Abstract
Previous studies have shown that exposure to early life stress (ELS) is associated with reduced volume of brain regions critical for information processing, memory and emotional function. Further, recent studies from our lab utilizing diffusion tensor imaging (DTI) have found alterations in the microstructural integrity of white matter pathways among adults exposed to ELS. However, it is not clear if these relationships extend to children and adolescents, and it is also unclear if these DTI abnormalities are associated with cognitive performance. The present study examined the relationship between ELS and the microstructural integrity of the corpus callosum among a sample of otherwise healthy controls between the ages of 8 and 73. The participants were subdivided into four age groups (8–12, 13–18, 19–50, 51–73). Individuals with three or more ELS events were compared to individuals with fewer than 3 ELS events on fractional anisotropy (FA) in the genu of the corpus callosum. Separate analyses examined the two groups on tests of verbal memory, information processing speed, psychomotor speed and cognitive flexibility. Results revealed that the youngest group and the oldest group of individuals with ELS exhibited significantly lower FA in the genu compared to individuals without ELS. However, there were no group differences on any of the cognitive tasks. Our results indicate that ELS is related to subtle alterations in brain structure, but not function. The effects found with regard to DTI occurred during periods of critical age-related developmental windows.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
While it is important for childhood and adolescence to be a period of healthy physical and mental development, exposure to serious psychological stress during these age ranges is common. Cohen et al. (2006b) reported that over one-third of the participants (n = 1659) in an international study on early life stress (ELS) reported experiencing three or more adverse childhood events. The most common events were divorce (22%), severe family conflict (20%), and being bullied/socially rejected (17%). Importantly, these percentages were observed among individuals who were closely screened for psychiatric disorders, and therefore the findings suggest that exposure to ELS is very common among otherwise healthy individuals.
Exposure to ELS can result in important physical sequelae that can continue through adulthood including decreased immune function and dysfunction of the stress response system (Graham et al. 2006). The stress response system is comprised of the sympathetic-adrenomedullary system responsible for releasing epinephrine (adrenaline) to initiate the fight/flight response, and the hypothalamic–pituitary–adrenocortical (HPA) system responsible for the production of steroid hormones including glucocorticoids (GCs), and more specifically cortisol (Gunnar and Quevedo 2006). Unlike epinephrine, GC’s easily cross the blood brain barrier and many studies have demonstrated that GC’s, and cortisol in particular, may be associated with alterations in brain structures critical to higher level cognitive functioning.
A study by Cohen et al. (2006a) found that healthy adults without psychopathology or brain disorders who had experienced adverse childhood events had a significant decrease in volume of the anterior cingulate cortex and caudate nucleus, two brain regions critical for emotional and cognitive behaviors. Further research focusing on adult women who had experienced childhood sexual or physical abuse found significant decreases in left hippocampal volumes in abused participants when compared to controls (Sapolsky 2000). Additional studies have revealed that the corpus callosum of children who were abused or neglected were 17% smaller than those in a nonexposed control group (Teicher et al. 2004).
The imaging studies noted above utilized traditional magnetic resonance imaging (MRI) to examine the macrostructural integrity of brain structures. Diffusion tensor imaging (DTI) is a relatively new technology that is more sensitive to brain pathology than traditional MRI scans (Neil et al. 2002). DTI measures the rate and directionality of water diffusion in the brain providing an index of the microstructural integrity of white matter pathways that form the neural circuits necessary for information processing. The structure of these pathways consists mainly of axons with varying degrees of length, diameter and myelination, and aging, disease or trauma can cause disruptions in the amount and/or linear movement of water. The traditional metric obtained from DTI is fractional anisotrophy (FA), which is a ratio of directional to nondirectional water movement in a single imaging voxel. Lower FA values reflect lower directional water movement, and this serves as a proxy measure of neuropathology or structural abnormalities in white matter pathways.
In a recent study, Paul et al. (2007) used DTI to identify alterations in white matter pathways among otherwise healthy adults who had been exposed to ELS. In this study, the total number of stressors experienced by individuals was significantly related to lower FA in the genu of the corpus callosum, a finding consistent with previous studies of macrostructural abnormalities in the corpus callosum among children who were abused or neglected (Teicher et al. 2004). It is important to note that children and adolescents were not included in the study conducted by Paul et al; and therefore it is unknown whether the impact of ELS on the microstructural integrity of the white matter extends to younger ages. Evidence of lower FA in the corpus callosum of children and adolescents exposed to ELS would indicate a strong temporal relationship between ELS and brain structure. In addition, it would indicate that the results found by Paul et al. (2007) in otherwise healthy adults are not simply delayed effects influenced by other external factors that occur later in adulthood (i.e., subsequent traumatic events, subclinical brain insults, etc.).
A separate literature has examined the relationship between ELS and cognitive function. The rationale for these studies is that the brain structures and regions impacted by ELS are known to mediate important higher-level mental functions including information processing speed, memory, verbal abilities and intelligence (for review see Goethals et al. 2003). Research examining memory and learning performance in children and adolescents with post traumatic stress disorder (PTSD) has reported that trauma-exposed youth suffering from PTSD score significantly lower on memory indices when compared to youth exposed to early trauma but without diagnoses of PTSD and a nontraumatized control group (Moradi et al. 1999). In addition, Perez and Widom (1994) reported that adult survivors of childhood abuse and neglect scored significantly lower on IQ and reading ability than nonvictimized controls. However, the results of research in this area have been inconsistent, and other studies examining cognition in children, adolescents, and adults with histories of childhood sexual abuse found no short- or long-term effects on learning and memory indices when compared to matched control groups (Porter et al. 2005; Stein et al. 1999).
The failure to find an effect of ELS on cognition may have resulted from differences in subject characteristics (e.g., whether or not they were receiving psychological/psychiatric treatment). In addition, an important limitation of previous studies examining relationships between ELS and cognition is the exclusion of cognitive measures sensitive to processing speed and executive function, both of which would be expected to be impacted by the alterations in white matter integrity observed by Teicher et al. (2004) and Paul et al. (2007).
As described above, previous studies have demonstrated that ELS is associated with alterations on neuroimaging in otherwise healthy adults. However, it remains unclear if these relationships extend to children and adolescents and whether any such effects negatively impact cognitive functioning. One purpose of this study is to determine whether ELS is associated with microstructural abnormalities as evidenced by DTI across the lifespan from childhood through adolescence and adulthood. We examined this issue using a cross-sectional method and archival data that allowed us to compare brain scans of individuals with and without histories of ELS based on their age at the time of the scan. We hypothesized that ELS would be related to significant deficits in the microstructural integrity of the white matter pathways as evidenced by lower FA values in the genu of the corpus callosum. Our focus on the corpus callosum and the genu in particular allows us to more closely examine microstructural abnormalities because of the fact that this area contains more linear white matter than other areas and the size of the bundle helps to minimize partial voluming effects. In addition, it is located in the prefrontal cortex, an area known to play an important part in higher level cognitive skills. This is important because the second purpose of this study is to determine whether ELS is associated with reduced cognitive functioning among a population of “healthy” children, adolescents and adults. This issue was examined in a separate cohort of individuals who had undergone cognitive assessment, but not neuroimaging. We hypothesized that ELS would be significantly associated with poorer performance on tests of cognitive function, specifically memory, information processing, response inhibition and cognitive flexibility.
Methods
Participants
The present study utilized archival data obtained from the Brain Resource International Database (BRID; Gordon et al. 2005). The BRID was developed as a multidisciplinary database of brain function. Six laboratories participated in the initial development of the BRID, including two sites located in Australia, two in the United States, one in the Netherlands, and one in the United Kingdom, although neuroimaging data were obtained at only one of the two laboratories in Australia. Both imaging and cognitive testing were completed at the age reported. The cognitive data were collected in all laboratories using identical methodology. Participants were excluded from the BRID if they had endorsed a history of drug or alcohol addiction and/or other serious medical condition, mental illness, physical brain injury, or a neurological disorder. In addition, participants were excluded if they reported a family history of Attention Deficit Hyperactivity Disorder (ADHD), Schizophrenia, Bipolar Disorder, or other genetic disorder. In order to screen for familiar undiagnosed anxiety or depressive disorders, participants completed the Somatic and Psychological Health Report (SPHERE; Hicki 1998) as well as the Depression, Anxiety and Stress Scale (DASS; Lovibond and Lovibond 1995).
For purposes of this study we included individuals between the ages of 8–73 years. We did not exclude individuals over 73, but rather this represented the oldest age in the imaging sample. Individuals younger than 8 were not included due to limited neuroimaging and cognitive data available in the database. As noted above, two samples were selected from the BRID, one sample from the BRID neuroimaging database (n = 117; 55% male, 44% female), and a separate sample from the BRID cognitive database (n = 621; 52% male, 48% female). While some participants within the two samples received both imaging and cognitive testing, the limited amount did not allow us to examine the relationship between FA and cognition. We initially planned on subdividing the group into four age bands (8–12, 13–18, 19–50 and 51–73). However, the distribution of ELS events among the 13–18-year old group was extremely limited in the DTI database, with only 1 participant reporting experiencing at least 3 ELS events. As such, we eliminated this group from both the imaging and cognitive analyses. Therefore, three groups of participants were examined including: children 8–12 years old (neuroimaging n = 9, cognitive n = 133), young adults19–50 years old (neuroimaging n = 72, cognitive n = 372), and older adults 51–73 years old (neuroimaging n = 26, cognitive n = 114). The neuroimaging sample had a mean age of 32.3 (SD = 17.7) and 12.4 (SD = 3.7) years of education. The cognitive sample had a mean age of 32.3 (SD = 16.9) and 12.9 (SD = 4.7) years of education.
The participants were further subdivided according to ELS exposure status. To maximize inclusion of data we classified individuals by whether or not they had been exposed to three or more ELS events. Individuals exposed to three or more ELS events were identified as the ELS-high group, and individuals with fewer than three events were classified as the ELS-low group (See Table 1). Grouping of participants by exposure to at least three ELS events as ELS-high is consistent with previous studies of ELS completed from this database (McFarlane et al. 2005; Paul et al. 2007) as well as the frequency of ELS as reported by Cohen et al. (2006b). While some studies (e.g., Cohen et al. 2006a) have used slightly different groupings, including a no exposure group, we elected not to classify participants based on no exposure vs a minimum number of ELS events in order to maximize data inclusion and ensure that individuals classified as ELS-high were in fact exposed to at least a moderate degree of ELS.
Procedures
All participants (or their guardians if under age 18) were required to provide signed informed consent before participation and refrain from caffeine and smoking for at least 2 h before testing began. Individuals under the age of 18 provided assent. Prior to carrying out experimental tasks, participants completed a 19 item web-based questionnaire designed to determine exposure to ELS based on questions developed by Sanders and Becker-Laursen (1995). Examples of items on the questionnaire included adoption, divorce, illness, and physical, sexual or emotional abuse. Participants responded either “yes” they had experienced the event, or “no” they had not. If they responded “yes”, they were then asked to indicate the age at which they had experienced the event using the following coding: 0–3 years, 4–7 years, 8–12 years and 13–17 years. Most individuals (75%) reported ELS onset before the age of 13. The total number of ELS events was summed for each participant to create a total ELS density score (see Table 2).
Image acquisition
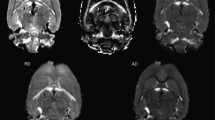
Neuroimaging was conducted using a 1.5 Tesla Siemens (Erlangen, Germany) Sonata at Perrett Imaging, Flinders University, Australia. DTI was acquired using a DTI-EPI sequence (TR: 160 ms; TE: 88 ms; Fat Saturation; NEX: 4). A baseline image (b = 0) and 12 different diffusion orientations were acquired with a b value of 1250. 32 contiguous slices of 6.5 mm were acquired with an in-plane matrix of 128 × 128 at a resolution of 1.72 × 1.72 mm. To define the region of the genu of the corpus callosum we first determined the anterior and posterior regions of the body were defined by the MNI co-ordinates y = +17 to −18 mm. The genu was then defined as the remaining anterior component of the whole corpus callosum region. This is consistent with our previous work (Paul et al. 2007) (Fig. 1).
Diffusion Tensor Analysis
DTI data were processed using a custom written routine (by author—SMG) in MATLAB 6.5 (MathWorks, Natick, USA). Trace apparent diffusion co-efficient (TrADC) and FA images were calculated in native space from the b = 0 image and 12 diffusion weighted imaged images (b = 1250 s cm−2). FA values range from 0–1.0 with a value of 1.0 reflecting more highly organized tissue. This procedure has been described in previous work (Grieve et al. 2007).
Materials and equipment
Participants completed tasks utilizing a touchscreen computer (NEC MultiSync LCD 1530) centrally located on a desktop in a sound-attenuated room regulated for ambient light. Instructions and materials for each task were pre-recorded on computer ‘.wav’ files and administered to participants via headphones and visual displays on the touch-screen computer. Both verbal and written instructions were used in order to maximize the participant’s ability to understand the task instructions and complete the battery regardless of their age. In order to ensure comprehension of the task and compliance, an automated protocol was used that directed participants through several practice trials. If three practice trials were failed, the participant was automatically taken to the next test in the battery.
Integneuro test battery
The initial test battery was made up of 12 tasks that take approximately 50 min to complete. The integneuro battery has previously been shown to consist of strong validity and reliability (McFarlane et al. 2005; Paul et al. 2005; Williams et al. 2005), and has established norms (Clark et al. 2006). The instructions and practice trials were administered immediately before completing each task. For purposes of this study we utilized 9 of the 12 tasks initially administered. These tasks tap cognitive domains subserved by brain regions impacted by ELS and/or trauma/PTSD (Cohen et al. 2006a; Sapolsky 2000; Teicher et al. 2004).
Tasks
Attention
The Span of Visual Memory task is an adaptation of the Corsi Blocks task (Milner 1970) and it provides a measure of visual short-term attention. Participants are shown a touchscreen with nine squares positioned asymmetrically on the screen. The squares flash in sequence, with two to nine squares per sequence, followed by a tone. After the tone, participants touch the squares in the order previously flashed. Trials are presented in ascending order with two trials per length. The task ends if the participant fails two trials, or successfully completes all trials. The longest sequence length completed was recorded.
The Digit Span task is a two-part test used to determine attention and immediate memory recall. Participants are shown a sequence of numbers presented at consistent intervals in a box on the computer screen. The number of items per sequence ranges from three to nine, and participants are presented two trials for each length presented in ascending order. They are then asked to recall the numbers in the order presented by touching the numbers on a screen keypad. The task ends after 2 failed trials or the completion of all 18 trials. The second part of the test required participants to recall the numbers in reverse order of presentation. The recorded measure was the longest sequence length successfully completed for each part.
Motor Speed
In order to provide a measure of manual dexterity, participants completed The Motor Tapping Test, which is a variation of the Finger Tapping test (Halstead 1947; Lezak 2004). Participants placed the palm of their hand on the touch-screen and tapped their index finger as quickly as they could for 30 s. The number of taps from the dominant hand was recorded.
In addition, participants completed The Choice Reaction Time test. On the touch-screen, four black circles are evenly placed along a semicircular arc with two circles to the left of midline and two to the right. Participants rest the pointing finger of their dominant hand on a white circle equally positioned below each of the black circles at the midline. At equal intervals, one of the black circles is changed to green and participants are required to touch the green circle with their pointing finger. The average speed of their response was recorded.
Memory
Assessment of auditory-verbal learning, recall and recognition were examined with The Memory Recall and Recognition test, a version of the Rey Auditory Verbal Learning and Memory task (Geffen et al. 1990). Utilizing headphones, participants listened to a series of 12 words presented at one second intervals in both ears, and were instructed to immediately recall as many of the words from the list as possible in any order. The learning trials were administered on four trials, and the total number of words recalled for all four trials was recorded. As a means of distraction, in the same manner as previously described, the participant then listened to a second list of words that were unrelated to the first list and they were asked to recall as many of the words as possible from the list. Immediately after completion of the distraction trial, participants were then asked to again recall as many of the words from the first list as possible in any order (Immediate Recall Trial). Following a 25-min filled delay the participants were required to recall as many words from the first list as possible (Delayed Recall Trial). In the final recognition trial the participants were presented with a list of 24 words one at a time in random order via computer screen. Half of the words were from the first list and the other half were new words. Participants indicated by touching a “Yes” or “No” button as to whether to word appeared on the first list. The dependent measures were the total number of words recalled across the learning trials and total number of words recalled on the delayed trial. Previous studies have demonstrated that performance on delayed verbal memory is sensitive to brain regions implicated in trauma research (Yasik et al. 2007; Lindauer et al. 2006).
Executive Function
Participants were asked to complete and remember how to solve a complex maze (The Maze task). The maze consisted of an 8 × 8 grid of red circles that appeared on a computer screen in a square arrangement with a yellow marked circle at the beginning and a blue marked circle indicating the end. Utilizing arrow keys displayed on the touch-screen, participants moved through the maze until completion. An audible and visual signal alerted them to incorrect moves through the maze. They were required to continue the test until they completed the maze on two consecutive trials without error, or until 7 min had elapsed (whichever came first). The dependent measure was the number of errors on the task.
Using an adaptation of the Stroop test (Stroop 1935), participants were presented a list of color words (e.g., red, blue, yellow and green) in contrasting colors one at a time on the computer screen in pseudo random order with the words red, yellow, green and blue presented across the bottom of the screen in black font. The participants were required to name the color word after it was presented on the screen, and the number of correctly identified words was recorded. In part 2 of the Word Interference test participants must name the color of each word presented on the computer screen (as opposed to naming the color word) as quickly as possible. The number of words correctly identified on part 2 was the measured variable.
Assessment of mental flexibility and concept structure was measured utilizing the Switching of Attention test, a computerized version of the Trail Making test (Reitan 1955, 1958). Participants were presented the numbers 1 to 25 in pseudo random order on the computer screen and required to touch the numbers in ascending order as quickly as possible. Time to completion was the dependent measure. Part 2 of the Switching of Attention test presents participants with a series of numbers (1–13) and 12 letters (A–L) on the computer screen. Alternating between numbers and letters, participants are required to put all figures in sequential order (i.e. 1 A 2 B 3 C). The time to completion was recorded for each trial and the dependent measure for the study was the difference between time on part 1 and the time to complete on part 2. This allows examination of cognitive flexibility independent of the psychomotor aspects of the task.
Statistical Analysis
ANOVAs were computed to contrast the ELS-high vs the ELS-low group on FA in the genu. In addition, separate MANOVAs were computed for the three cognitive domains of Attention/Motor Speed, Verbal Memory, and Executive Function. Independent t tests were computed to contrast the groups when the omnibus MANOVA was significant. Pearson correlation analyses were also computed to examine total ELS exposure as a continuous variable, and both FA in the genu and performance on each of the cognitive tasks. The relationship between FA and cognition was not examined as data for these two variables were obtained from separate databases.
Results
An ANOVA was conducted to contrast the ELS-high group and ELS-low group on FA in the genu of the corpus callosum. Results of the omnibus analysis revealed a significant main effect for ELS group (F(1,101) = 16.5, p < 0.01), a main effect for age group(F(1,101) = 0.8, p < 0.05) and a significant interaction between ELS group and age group (F(2,101) = 3.6, p < 0.05). The univariate analyses revealed that children in the ELS-high group exhibited significantly lower FA in the genu than the children in the ELS-low group (t(7) = −2.5, p < 0.05). In addition, the older adult ELS-high group exhibited significantly lower FA in the genu than the older adult ELS-low group (t(24) = −2.2, p < 0.05). By contrast, the young adults classified as ELS-high did not differ significantly from the young adults classified as ELS-low on FA (t(70) = −0.88, p > 0.05; see Table 1).
To ensure that the differences in FA among the oldest group were not driven by lower education among the ELS-high group, we examined education differences between these two groups using an independent t test. Results of this analysis revealed no significant differences, and in fact an inspection of the means revealed that individuals in the ELS-high group were slightly more educated than individuals in the ELS-low group (12.6 vs 10.9).
MANOVAs were conducted to contrast differences in cognitive performance among the ELS-high group and the ELS-low group. As noted above, separate analyses were conducted for each of the three cognitive domains (attention/motor speed, memory, executive function). For the Attention/Motor speed domain, we observed a significant main effect for age [Wilks’ lambda = 0.80 (8, 734) = 10.61, p < 0.01], a significant main effect for ELS group [Wilks’ lambda = 0.97 (4, 367) = 2.48, p < 0.05], and a significant Age × ELS interaction [Wilks’ lambda = 0.95 (8, 734) = 2.01, p < 0.05]. Posthoc analyses revealed that the ELS and ELS × Age interaction were evident on the Choice Reaction Time test, with the children in the high ELS group exhibiting significantly slower choice reaction times compared to children in the low ELS group. Because the sample sizes were markedly different between the groups we randomly selected individuals from the ELS-low group to match the ELS-high group on subject numbers. A Student t test was computed between these two matched groups on Choice Reaction Time and the results were no longer statistically significant [t(12) = 0.31, p > 0.05; see Table 3].
In the memory domain, we observed a significant main effect for age (Wilks’ lambda = 0.94 (4, 744) = 5.36, p < 0.01] but no significant effect for ELS [Wilks’ lambda = 0.94 (4, 372) = 1.19, p > 0.05] or Age × ELS [Wilks’ lambda = 0.98 (4, 744) = 1.77, p > 0.05]. In the Executive domain we observed a significant main effect for age [Wilks’ lambda = 0.79 (6, 1220) = 5.36, p < 0.01] but no significant effect for ELS [Wilks’ lambda = 0.94 (4, 372) = 1.19, p > 0.05] or Age × ELS [Wilks’ lambda = 0.98 (4, 744) = 1.77, p > 0.05; see Table 3).
Separate correlation analyses were conducted for each age band to determine if total number of ELS events covaried with FA in the genu and with performance on the cognitive tests. These results revealed a strong correlation between total ELS and FA in the genu among the children in the youngest age group (r = −0.81), with higher ELS associated with lower FA. By contrast, there was no significant correlation between total ELS and FA in the genu among the young adults (r = −0.16) or the older adults (r = −0.29). Several statistically significant correlations were observed between total ELS and performance on the cognitive tests for the children (FAS, r = 0.26 p < 0.01 and choice reaction time, r = −0.21 p < 0.01) but the overall amount of variance shared between these variables is quite limited. Similarly, two statistically significant correlations were noted among the young adults between total ELS and cognitive performance on total delayed recall on verbal memory (r = −0.13, p < 0.05) and the difference score on Switching of Attention (r = −0.16, p < 0.05), but again the overall degree of shared variance is limited. No statistically significant correlations were noted between total ELS and cognitive performance among the older adults (see Table 3).
Independent t tests were also computed to determine if the ELS-high group and ELS-low group differed significantly on depression, anxiety and stress scores. Results of these analyses revealed no significant differences between the groups.
Discussion
In this present study we sought to determine whether exposure to ELS is associated with structural and functional brain changes among children and both young and older adults. Consistent with our predictions, we observed lower FA values in the genu of the corpus callosum among the children (age 8–12) and older adults (age 51–73) of individuals exposed to ELS. By contrast, FA levels were not significantly lower among the young adults (age 19–50). With regard to cognitive processes, we found no group differences between those in the high ELS group and low ELS group for any of the age ranges. Overall these findings are consistent with our previous reports of alterations in brain structure (Cohen et al. 2006a, b) and electrophysiological recordings (McFarlane et al. 2005) associated with ELS. Importantly our sample that underwent DTI consisted of minimal subject overlap with these two previous studies (less than 20%), providing support for independent outcomes.
While other studies focusing on ELS and brain structure have utilized traditional MRI scans to examine the volume of specific brain regions, this is one of the first studies to use DTI to examine the integrity of white matter pathways with regard to this relationship. Our results are consistent with the findings by Teicher et al. (2004) who, using MRI technology, found an average 17% reduction in the volume of the corpus callosum of children and adolescents who had experienced childhood neglect. Since neglect can include neglect of basic biological needs for optimum brain development (e.g., nutrition), our findings extend those of Teicher et al. (2004) by demonstrating alterations in white matter integrity when the ELS events are more predominately psychological in nature.
The findings in our study of significantly lower FA values among children (age 8–13) and older adults (age 51–73) exposed to three or more ELS events may be a result of the sensitivity of the brain during these critical windows of brain development. Studies utilizing DTI to examine neurodevelopment in a healthy population of individuals found that FA values in the genu of the corpus callosum increase with age from 8–13 years, and these increases are thought to occur as part of normal maturational changes in the brain (Snook et al. 2005). In addition, researchers mapping brain development have found a similar pattern with regard to gray matter volume. MRI images reveal increases in gray matter volume at earlier ages, followed by a decline beginning around age 6 or 7 with sustained loss beginning around puberty (Gogtay et al. 2004 and Toga et al. 2006). More recent DTI studies have identified more subtle microstructural changes such as reduced myelin density, reductions in the number of myelinated fibers and gradual reductions in FA in the genu of the corpus callosum beginning around age 20 (Salat et al. 2005; Sullivan and Pfefferbaum 2006).
The significantly lower levels of FA among older adults found in our study are consistent with findings by Paul et al. (2007). However, it is interesting to note that while the age range used by Paul et al. (2007) was 18–80, our study had two groups for this one age range but only found significant results for the oldest group (51–73). Therefore, the results of our study narrow the age range of those significantly affected by ELS to older adults, indicating that the results found by Paul et al. (2007) may not have been confounded by events experienced after ELS onset and the time of imaging since we found the same effects in the young age group as well.
While the results of the youngest and older age groups were consistent with our hypothesis, our DTI findings did not extend to young adults. This observation suggests that the brain may be better able to compensate for the loss during young adulthood since this is not typically a time of critical development or a period of typical degeneration. The possibility that exposure to ELS may impact the brain during select developmental windows is supported by animal studies. Andersen and Teicher (2004) found significant reductions of synaptic overproduction in the hippocampus of rats subjected to early maternal separation and isolation as pups versus pups left with their dams and litters. Synaptic density was measured at 20, 40, 60, 80 and 100 days, with significant reductions observed at 60 and again at 100 days of age in those pups who experienced separation and isolation. In addition, these results are consistent with other studies demonstrating structural alterations in adult brains following early life stress. For example, Brunson et al. (2005) reported significant dendritic atrophy in the hippocampus of middle aged rats provided with limited nesting material compared to rats with more significant nesting material. These data indicate that stressful events experienced early in life may have prolonged effects that may not develop until later in life. The mechanisms associated with this outcome have not been identified, but may represent a complex interaction between circulating GCs and related stress hormones and the integrity of cellular systems that regulate myelin across the lifespan.
Frontal lobe executive functions include working memory, problem solving, attention and processing speed among others. The ability of white matter pathways to efficiently communicate information to cortical and subcortical areas is important for effective higher level processing skills. In addition, the corpus callosum plays a key role in the transfer of information between hemispheres. However, in contrast to our expectations we did not observe significant effects of ELS on cognitive function among any of our age groups. While we extended our battery of tasks to include measures of attention, motor speed and executive function, our findings were still consistent with the study by Porter et al. (2005) who examined several cognitive measures focusing on memory in a population of children aged 8–14 with a history of sexual abuse. Their results showed no distinct cognitive memory deficits when compared to a healthy control group free of psychiatric disorders and matched for age, race, gender, handedness, grade and SES. In another study focusing on adult female survivors of childhood sexual abuse, Stein et al. (1999) found no memory differences between adult survivors of childhood sexual abuse and a control group.
However, studies that focused on memory and learning performance in children and adolescents suffering from PTSD found significant reductions in reading ability and general memory performance in diagnosed patients versus controls (Moradi et al. 1999). In addition, Yasik et al. (2007) compared trauma exposed children with PTSD to trauma exposed children without PTSD and children without trauma. They found reductions in verbal memory among those suffering from PTSD but not the trauma exposed without PTSD. While we used two separate sample populations (imaging and cognition), the results of our study indicate that cognitive function seems to be minimally affected in those healthy individuals who have experienced three or more ELS events, despite having lower FA values. Collectively our data, in conjunction with the results of other studies, suggest that cognitive consequences of ELS may not be apparent on formal neuropsychological measures until a threshold of severity is reached, at which point the clinical diagnosis of PTSD may be more likely.
The lack of significant findings with regard to cognitive functioning found in our study may be a result of the screening process used to acquire participants. It is important to remember that while the participants in our study may have experienced ELS, none of them were currently suffering from any clinical psychological problems as a result of ELS, indicating that they may be fairly resilient to stress. Many studies examining stress and cognition include many participants who are suffering from PTSD, depression, anxiety, high alcohol or drug use, or a combination of two or more of these psychological disorders. Our study utilized a population of individuals who were considered to be both physically as well as psychologically healthy. Given the health of our sample, it might also be possible that individuals with histories of ELS are capable of compensating for subtle brain alterations via increased cognitive effort which results in generally average cognitive performance. Future studies that incorporate more detailed analysis of the cognitive performances (e.g., sustained performances across quartiles of the verbal fluency task) may help to elucidate this possibility.
Although there is a large body of research linking cognitive difficulties to PTSD, there are several explanations describing this relationship. One such interpretation is that cognitive impairment may exist before the traumatic event and may be a predisposing and contributing factor in the development of PTSD (Brandes et al. 2002; Macklin et al. 1998; McNally and Shin 1995). Thus, significant cognitive impairment may increase the risk of developing PTSD after a traumatic event(s), thereby decreasing the likelihood that individuals with cognitive impairments would be included in this study sample.
While the overall number of participants who underwent cognitive assessment was large, the number of participants within the imaging sample was relatively limited. The reduced number of participants raises a question as to whether or not the effects we see in the youngest and oldest age groups with regard to lower FA values would remain consistent with a larger number of participants. As such it is important that our results be viewed as preliminary until further studies have been completed. In addition, because of the limited number of participants who received DTI imaging, we were unable to directly assess relationships between DTI abnormalities and cognitive performance. In the absence of significant cognitive findings in the present study this is not a major limitation. Nevertheless, it will be important for future studies to increase the DTI sample size in order to be able to use the same participants for both imaging and cognition. In addition, larger samples will allow for the examination of relationships between specific stressors (abuse, bullying or neglect) and brain structure and function.
The cross-sectional design and use of two separate groups of participants (imaging and cognition) in this study reduces the ability to determine a strong link between ELS, lower FA values and cognition. The ability to scan and test the same participants across their lifespan would allow us to more closely look at lower FA values and cognition, as well as to be able to look at specific stressors and their relationship to FA and cognition.
Finally, in order to maximize data inclusion, particularly for the youngest age group, we divided our sample into a high-ELS group (those reporting three or more events) and a low-ELS group (those reporting two or less). However, in doing this we were unable to analyze the relationship based on individual number of stressors and FA values. In addition, because only one participant among the 13–18-year old age group reported experiencing at least 3 ELS events, we limited our groupings from four to three. Therefore, we were unable to determine whether or not ELS affects teenage children in both areas of FA value and cognition. It is unclear why the number of ELS events in this age group was low relative to the other age groups. One explanation may be the reluctance of teenagers to acknowledge stressful events during childhood, especially those of a very personal nature.
The results of this study indicate that ELS may play a role in alterations of white matter pathways in the brain. Lower FA values among the youngest and oldest age groups exposed to three or more ELS events prior to age 13 may indicate a vulnerability of the brain during these critical periods of development. While we did not find significant alterations in cognitive measures, we did observe correlations with regard to the high-ELS group and cognitive tasks among the children and young adults. The use of a population of physically and psychologically healthy participants may have had an effect on our outcomes.
References
Andersen, S. L., & Teicher, M. H. (2004). Delayed effects of early stress on hippocampal development. Neuropsychopharmacology, 29, 1988–1993.
Brandes, D., Ben-Schachar, G., Gilboa, A., Bonne, O., Freedman, S., & Shalev, A. Y. (2002). PTSD symptoms and cognitive performance in recent trauma survivors. Psychiatry Research, 110(3), 231–238.
Brunson, K. L., Kramar, E., Lin, B., Chen, Y., Colgin, L. L., Yanagihara, K., et al. (2005). Mechanisms of late-onset cognitive decline after early-life stress. The Journal of Neuroscience, 25(41), 9328–9338.
Clark, C. R., Paul, R. H., Williams, L. M., Arns, M., Fallahpour, K., Handmer, C., et al. (2006). Standardized assessment of cognitive functioning during development and aging using an automated touchscreen battery. Archives of Clinical Neuropsychology, 21, 449–467.
Cohen, R. A., Grieve, S., Hoth, K. F., Paul, R. H., Sweet, L., Tate, D., et al. (2006a). Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry, 59, 975–982.
Cohen, R. A., Hitsman, B. L., Paul, R. H., McCaffery, J., Stroud, L., Sweet, L., et al. (2006b). Early life stress and adult emotional experience: An international perspective. International Journal of Psychiatry in Medicine, 36(1), 35–52.
Geffen, G. M., Moar, K. J., Hanlon, A. P., Clark, C. R., & Geffen, L. B. (1990). Performance measures of 16- to 86-year old males and females on the Auditory Verbal Learning test. The Clinical Neuropsychologist, 4, 45–63.
Goethals, I., Audenaert, K., Van De Wiele, C., & Dierckx, R. (2003). The prefrontal cortex: insights from functional neuroimaging using cognitive activation tasks. European Journal of Nuclear Medicine and Molecular Imaging, 31(3), 408–416.
Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, C., et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. PNAS, 101(21), 8174–8179.
Gordon, E., Cooper, N., Rennie, C., Hermens, D., & Williams, L. M. (2005). Integrative neuroscience: The role of a standardized database. Clinical EEG and Neuroscience, 36(2), 64–75.
Graham, J. E., Christian, L. M., & Kiecolt-Glaser, J. K. (2006). Stress, age, and immune function: Toward a lifespan approach. Journal of Behavioral Medicine, 29(4), 389–400.
Grieve, S. M., Williams, L. M., Paul, R. H., Clark, C. R., & Gordon, E. (2007). Cognitive aging, executive function, and fractional anisotropy: A diffusion tensor MR imaging study. American Journal of NeuroRadiology, 28, 226–235.
Gunnar, M., & Quevedo. K. (2006). The neurobiology of stress and development. Annual Review of Psychology[online]. Accessed 29 September 2006. URL: http://pych.annualreviews.org.
Halstead, W. C. (1947). Brain and intelligence. Chicago: University of Chicago Press.
Hicki, I. (1998). SPHERE: A National Depression Project. Australasian Psychiatry, 6, 248–250.
Lezak, M. (2004). Neuropsychological assessment. New York: OUP.
Lindauer, R. J. L., Olff, M., van Meijel, E. P. M., Carlier, I. V. E., & Gersons, B. P. R. (2006). Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biological Psychiatry, 59(2), 171–177.
Lovibond, P., & Lovibond, S. (1995). The structure of negative emotional sates: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Journal of Beaviour Research Therapy, 33, 335–343.
Macklin, M. L., Metzger, L. J., Litz, B. T., et al. (1998). Lower precombat intelligence is a risk factor for posttraumatic stress disorder. Journal of Consulting and Clinical Psychology, 66(2), 323–326.
McFarlane, A., Clark, C. R., Bryant, R. A., Williams, L. M., Niaura, R., Paul, R. H., et al. (2005). The impact of early life stress on psychophysiological, personality and behavioral measures in 740 non-clinical subjects. Journal of Integrative Neuroscience, 4(1), 27–40.
McNally, R. J., & Shin, L. M. (1995). Association of intelligence with severity of posttraumatic stress disorder symptoms in Vietnam combat veterans. American Journal of Psychiatry, 152(6), 936–938.
Milner, B. (1970). Memory and the medial temporal regions of the brain. Biology of memory. New York: Academic Press.
Moradi, A. R., Neshat Doost, H. T., Taghavi, M. R., Yule, W., & Dalgleish, T. (1999). Everyday memory deficits in children and adolescents with PTSD: Performance on the Rivermead Behavioural Memory Test. Journal of Child Psychology, 40(3), 357–361.
Neil, J., Miller, J., Mukherjee, P., & Huppi, P. S. (2002). Diffusion tensor imaging of normal and injured developing human brain: A technical review. NMR Biomedicine, 15, 543–552.
Paul, R., Henry, L., Grieve, S. M., Guilmete, T. J., Niaura, R., Bryant, R., et al. (2007). The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatric Disease and Treatment (in press).
Paul, R. H., Lawrence, J., Williams, L. M., Richard, C. C., Cooper, N., & Gordon, E. (2005). Preliminary validity of “integneuro”: a new computerized battery of neurocognitive tests. International Journal of Neuroscience, 115, 1549–1567.
Perez, C. M., & Widom, C. S. (1994). Childhood victimization and long-term intellectual and academic outcomes. Child Abuse & Neglect, 18(8), 617–633.
Porter, J., Lawson, J. S., & Bigler, E. D. (2005). Neurobehavioral sequelae of childhood sexual abuse. Child Neuropsychology, 11, 203–220.
Reitan, R. M. (1955). The relation of the Trail Making test to organic brain damage. Journal of Consultation Psychology, 5, 393–394.
Reitan, R. M. (1958). Validity of the Trail Making test as a indicator of organic brain damage. Perceptual & Motor Skills, 8, 271–276.
Salat, D. H., Tuch, D. S., Greve, D. N., van der Kouwe, A. J. W., Hevelone, N. D., Zaleta, A. K., et al. (2005). Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiology of Aging, 26, 1215–1227.
Sanders, B., & Becker-Lausen, E. (1995). The measurement of psychological maltreatment. Child Abuse and Neglect, 19, 315–323.
Sapolsky, R. M. (2000). Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. General Psychiatry, 57(10), 925–935.
Snook, L., Paulson, L., Roy, D., Phillips, L., & Beaulieu, C. (2005). Diffusion tensor imaging of neurodevelopment in children and young adults. NeuroImage, 26, 1164–1173.
Stein, M. B., Hanna, C., Vaerum, V., & Koverola, C. (1999). Memory functioning in adult women traumatized by childhood sexual abuse. Journal of Traumatic Stress, 12(3), 527–534.
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18, 643–662.
Sullivan, E., & Pfefferbaum, A. (2006). Diffusion tensor imaging and aging. Neuroscience and Biobehavioral Reviews, 30, 749–761.
Teicher, M. H., Dumont, N. L., Ito, Y., Vaituzis, C., Giedd, J. N., & Andersen, S. L. (2004). Childhood neglect is associated with reduced corpus callosum area. Biological Psychiatry, 56(2), 80–85.
Toga, A. W., Thompson, P. M., & Sowell, E. R. (2006). Mapping brain maturation. Trends in Neurosciences, 29(3), 148–158.
Williams, L. J., Paul, R. H., Lawrence, J., Clark, C. R., Cooper, N., & Gordon, G. (2005). The test-retest reliability a Standardized Neurocognitive and Neurophysiological test battery: “Neuromarker”. International Journal of Neuroscience, 115(12), 1605–1630.
Yasik, A. E., Saigh, P. A., Oberfield, R. A., & Halamandaris, P. V. (2007). Posttraumatic stress disorder: Memory and learning performance in children and adolescents. Biological Psychiatry, 61(3), 382–388.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seckfort, D.L., Paul, R., Grieve, S.M. et al. Early Life Stress on Brain Structure and Function Across the Lifespan: A Preliminary Study. Brain Imaging and Behavior 2, 49–58 (2008). https://doi.org/10.1007/s11682-007-9015-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-007-9015-y