Abstract
Traumatic brain injury (TBI) is a leading cause of death and disability in children, yet little is known regarding the pattern of TBI-related microstructural change and its impact on subsequent development. Diffusion tensor imaging (DTI) was used to examine between-group differences at two time points (planned intervals of 3 months and 18 months post-injury) and within-group longitudinal change in a group of children and adolescents aged 7–17 years with moderate-to-severe TBI (n = 20) and a comparison group of children with orthopedic injury (OI) (n = 21). In the 3- and 18-month cross-sectional analyses, tract-based spatial statistics (TBSS) generally revealed decreased fractional anisotropy (FA) and increased apparent diffusion coefficient (ADC) in the TBI group in regions of frontal, temporal, parietal, and occipital white matter as well as several deep subcortical structures, though areas of FA decrease were more prominent at the 3-month assessment, and areas of ADC increase were more prominent at the 18 month assessment, particularly in the frontal regions. In terms of the within-group changes over time, the OI group demonstrated primarily diffuse increases in FA over time, consistent with previous findings of DTI-measured white matter developmental change. The TBI group demonstrated primarily regions of FA decrease and ADC increase over time, consistent with presumed continued degenerative change, though regions of ADC decrease were also appreciated. These results suggest that TBI-related microstructural changes are dynamic in children and continue until at least 18 months post-injury. Understanding the course of these changes in DTI metrics may be important in TBI for facilitating advances in management and intervention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pediatric traumatic brain injury (TBI) is the most common cause of death and disability among children. Nearly half a million children aged 0–14 years in the United States visit the emergency department each year for TBI-related injuries, with about 25% of these injuries considered to be moderate to severe (Faul et al. 2010). Despite the prevalence of pediatric TBI, information is limited regarding the course of recovery within brain parenchyma following head injury in the developing brain.
Neuroimaging has been considered an important tool in better understanding the sequelae of injury, as it allows in vivo examination of the consequences of injury. However, studies using standard imaging techniques such as computed tomography (CT) and conventional magnetic resonance imaging (MRI) sequences to perform lesion analysis are typically limited to selecting specific regions of interest for analysis (Gale and Prigatano 2010; Schonberger et al. 2009) that only partially address the relation between more diffuse injury to brain parenchyma and outcome, with some studies also demonstrating the limits of simply measuring the size or location of focal injury and later outcome (Chastain et al. 2009; Salorio et al. 2005).
Studies utilizing more advanced neuroimaging techniques such as diffusion tensor imaging (DTI) of white matter have more consistently reported stronger relations with outcome variables, suggesting that more diffuse injury to white matter, likely via detection of traumatic axonal injury, may be a better predictor than focal injury of outcome in global assessment of functioning (Kinnunen et al. 2010; Marquez de la Plata et al. 2010; Warner et al. 2010a; b). DTI has been used to probe the integrity of white matter in specific brain areas through common DTI-derived metrics such as fractional anisotropy (FA) and apparent diffusion coefficient (ADC; also referred to as mean diffusivity or MD) (Huisman et al. 2004; Alexander et al. 2007). FA is derived from the tendency of water molecules to move preferentially in parallel (rather than perpendicular) to barriers to free diffusion such as fibers, axons, or other support cells. A high degree of anisotropic diffusion has been shown to be related to homogeneity in fiber orientation, increased fiber density or axonal diameter, and the ratio of intracellular/extracellular space. ADC represents the average diffusivity of free water movement within tissue, which also enables inferences regarding the microstructure in tissue; generally higher average diffusivity is indicative of decreased fiber density, axonal diameter, decreased myelination and/or increased extracellular space.
The validity of DTI metrics in the study of TBI-related pathology has already been established through significant correlation of FA and ADC (or mean diffusivity) values as well as other DTI-related metrics with injury indicators such as the Glasgow Coma Scale (GCS) and outcome measures in adult patients with head trauma (Kumar et al. 2009; Benson et al. 2007; Kraus et al. 2007; Huisman et al. 2004; Sidaros et al. 2008; Perlbarg et al. 2009). Additionally, this has been established in limited studies involving children with TBI using FA (Wozniak et al. 2007; Yuan et al. 2007) as well as both FA and ADC, or other DTI metrics (Levin et al. 2008; Ewing-Cobbs et al. 2008; Yuan et al. 2007; Wozniak et al. 2007; Wilde et al. 2006). FA reductions, ADC increases, and/or changes in other DTI-derived metrics following TBI in children and adolescents include, but are not necessarily limited to, the corpus callosum, uncinate fasciculus, cingulum bundle, corticospinal tract, posterior limb of the internal capsule, and the external capsule (Arfanakis et al. 2002; Huisman et al. 2004; Inglese et al. 2005; Wilde et al. 2006; Ewing-Cobbs et al. 2008). However, only a few studies have utilized whole brain techniques in children to uncover changes occurring throughout the brain on a voxel by voxel basis (Yuan et al. 2007). Additionally, despite consistent evidence of widespread degenerative changes detected with DTI in adult and child patients with moderate to severe TBI in the chronic post-injury period, relatively few longitudinal studies have been undertaken, meaning that the nature, degree, and course of degenerative TBI-related changes in the developing brain remain poorly understood. The purpose of the current study is to examine differences in white matter using tract-based spatial statistics (TBSS), a method of global brain analysis through white matter skeletonization, to examine injury-related differences in children and adolescents with TBI in comparison to a group of orthopedically-injured (OI) children at both 3 and 18 months post-injury, and within-group changes longitudinally. Because FA and ADC may be differentially sensitive to factors presumed to underlie white matter integrity following TBI (e.g., demyelination or dysmyelination, neural degeneration, etc.) and because previous TBI studies have reported variation in the degree of group differences observed between these metrics as well as differences in the magnitude of relation with outcome measures, we examined both DTI metrics. We hypothesized that the TBI group would demonstrate multiple regions of significantly lower FA and increased ADC at both assessments, reflecting decreased fiber integrity, in relation to the OI group, that would be particularly apparent in the frontal and temporal regions as these are highly vulnerable to TBI-related injury. Next, we wished to explore changes within each group between the 3- and 18-month assessments to determine the locations and degree of change evident in this recovery interval. We hypothesized that within the OI group, regions of relative increase in FA and decrease in ADC would emerge by 18 months, particularly in frontal and temporal regions, presumed to reflect developmental myelination in these brain areas in children and adolescents within this age range (see Lenroot & Giedd, 2006). In the TBI group, we hypothesized that we would see evidence for continued degenerative change in these regions manifest by decreased FA and increased ADC by 18 months secondary to TBI-related disruption of normal development.
Methods and materials
Participants
This research protocol was approved by the Institutional Review Board of the Baylor College of Medicine and other affiliated institutions involved in this project. Informed consent was obtained from each participant or his/her legal guardian prior to enrollment in the study. The cohort of participants included in this study was selected from a larger sample of participants engaged in a longitudinal examination of the cognitive and imaging effects of pediatric brain injury (see also Levin et al. 2011; McCauley et al. 2011; Oni et al. 2010; Wilde et al. 2010; Wu et al. 2010). In the current report, where the objective was to examine the effects of TBI on brain tissue over time as assessed by DTI, we included only those participants who had useable DTI data at both 3 and 18 months post-injury time points. Although the actual mean post-injury interval for the initial assessment was closer to 4 months than 3 months, the study design included a planned assessment at 3 months post-injury, and we refer to the initial assessment as the “3-month” assessment.
The TBI group was composed of 20 participants (11 males and 9 females) between the ages of 8.2 and 17.5 years (mean = 13.6. ± 2.9) who sustained either complicated mild or moderate-to-severe TBI. The severity of the injury was determined by the lowest postresuscitation Glasgow Coma Scale (GCS) scores (Teasdale and Jennett 1974). Severe TBI was defined as a GCS score of 3–8, and moderate TBI as GCS score of 9–12. A complicated mild TBI was defined as a GCS score of 13–15, but with acute (within 24 h post-injury) trauma-related CT findings (e.g., contusion, hematoma, etc.). Participants with complicated mild TBI were included in our “moderate to severe” patient cohort because the presence of cerebral lesions on CT has been demonstrated to be predictive of poor cognitive outcome at 12 months post-injury, and more similar to the outcome of individuals who experience moderate TBI than those who experience uncomplicated mild TBI (Levin et al. 2009). Given these classifications, there were 13 severe, 4 moderate and 3 complicated mild cases. The mean modified Injury Severity Scale (m-ISS; not including the head region) score for individuals with TBI was 22.6 ± 11.6 (range 12.0–50.0). All participants in the TBI group had at least one focal lesion; the most common site for focal lesion was in the frontal lobes, followed by the temporal lobes and parietal lobes. Lesion volume for lesions in the frontal lobes ranged from 0.06 to 23.02 cubic centimeters. Table 1 lists focal lesion location for each participant used in this analysis.
Participants in the OI group included 15 males and 6 females, and had a mean age of 12.1 ± 2.5 years. The mean m-ISS score for individuals with OI was 6.0 ± 2.5 (range 4.0–9.0).
The selection of participants with OI was designed to equate the groups by controlling for risk factors that predispose individuals to accidents including pre-existing behavioral problems, family variables, and subtle learning disabilities (Stancin et al. 1998). The OI group included participants who were hospitalized overnight for bone fractures of the upper or lower extremities. Mechanisms of injury for this group included predominantly sports and play-related falls. A detailed developmental questionnaire was administered to legal guardians to confirm the absence of significant previous head trauma in the OI children and was cross-referenced with medical records and/or physician report of relevant history and, when available, clinical imaging results that include negative CT scans for the OI group.
All participants in both the TBI and OI groups had no pre-injury history of neurologic or psychiatric disorders and were recruited from consecutive admissions to emergency rooms at Level-1 trauma centers in Houston, Dallas, and Miami. In both groups, participants were excluded if there was any evidence of previous head injury, child abuse, pre-existing neurologic disorders (e.g. epilepsy), diagnosed learning disabilities, pre-existing severe psychiatric disorders (e.g. schizophrenia), prematurity or low birth weight (birth weight <2,500 g and gestation <37 weeks), penetrating injury, hypoxia (PO2 <96 mmHg) or hypotension (systolic blood pressure 2 standard deviations below the mean for the age group). Detailed information for demographic and injury characteristics for both groups appears in Table 2.
Imaging protocols
All participants underwent MRI without sedation on Philips 1.5 Tesla Intera or Achieva scanners (Philips, Cleveland, OH) at Texas Children’s Hospital in Houston, the Rogers MRI Center or University of Texas Southwestern Medical Center in Dallas, or the Miami Children’s Hospital in Miami using comparable platforms and software. Regular quality assurance testing was performed on all three scanners including American College of Radiology (ACR) phantom testing, and all scanners were consistently noted to be within a range considered acceptable throughout the course of the study. Additionally, similar ranges of values for Weisskoff stability measurements (Weisskoff 1996) (minimum 1/SNR index, peak-to-peak and RMS stability) taken on the day of scan indicated stability of scanners over time. Participants from both the OI and TBI groups were scanned at each site (Dallas: 5 TBI, 12 OI; Houston: 6 TBI, 7 OI; Miami: 9 TBI, 2 OI), and no systematic differences were detected in the quantitative DTI values derived from each site for the OI participants.
DTI parameters included an axial single-shot spin-echo echo-planar imaging sequence with 15 diffusion-encoding directions; 256-mm field of view (FOV); acquisition voxel size, 2.69 × 2.69 × 2.7 mm3; repetition time (TR), 6318.0 ms; echo time (TE), 51 ms, sensitivity encoding (SENSE) reduction factor of 2; 2 B factors with 0 s/mm2, low B, and 860 s/mm2, high B), with 2 acquisitions to average the signal of the two DTI scans in order to ensure better signal-to-noise ratio. Acquisitions consisted of 55 slices. A SENSE 8-channel head coil was used.
Image processing
All pre-processing was completed with the Functional MRI of the Brain (FMRIB) diffusion toolbox (FDT) software included in the FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl) (see also Smith et al. 2006; Smith et al. 2007 for additional detail). Head motion and eddy current artifact were corrected via a linear, affine registration algorithm, and DTI metrics (e.g. FA and ADC) were performed using FDT’s automated calculation. FSL’s brain extraction tool (BET) was used to remove all non-brain voxels from the zero-diffusion weighted image of each tensor of interest (i.e., fractional anisotropy (FA) and apparent diffusion coefficient (ADC) (Smith, 2002).
Tract-based spatial statistics analysis
All subjects' FA data were then aligned into a common space using the nonlinear registration tool FNIRT, which uses a b-spline representation of the registration warp field. The target image used in the registrations was chosen to be the most “typical” subject in the study in order to generate a study-specific FA target image. This target image was then affine-aligned into MNI152 standard space. All participants’ images were then transformed into standard 1 × 1 × 1 mm3 MNI152 space by combining the nonlinear transform to the target FA image with the affine transform from that target to MNI152 space. This resulted in a standard-space version of each subject’s FA image, which was then merged into a single FA 4D image containing all participant data. For ADC images, the FA transformation matrices were utilized to achieve the same nonlinear registration. All subjects’ ADC data were then merged into a single 4D image volume. A general linear model was used to analyze group differences (cross-sectional and longitudinal) using a voxel-wise analysis utilizing permutation-based testing to analyze differences in FA and ADC. Threshold free cluster enhancement was used as a correction tool for multiple comparisons.
Other statistical analyses
Demographic statistics between the TBI and OI group were compared using Chi-square testing (for gender, race/ethnicity, high-versus low-velocity mechanism of injury) or Fisher’s exact testing (for handedness and mechanism of injury) for categorical variables. A Student t-test analysis was performed for: 1) age at injury at both 3- and 18-month assessments, 2) post-injury interval for both 3- and 18-month assessments, and 3) Socioeconomic Composite Index (SCI) z-score (Yeates et al. 1997). The threshold for statistical significance was set at p < 0.05.
Results
Demographic results
The two groups did not differ significantly for post-injury interval for either 3- or 18-month assessment, handedness, gender, or socioeconomic status as measured by SCI z-score. The TBI and OI groups differed significantly in the mechanism of injury with TBI patients sustaining a majority of motor vehicle accidents, and OI participants sustaining a majority of sports- and play-related injuries; when categorized as high- versus low-speed injuries, the groups significantly differed (χ²(1) = 15.7, p = 0. 0001). As expected, m-ISS scores also significantly differed between groups (t(18.43) = -5.92), p = <0.0001), with the TBI group having a higher mean m-ISS score. Groups tended to differ for age at injury at both the 3-month assessment (t(39) = -1.85, p = 0.07) and the 18-month (t(39) = -1.89, p = 0.07) assessments, with the TBI group being slightly older than the OI group. Groups also differed in race/ethnicity (χ²(2) = 5.7, p = 0.06), with the TBI group having a higher proportion of Hispanic participants, and the OI group having a higher proportion of African-American participants. For a detailed list of means and demographic and injury characteristics, please see Table 2.
TBSS results
All results are bilateral unless otherwise specified.
Group differences between TBI and OI at 3 months
Regions of significantly lower FA in the TBI as compared to the OI group included: frontal, superior temporal, parietal, and occipital white matter, temporal stem, left peri-insular white matter, corpus callosum, cingulum bundle, fornix, thalamus, dorsal pons, and cerebellar white matter. Regions of increase in the FA of the TBI group compared to the OI group were found in the occipital white matter, left putamen, inferior temporal white matter, and thalamus.
Regions of significantly higher ADC in the TBI as compared to the OI group included: frontal, posterior temporal, and parietal white matter, genu and midbody of the corpus callosum, midbody and posterior cingulum bundle, visual cortical white matter, centrum semiovale, thalamus, internal capsule, left lateral midbrain, and cerebellar white matter. Regions of lower ADC at 3 months post-injury in the TBI group compared to the OI group included: pons, middle temporal white matter, left putamen, anterior cingulum bundle, caudate nucleus, and splenium of the corpus callosum. These findings are summarized in Table 3.
Group differences between TBI and OI at 18 months
Regions of significantly lower FA in the TBI as compared to the OI group included: frontal, temporal, occipital and parietal white matter, corpus callosum, cingulum bundle, anterior commissure, right thalamus, fornix, and cerebellar white matter. Regions of significantly higher FA in the TBI group as compared to the OI group include: pons, midbrain, visual cortical white matter, left thalamus, and parietal white matter (not overlapping with the decreases in FA in the same lobe of the brain).
Regions of significantly higher ADC in the TBI as compared to the OI group included: frontal, right temporal, and parietal white matter, genu, midbody, and splenium of the corpus callosum, thalamus, midbrain and right cerebellar white matter. The TBI group showed significantly lower regions of ADC at 18 months post-injury in the pons, left temporal, caudate nucleus, and parietal white matter. As with the 3-month assessment data, corresponding brain structures that exhibited group differences in FA and ADC were not necessarily overlapping within the same general region; for example, differences in ADC in the temporal lobes were located anterior to differences in FA. Findings are summarized in Table 4.
Longitudinal analyses: TBI group
Regions of significantly increased FA were seen from 3 to 18 months post-injury in the TBI group in the putamen, posterior temporal lobe, and thalamus. Regions of significantly decreased FA included frontal, anterior and central temporal, occipital and parietal white matter, genu and splenium of the corpus callosum, cerebellum, brainstem, and cingulum bundle.
Regions of significantly decreased ADC included frontal and parietal white matter, pons, posterior temporal, thalamus, splenium and body of the corpus callosum, and cerebellar white matter. Regions of significantly increased ADC could be seen over time in the parahippocampal and anterior temporal white matter, genu of the corpus callosum, and parietal white matter. Findings are summarized in Table 5.
Longitudinal analysis: OI group
The OI group showed significant increases in FA between the two time points in the cerebellum, temporal, frontal, occipital, and parietal lobe white matter, midbrain, fornix, thalamus, insula, putamen, and whole corpus callosum.Very limited regions of FA decrease between 3 to 18 months post-injury were seen in the temporal stem and scattered throughout the subcortical parietal white matter.
ADC was significantly increased in the OI group in regions of the cerebellar white matter, thalamus, and caudate. Both increases and decreases in ADC were found in the temporal, frontal, occipital, and parietal lobe white matter. Regions with significantly decreased ADC included anterior and posterior temporal white matter, temporal stem, genu and splenium of the corpus callosum. Findings are summarized in Table 6.
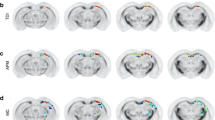
Figure 1 illustrates results of between-group comparisons on multiple 2D slices as well as within-group changes for both FA and ADC for all analyses described above. Figure 2 illustrates these changes in a 3D format to better appreciate the pattern and extent of change in context of the whole brain. Table 7 provides a listing of the 10 largest cluster sizes (with number of voxels in the cluster and location) for FA-related changes (increases and decreases) in the TBI group at 3- and 18-month assessments.
Images are derived from TBSS results and rendered upon a series of two-dimensional axial T1-weighted images from a template image. Part A illustrates group differences at 3 months post-injury in TBI versus OI participants. Part B illustrates group TBI versus OI group differences at 18 months post-injury. Part C reflects changes within the TBI group (3 month–18 month), and part D reflects changes within the OI group over time. The following is applied to the TBI group in all comparisons a-d: areas denoted in red represent areas of lower ADC, areas in blue indicate areas of higher ADC, areas in green represent areas of lower FA, and areas in yellow represent areas of higher FA. For each image, the right hemisphere of the brain is portrayed on the left hand side of each slice, consistent with radiologic convention
Part a illustrates group differences at 3 months post-injury in TBI versus OI participants derived from TBSS and rendered upon a series of three-dimensional T1-weighted images from a template image as this allows a more global perspective of changes occurring throughout the brain. Part b illustrates group TBI versus OI group differences at 18 months post-injury. Part c reflects changes within the TBI group (18 month–3 month), and part d reflects changes within the OI group over time. The following is applied to the TBI group in all comparisons (a-d): areas denoted in red represent areas of lower ADC, areas in blue indicate areas of higher ADC, areas in green represent areas of lower FA, and areas in yellow represent areas of higher FA
Discussion
Consistent with our expectation, the children and adolescents with moderate–to-severe TBI demonstrated multiple regions of significantly lower FA and increased ADC at both the planned 3- and 18-month assessments in relation to the OI group. Our findings are consistent with previous reports using participants with moderate-to-severe TBI in chronic post-injury intervals using voxel-based DTI analytic techniques (Bendlin et al. 2008; Xu et al. 2007; Salmond et al. 2006; Sidaros et al. 2008; Arfanakis et al. 2002; Huisman et al. 2004). For example, Xu et al. and Salmond et al. both used holistic voxel-based analysis to report decreased anisotropy in major white matter tracts of adult TBI patients in regions of the corpus callosum, internal and external capsules, and cortical white matter (Salmond et al. 2006; Xu et al. 2007). Our results are also consistent with those reported in region of interest (ROI) approaches to DTI analyses which found decreased FA in the corpus callosum, posterior limb of the internal capsule, centrum semiovale, and the cerebral peduncles in adults with TBI as compared to OIs (Sidaros et al. 2008). Finally, these findings are consistent with changes in both adults and children following TBI using other advanced imaging modalities such as volumetric analysis of white matter, suggesting that TBI affects virtually all major regions of the brain (Bendlin et al. 2008; Bigler et al. 2010).
We had hypothesized that TBI-related DTI changes would be most apparent in the frontal and temporal regions of the brain. Acknowledging that the exact areas of identified injury for a cohort in group analyses of moderate-to-severe TBI will differ by study due to the location, extent, severity, and mechanism of focal injury in the individuals within that particular cohort, our study indicated prominent involvement of frontal regions in our pediatric TBI group. DTI-related changes such as those demonstrated in the frontal lobes on the between-group analyses at 3 and 18 months are expected given the known vulnerability of this region to mechanical forces during TBI and the prevelance of resulting focal lesions, as evidenced in our sample. However, there was evidence for continued deleterious change as measured by decreases in FA and increases in ADC in this region over time in the within-group analysis of the TBI group as illustrated in Figs. 1 and 2. It is possible that injury sustained during childhood and adolescence, a period where frontal white matter is still maturing structurally and physiologically, may alter the course of normal development in this important region presumed to be involved in executive functioning, attention and cognitive and behavioral control (Levin and Hanten 2005; Liston et al. 2006; Wozniak et al. 2007).
Next, we had anticipated that within the OI group, regions of relative increase in FA and decrease in ADC would emerge by 18 months post-injury, particularly in frontal and temporal regions, presumably reflecting developmental myelination in these brain areas in children and adolescents within this age range. Indeed, increased FA was found in several regions over time in the OI group. Generally, studies related to white matter development utilizing DTI have demonstrated age-related increases in FA with concomitant decreases in ADC, most likely reflecting the ongoing process of myelination, though synaptogenesis and synaptic pruning continue throughout childhood and may also contribute to changes in these metrics. Various white matter structures have been reported to show changes in DTI-derived metrics in studies of normal development, consistent with several of the regions that were demonstrated in the current study (Ashtari et al. 2007; Barnea-Goraly et al. 2005; Bonekamp et al. 2007; Clayden et al. 2011; Eluvathingal et al. 2007; Giorgio et al. 2010; Lebel et al. 2008; Muetzel et al. 2008; Schmithorst et al. 2002). We note that FA increases were not as restricted to the frontal and temporal white matter as we predicted, but were located diffusely throughout the brain. Additionally, we note that the within-group changes predominantly manifested as increased FA, with fewer than expected concomitant changes in decreased ADC. The increases in FA were notably absent in the TBI longitudinal analysis, potentially indicating alteration in the timing or the course of the expected developmental trajectory in participants with TBI. Additional research will need to be conducted to more precisely determine the mechanism underlying these white matter changes and the course these changes follow.
Finally, in the within-group analysis of the TBI group, we anticipated finding evidence for continued degenerative change in brain regions, as manifest by decreased FA and increased ADC over time, particularly in the frontal and temporal lobes. We note that few longitudinal studies using DTI in moderate-to-severe TBI have been reported. Bendlin et al. reported that adult patients with moderate-to-severe TBI exhibited decreased FA and increased mean diffusivity (MD) over time in several major fiber bundles including the corpus callosum, cingulum bundle, and the inferior fronto-occipital fasciculus (Bendlin et al. 2008). Similarly, Sidaros and colleagues reported that adult patients with TBI evidenced decreased FA and increased ADC over time in the splenium of the corpus callosum and cerebral peduncle (Sidaros et al. 2008). Kumar et al. reported persistent decreases in FA at 6 months post-injury in the genu of the corpus callosum and the anterior and posterior internal capsule as well as additional areas of FA decrease in other aspects of the corpus callosum in participants with TBI as compared to controls (Kumar et al. 2009). In the only other study reporting longitudinal changes in a pediatric cohort of moderate-to-severe TBI, Wu and colleagues restricted analysis to tractography in the corpus callosum and found ADC increases in the corpus callosum in TBI and OI groups at 18 months versus 3 months post-injury (Wu et al. 2010). Our findings were generally consistent with these previous reports.
However, it is important to note that at both time points, and longitudinally within the TBI group, both increases and decreases in the FA and ADC were observed. Generally, the expected degenerative changes (i.e., lower FA and higher ADC in the TBI group) were predominant in the TBI group in between-group comparisons at both assessments, but were interspersed with additional relatively small regions of change in the opposite direction (e.g., higher FA in certain regions in the TBI group). Interestingly, in some of the studies referenced above, each described evidence of deleterious change, but also findings suggestive of modest degrees of apparent recovery when the later time point was compared to the earlier one, as was observed in our current study. For example, Sidaros and colleagues found increased FA in the adult TBI group compared to the OI group in the centrum semiovale and internal capsule (Sidaros et al. 2008; Sidaros et al. 2009). Bendlin et al. reported decreased MD in the adult TBI group over time in regions including the internal capsule and portions of the occipital white matter (Bendlin et al. 2008). Finally, Wu and colleagues also described an increase in FA over time in the pediatric TBI group (Wu et al. 2010). The presence of apparent non-degenerative additional changes in our study is consistent with previous studies despite differences in analysis techniques and methodology. Wu et al. utilized a tractography-based approach to DTI analysis of pediatric patients with TBI and Sidaros et al. analyzed adult TBI patients with a smaller post-injury time interval and using an ROI-based approach (Sidaros et al. 2008; Wu et al. 2010). In our study, particularly in non-frontal regions, we note that group differences for FA decrease and ADC increase in the cross-sectional analyses generally appear more to be diminished in size and distribution at 18 months as compared to 3 months, presumably indicating some seeming amelioration of the initial deleterious changes. In fact, there are regions of ADC decrease in parietal and occipital areas as well as in the midbrain (see Fig. 1). Admittedly, these changes are less visible than the degenerative changes, but highlight the complex and dynamic aspect of the changes subsequent to TBI in the immature brain.
Although there were some small areas of commonality between DTI-related group differences in FA and ADC, the extent and location of differences in these metrics did not necessarily overlap at either time point, suggesting that each metric reveals somewhat unique information about the microstructural environment. Although several published studies have included two or more DTI metrics (e.g., FA, MD, ADC, axial diffusivity or AD, or radial diffusivity or RD), the relation of these metrics to each other and also to outcome or cognition in different states of pathology is not fully understood, and few researchers have addressed this discrepancy. The variation in which DTI metrics are utilized, as well as the time at which they are measured, may partially account for reported differences in the relation of DTI findings to cognitive and clinical outcome. For example, Perlbarg and colleagues (2009) reported that ADC in the cerebral peduncles, inferior longitudinal fasciculus, posterior limb of internal capsule and posterior corpus callosum measured at 5–53 days did not significantly differ between groups of TBI participants with favorable versus unfavorable outcome though FA did differ between groups at this post-injury interval (Perlbarg et al. 2009). Moreover, in this study, FA was related to one-year outcome, though ADC was not related to outcome and did not significantly differ between groups. Similarly, Sidaros et al. (2008) found no ADC changes at 5–11 weeks despite having seen widespread white matter differences in FA, and limited differences in ADC were seen at 12 months post-injury (i.e., only in the posterior corpus callosum) despite multiple regions of change in FA at this post-injury interval (Sidaros et al. 2008). Salmond et al. (2006) reported a slight difference in regions of change between DTI metrics. Whereas anisotropy decreases were apparent in deep white matter and spread throughout the cortical white matter, diffusivity increases primarily manifested in the TBI group in the cerebellum, insula, cingulate, and some cortical white matter (Salmond et al. 2006). Clearly these metrics may demonstrate sensitivity to microstructural changes within brain tissue that may follow differing time courses, vary by region due to site-specific structural organization, and are influenced by different forms of pathology. A better understanding of metric-specific changes in different regions of the brain will be important in elucidating and monitoring underlying pathological processes in vivo and how these contribute to brain function and recovery following TBI.
Unlike ROI approaches to DTI analysis, TBSS is an automated technique that allows for analysis of potential regions of significance throughout the brain, rather than limiting analysis to specific hypotheses and predefined ROI. TBSS provides important information regarding the overall pattern of microstructural change. The use of a combination of linear and non-linear registration combined with projection onto a skeletal tract of the pooled subjects such that only the white matter tracts common to all subjects in the study are analyzed, minimizes the former concern regarding registration. DTI in general has known limitations relating to the accuracy of FA and ADC in regions with a great deal of crossing fibers, and newer techniques that can better resolve this issue could be applied in the future (e.g., diffusion spectrum imaging).
This study represents the first prospective study utilizing TBSS DTI analysis in a cohort of children and adolescents with moderate to severe TBI at two fixed time points. The relatively large sample size compared to previous studies and inclusion of a comparison cohort of demographically-similar children with orthopedic injury represent additional strengths of the study. Finally, our study included comparison of two DTI parameters (i.e., FA and ADC) to examine microstructural changes occurring at these time intervals.
Limitations of the study include heterogeneity in injury severity as well as the location, extent and nature of focal pathology. An additional limitation involves the potential influence of age in the group comparisons. While the groups did not technically differ in terms of mean age, it should be noted that the difference was marginally significant, with the mean age of TBI group being slightly higher than the OI group. In the current report, we included all subjects with complete imaging data of sufficient quality for both 3 and 18 month imaging occasions (i.e., no missing imaging data) that were between the ages of 8–17 years. Since developmental changes were of interest, we elected not to control age in the analyses as this would potentially eliminate some of the expected age-related change we were attempting to examine in the OI group. However, we do acknowledge that the marginal differences between groups in terms of age or the precise distribution of age within each group could also contribute to our findings. Ideally, future studies would examine the impact of TBI in a true longitudinal design with stratification by age. Additionally, we note that race/ethnicity differed between our TBI and OI groups. Our recruitment strategy was to enroll every eligble participant from a prospective consecutive sample, regardless of the specifics of the demographic data. The inclusion of a relatively high number of “minority” participants is reflective of the samples we recruited from, where Houston and Miami in particular have higher numbers of Hispanic and African American subjects. We know of no particular data that reveals a systematic bias in DTI data due to race/ethnicity per se, and more important estimates of socioeconomic status, such as the Social Composite Index were comparable between groups.
Future research is necessary to investigate the injury mechanisms underlying the different metrics in DTI-related changes in TBI, as understanding of the dynamic post-injury changes following TBI remains incomplete. We also acknowledge that the pattern of DTI-related changes and their persistence over time may differ for patients with mild TBI (see Mayer et al., 2010). Additionally, the present study is limited to comparison of white matter and subcortical gray matter structures that also contain white matter, such as the thalamus and the basal ganglia; future analysis of cortical gray matter changes in diffusivity may also be of importance. Future studies that incorporate multi-modality imaging in pediatric TBI should also be performed. This study was intentionally limited to structural imaging-related changes, but studies exploring the complex relation of these changes to other medical injury variables, outcome and cognition are underway.
Conclusions
In this study we report that TBI-related changes continue until at least 18 months post-injury as indicated by significant changes in FA and ADC. These results are generally consistent with other studies revealing decreased FA and increased ADC following TBI at different chronic post-injury intervals in both children and adults. Children and adolescents with TBI failed to demonstrate the expected pattern of FA increases that was observed in the OI group, suggesting that TBI may disrupt the timing or course of development subsequent to TBI. We observed that DTI-related changes between FA and ADC metrics did not overlap perfectly; they were affected in different regions, and the extent and location of their change also varied with time between 3 and 18 months post-injury. A better understanding of the long-term dynamic changes occurring within the immature brain following TBI may increase our understanding of neuroplasticity and continuing degenerative change, which in turn, may facilitate advances in management and intervention. Additionally, our study reaffirms the need to examine multiple DTI metrics and to further investigate the relation of these metrics to each other. Future analyses will include additional examination of the relation of imaging changes to cognitive and functional outcome as well as multi-modal imaging analyses of pediatric TBI.
References
Alexander, A. L., Lee, J. E., Lazar, M., & Field, A. S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics, 4(3), 316–329. doi:10.1016/j.nurt.2007.05.011.
Arfanakis, K., Haughton, V. M., Carew, J. D., Rogers, B. P., Dempsey, R. J., & Meyerand, M. E. (2002). Diffusion tensor MR imaging in diffuse axonal injury. AJNR. American Journal of Neuroradiology, 23(5), 794–802.
Ashtari, M., Cervellione, K. L., Hasan, K. M., Wu, J., McIlree, C., Kester, H., et al. (2007). White matter development during late adolescence in healthy males: A cross-sectional diffusion tensor imaging study. NeuroImage, 35(2), 501–510.
Barnea-Goraly, N., Menon, V., Eckert, M., Tamm, L., Bammer, R., Karchemskiy, A., et al. (2005). White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cereb Cortex, 15(12), 1848–1854.
Bendlin, B. B., Ries, M. L., Lazar, M., Alexander, A. L., Dempsey, R. J., Rowley, H. A., et al. (2008). Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. NeuroImage, 42(2), 503–514. doi:10.1016/j.neuroimage.2008.04.254.
Benson, R. R., Meda, S. A., Vasudevan, S., Kou, Z., Govindarajan, K. A., Hanks, R. A., et al. (2007). Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. Journal of Neurotrauma, 24(3), 446–459.
Bigler, E. D., Abildskov, T. J., Wilde, E. A., McCauley, S. R., Li, X., Merkley, T. L., et al. (2010). Diffuse damage in pediatric traumatic brain injury: a comparison of automated versus operator-controlled quantification methods. NeuroImage, 50(3), 1017–1026. doi:10.1016/j.neuroimage.2010.01.003.
Bonekamp, D., Nagae, L. M., Degaonkar, M., Matson, M., Abdalla, W. M., Barker, P. B., et al. (2007). Diffusion tensor imaging in children and adolescents: Reproducibility, hemispheric, and age-related differences. NeuroImage, 34(2), 733–742.
Chastain, C. A., Oyoyo, U. E., Zipperman, M., Joo, E., Ashwal, S., Shutter, L. A., et al. (2009). Predicting outcomes of traumatic brain injury by imaging modality and injury distribution. Journal of Neurotrauma, 26(8), 1183–1196. doi:10.1089/neu.2008.0650.
Clayden, J. D., Jentschke, S., Munoz, M., Cooper, J. M., Chadwick, M. J., Banks, T., et al. (2011). Normative development of white matter tracts: Similarities and differences in relation to age, gender, and intelligence. Cereb Cortex, In press.
Eluvathingal, T. J., Hasan, K. M., Kramer, L., Fletcher, J. M., & Ewing-Cobbs, L. (2007). Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex, 17(12), 2760–2768.
Ewing-Cobbs, L., Prasad, M. R., Swank, P., Kramer, L., Cox, C. S., Jr., Fletcher, J. M., et al. (2008). Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. NeuroImage, 42(4), 1305–1315. doi:10.1016/j.neuroimage.2008.06.031.
Faul, M., Xu, L., Wald, M., & al., e. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths, 2002-2006. (pp. 1-74): US Department of Health and Human Services, Centers for Disease Control and Prevention.
Gale, S. D., & Prigatano, G. P. (2010). Deep white matter volume loss and social reintegration after traumatic brain injury in children. The Journal of Head Trauma Rehabilitation, 25(1), 15–22. doi:10.1097/HTR.0b013e3181c39960.
Giorgio, A., Watkins, K. E., Chadwick, M., James, S., Winmill, L., Douaud, G., et al. (2010). Longitudinal changes in grey and white matter during adolescence. NeuroImage, 49(1), 94–103.
Huisman, T. A., Schwamm, L. H., Schaefer, P. W., Koroshetz, W. J., Shetty-Alva, N., Ozsunar, Y., et al. (2004). Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR. American Journal of Neuroradiology, 25(3), 370–376.
Inglese, M., Makani, S., Johnson, G., Cohen, B. A., Silver, J. A., Gonen, O., et al. (2005). Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. Journal of Neurosurgery, 103(2), 298–303.
Kinnunen, K. M., Greenwood, R., Powell, J. H., Leech, R., Hawkins, P. C., Bonnelle, V., et al. (2010). White matter damage and cognitive impairment after traumatic brain injury. Brain. doi:10.1093/brain/awq347.
Kraus, M. F., Susmaras, T., Caughlin, B. P., Walker, C. J., Sweeney, J. A., & Little, D. M. (2007). White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain, 130(Pt 10), 2508–2519.
Kumar, R., Gupta, R. K., Husain, M., Chaudhry, C., Srivastava, A., Saksena, S., et al. (2009). Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: its correlation with neuropsychometric tests. Brain Injury, 23(7), 675–685. doi:10.1080/02699050903014915.
Lebel, C., Walker, L., Leemans, A., Phillips, L., & Beaulieu, C. (2008). Microstructural maturation of the human brain from childhood to adulthood. NeuroImage, 40(3), 1044–1055.
Lenroot, R. K., Giedd, J. N. (2006). Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Reviews, 30(6):718–29. doi:10.1016/j.neubiorev.2006.06.001
Levin, H. S., & Hanten, G. (2005). Executive functions after traumatic brain injury in children. Pediatric Neurology, 33(2), 79–93. doi:10.1016/j.pediatrneurol.2005.02.002.
Levin, H. S., Wilde, E. A., Chu, Z., Yallampalli, R., Hanten, G. R., Li, X., et al. (2008). Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. The Journal of Head Trauma Rehabilitation, 23(4), 197–208. doi:10.1097/01.HTR.0000327252.54128.7c.
Levin, H. S., Hanten, G., & Li, X. (2009). The relation of cognitive control to social outcome after paediatric TBI: Implications for intervention. Developmental Neurorehabilitation, 12(5), 320–329. doi:10.3109/17518420903087673.
Levin, H. S., Wilde, E. A., Hanten, G., Li, X., Chu, Z. D., Vasquez, A. C., et al. (2011). Mental state attributions and diffusion tensor imaging after traumatic brain injury in children. Developmental Neuropsychology, 36(3), 273–287.
Liston, C., Watts, R., Tottenham, N., Davidson, M. C., Niogi, S., Ulug, A. M., et al. (2006). Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex, 16(4), 553–560. doi:10.1093/cercor/bhj003.
Marquez de la Plata, C. D., Yang, F. G., Wang, J. Y., Krishnan, K., Bakhadirov, K., Paliotta, C., et al. (2010). Diffusion tensor imaging biomarkers for traumatic axonal injury: analysis of three analytic methods. Journal of International Neuropsychological Society, 17(1), 24–35. doi:10.1017/S1355617710001189.
Mayer, A.R., Ling, J., Mannell, M.V., Gasparovic, C., Phillips, J.P., Doezema, D., Reichard, R., and Yeo,R.A. (2010). A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 74, 643–650.
McCauley, S. R., Wilde, E. A., Bigler, E. D., Chu, Z., Yallampalli, R., Oni, M. B., et al. (2011). Diffusion tensor imaging of incentive effects in prospective memory after pediatric traumatic brain injury. Journal of Neurotrauma, 28(4), 503–516.
Muetzel, R. L., Collins, P. F., Mueller, B. A. M., Schissel, A., Lim, K. O, & Luciana, M. (2008). The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. NeuroImage, 39(4), 1918–1925.
Oni, M. B., Wilde, E. A., Bigler, E. D., McCauley, S. R., Wu, T. C., Yallampalli, R., et al. (2010). Diffusion tensor imaging analysis of frontal lobes in pediatric traumatic brain injury. Journal of Child Neurology, 25(8), 976–984.
Perlbarg, V., Puybasset, L., Tollard, E., Lehericy, S., Benali, H., & Galanaud, D. (2009). Relation between brain lesion location and clinical outcome in patients with severe traumatic brain injury: A diffusion tensor imaging study using voxel-based approaches. Human Brain Mapping, 30(12), 3924–3933.
Salmond, C. H., Menon, D. K., Chatfield, D. A., Williams, G. B., Pena, A., Sahakian, B. J., et al. (2006). Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. NeuroImage, 29(1), 117–124. doi:10.1016/j.neuroimage.2005.07.012.
Salorio, C. F., Slomine, B. S., Grados, M. A., Vasa, R. A., Christensen, J. R., & Gerring, J. P. (2005). Neuroanatomic correlates of CVLT-C performance following pediatric traumatic brain injury. Journal of International Neuropsychological Society, 11(6), 686–696. doi:10.1017/S1355617705050885.
Schmithorst, V. J., Wilke, M., Dardzinski, B. J., & Holland, S. K. (2002). Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: A cross-sectional diffusion-tensor MR imaging study. Radiology, 222(1), 212–218.
Schonberger, M., Ponsford, J., Reutens, D., Beare, R., & O'Sullivan, R. (2009). The Relationship between age, injury severity, and MRI findings after traumatic brain injury. Journal of Neurotrauma, 26(12), 2157–2167. doi:10.1089/neu.2009.0939.
Sidaros, A., Engberg, A. W., Sidaros, K., Liptrot, M. G., Herning, M., Petersen, P., et al. (2008). Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain, 131(Pt 2), 559–572. doi:10.1093/brain/awm294.
Sidaros, A., Skimminge, A., Liptrot, M. G., Sidaros, K., Engberg, A. W., Herning, M., et al. (2009). Long-term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. NeuroImage, 44(1), 1–8. doi:10.1016/j.neuroimage.2008.08.030.
Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3):143–155.
Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., et al. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage, 31(4), 1487–1505.
Smith, S. M., Johansen-Berg, H., Jenkinson, M., Rueckert, D., Nichols, T. E., Miller, K. L., et al. (2007). Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc, 2(3), 499-503.
Stancin, T., Taylor, H. G., Thompson, G. H., Wade, S., Drotar, D., & Yeates, K. O. (1998). Acute psychosocial impact of pediatric orthopedic trauma with and without accompanying brain injuries. The Journal of Trauma, 45(6), 1031–1038.
Teasdale, G., & Jennett, B. (1974). Assessment of coma and impaired consciousness. A pratical scale. Lancet, 13(7872), 81–84.
Warner, M. A., Marquez de la Plata, C., Spence, J., Wang, J. Y., Harper, C., Moore, C., et al. (2010). Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. Journal of Neurotrauma, 27(12), 2121–2130. doi:10.1089/neu.2010.1429.
Warner, M. A., Youn, T. S., Davis, T., Chandra, A., Marquez de la Plata, C., Moore, C., et al. (2010). Regionally selective atrophy after traumatic axonal injury. Archives of Neurology, 67(11), 1336–1344. doi:10.1001/archneurol.2010.149.
Weisskoff, R. M. (1996). Simple measurement of scanner stability for functional NMR imaging of activation in the brain. Magnetic Resonance in Medicine, 36(4), 643–645.
Wilde, E. A., Chu, Z., Bigler, E. D., Hunter, J. V., Fearing, M. A., Hanten, G., et al. (2006). Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. Journal of Neurotrauma, 23(10), 1412–1426.
Wilde, E. A., Ramos, M. A., Yallampalli, R., Bigler, E. D., McCauley, S. R., Chu, Z., et al. (2010). Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Developmental Neuropsychology, 35(3), 333–351.
Wozniak, J. R., Krach, L., Ward, E., Mueller, B. A., Muetzel, R., Schnoebelen, S., et al. (2007). Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Archives of Clinical Neuropsychology, 22(5), 555–568. doi:10.1016/j.acn.2007.03.004.
Wu, T. C., Wilde, E. A., Bigler, E. D., Li, X., Merkley, T. L., Yallampalli, R., et al. (2010). Longitudinal Changes in the Corpus Callosum following Pediatric Traumatic Brain Injury. Developmental Neuroscience. doi:10.1159/000317058.
Xu, J., Rasmussen, I. A., Lagopoulos, J., & Haberg, A. (2007). Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. Journal of Neurotrauma, 24(5), 753–765. doi:10.1089/neu.2006.0208.
Yeates, K. O., Taylor, H. G., Drotar, D., Wade, S. L., Klein, S., Stancin, T., et al. (1997). Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. Journal of International Neuropsychological Society, 3(6), 617–630.
Yuan, W., Holland, S. K., Schmithorst, V. J., Walz, N. C., Cecil, K. M., Jones, B. V., et al. (2007). Diffusion tensor MR imaging reveals persistent white matter alteration after traumatic brain injury experienced during early childhood. AJNR. American Journal of Neuroradiology, 28(10), 1919–1925.
Acknowledgements
This work was supported by the National Institute Neurological Disorders and Stroke grant R01-NS21889 (“Neurobehavioral outcome of head injury in children,” Levin, PI). We also acknowledge the generous contribution of Mission Connect of the TIRR Foundation. We gratefully acknowledge the contribution of Ana C. Vasquez, Deleene Menefee, PhD., Summer Lane, Lori Cook, Sandra B. Chapman, PhD., and Gillian Hotz, PhD. in data collection, and Joshua Cooper and Alyssa P. Ibarra in manuscript preparation. We thank the participants and their families for their participation in this research. None of the authors have any financial or other relationship(s) that could be construed as a conflict of interest with respect to the content of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Elisabeth A. Wilde and Kareem W. Ayoub contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wilde, E.A., Ayoub, K.W., Bigler, E.D. et al. Diffusion tensor imaging in moderate-to-severe pediatric traumatic brain injury: changes within an 18 month post-injury interval. Brain Imaging and Behavior 6, 404–416 (2012). https://doi.org/10.1007/s11682-012-9150-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-012-9150-y