1636–1641.
Mann, D. M., Brown, A. M., Prinja, D., Jones, D., & Davies, C. A. (1990). A morphological analysis of senile plaques in the brains of non-demented persons of different ages using sliver, immunocytochemical and lectin histochemical staining techniques. Neuropathology and Applied Neurobiology, 16, 17–25.
Martland, H. S. (1928). Punch drunk. JAMA, 91, 1103–1107.
Masterman, D. L., Mendez, M. F., Fairbanks, L. A., & Cummings, J. L. (1997). Sensitivity, specificity, and positive predictive value of technetium 99-HMPAO SPECT in discriminating Alzheimer’s disease from other dementias. Journal of Geriatric Psychiatry and Neurology, 10(1), 15–21.
McKee, A. C., Cantu, R. C., Nowinski, C. J., Hedley-Whyte, E. T., Gavett, B. E., Budson, A. E., et al. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. Journal of Neuropathology and Experimental Neurology, 68(7), 709–735.
McKee, A. C., Gavett, B. E., Stern, R. A., Nowinski, C. J., Cantu, R. C., Kowall, N. W., et al. (2010). TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. Journal of Neuropathology and Experimental Neurology, 69, 918–929.
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Jr., Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia, 7(3), 263–269.
Millspaugh, J. A. (1937). Dementia pugilistica. US Naval Medical Bulletin, 35, 297–303.
Omalu, B. I., DeKosky, S. T., Minster, R. L., Kamboh, M. I., Hamilton, R. L., & Wecht, C. H. (2005). Chronic traumatic encephalopathy in a National Football League player. Neurosurgery, 57, 128–134. discussion, 128–134.
Omalu, B. I., DeKosky, S. T., Hamilton, R. L., Minster, R. L., Kamboh, M. I., Shakir, A. M., et al. (2006). Chronic traumatic encephalopathy in a national football league player: part II. Neurosurgery, 59, 1086–1092. discussion, 1092–1093.
Omalu, B. I., Fitzsimmons, R. P., Hammers, J., & Bailes, J. (2010). Chronic traumatic encephalopathy in a professional American wrestler. Journal of Forensic Nursing, 6, 130–136.
Omalu, B., Bailes, J., Hamilton, R. L., Kamboh, M. I., Hammers, J., Case, M., et al. (2011). Emerging histomorphologic phenotypes of chronic traumatic encephalopathy [CTE] in American athletes. Neurosurgery, 69(1), 173–183. discussion 183.
Prabhu, S. P. (2011). The role of neuroimaging in sport-related concussion. Clinics in Sports Medicine, 1, 103–114.
Pullela, R., Raber, J., Pfankuch, T., Ferriero, D. M., Claus, C. P., Koh, S. E., et al. (2006). Traumatic injury to the immature brain results in progressive neuronal loss, hyperactivity and delayed cognitive impairments. Developmental Neuroscience, 28, 396–409.
Roberson, E. D., Scearce-Levie, K., Palop, J. J., Yan, F., Cheng, I. H., Wu, T., et al. (2007). Reducing endogenous tau ameliorates amyloid beta induced deficits in an Alzheimer’s disease mouse model. Science, 316, 750–754.
Roberts, G. W., Whitwell, H. L., Acland, P. R., & Bruton, C. J. (1990). Dementia in a punch-drunk wife. Lancet, 335, 918–919.
Ross, B. D., Ernst, T., Kreis, R., Haseler, L. J., Bayer, S., Danielsen, E., et al. (1998). 1 H MRS in acute traumatic brain injury. Journal Magnetic Resonance Imaging, 8(4), 829–840.
Schmidt, M. L., Zhukareva, V., Newell, K. L., Lee, V. M.-Y., & Trojanowski, J. Q. (2001). Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer’s disease. Acta Neuropathologica, 101, 518–524.
Schneider, G. E. (1979). Is it really better to have your brain lesion early? A revision of the “Kennard principle”. Neuropsychologia, 17, 557–583.
Seeley, W. W., Crawford, R. K., Zhou, J., Miller, B. L., & Greicius, M. D. (2009). Neurodegenerative diseases target large-scale human brain networks. Neuron, 62(1), 42–52.
Shutter, L., Tong, K. A., & Holshouser, B. A. (2004). Proton MRS in acute traumatic brain injury: role for glutamate/glutamine and choline for outcome prediction. Journal of Neurotrauma, 21(12), 1693–1705.
Sperling, R. A., Dickerson, B. C., Pihlajamaki, M., Vannini, P., LaViolette, P. S., Vitolo, O. V., et al. (2010). Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Medicine, 12(1), 27–43.
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., et al. (2011). Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia, 7(3), 280–292.
Spiotta, A. M., Shin, J. H., Bartsch, A. J., & Benzel, E. C. (2011). Subconcussive impact in sports: a new era of awareness. World Neurosurgery, 75(2), 175–178.
Stern, Y. (2007). Cognitive reserve: Theory and applications. Philadelphia: Taylor & Francis.
Stern, R. A., Riley, D. O., Daneshvar, D. H., Nowinski, C. J., Cantu, R. C., McKee, A. C. (2011a). Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM&R, 10 Suppl 2, S460–467.
Stern, R. A., Anderson, S., & Gavett, B. (2011b). Executive functioning. In A. E. Budson & N. W. Kowell (Eds.), The handbook of Alzheimer’s disease and other dementias (pp. 369–415). New York: Wiley-Blackwell.
Talavage, T. M., Nauman, E. A., Breedlove, E. L., Yoruk, U., Dye, A. E., Morigaki, K., Feuer, H., Leverenz, L. J. (2010). Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. Journal of Neurotrauma, [Epub ahead of print.].
Teasdale, G. M., Nicoll, J. A., Murray, G., & Fiddes, M. (1997). Association of Apolipoprotein E polymorphism with outcome after head injury. Lancet, 350, 1069–1071.
Tubbs, R. S., Krishnamurthy, S., Verma, K., Shoja, M. M., Loukas, M., Mortazavi, M. M., et al. (2011). Cavum velum, interpositum, cavum septum pellucidum, and cavum vergae: a review. Childs Nervous System, 27, 1927–1930.
Vagnozzi, R., Signoretti, S., & Tavazzi, B. (2008). Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes-part III. Neurosurgery, 62(6), 1286–1296.
Zhou, J., Greicius, M. D., Gennatas, E. D., Growdon, M. E., Jang, E., Jang, J. Y., Rabinovici, G. D., et al. (2010). Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain, 133(5), 1352–1367.
Acknowledgments
This work was supported by grants from the National Institutes of Health (P30 AG13846; R01 NS078337), as well as a grant from the National Operating Committee on Standards for Athletic Equipment, and an unrestricted gift from the National Football League.
Conflict of interest
No authors on this paper have conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baugh, C.M., Stamm, J.M., Riley, D.O. et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging and Behavior 6, 244–254 (2012). https://doi.org/10.1007/s11682-012-9164-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-012-9164-5