Abstract
Diffusion tensor imaging was used to study the combined effects of HIV-infection and alcoholism (ALC) on corpus callosum (CC) integrity in relation to processes of attentional allocation and conflict resolution assessed by a novel Stroop Match-to-Sample task. We tested 16 ALC, 19 HIV, 20 subjects with combined disorder and 17 controls. In ALC, low fractional anisotropy and high mean diffusivity throughout the CC correlated with poor Stroop-match performance, i.e., when the cue-color matched the color of the Stroop stimulus. By contrast, in the two HIV groups DTI relations were restricted to the genu and poor Stroop-nonmatch performance, i.e., when the cue-color was in conflict with the Stroop stimulus color. These results suggest that disruption of callosal integrity in HIV-infection and alcoholism differentially affects regionally-selective interhemispheric-dependent attentional processing. We speculate that callosal degradation in these diseases curtails the opportunity for collaboration between the two hemispheres that contributes to normal performance in HIV or alcoholic patients with higher callosal integrity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A frequent comorbidity in individuals infected with human immunodeficiency virus (HIV) is alcohol use disorder (Justice et al. 2006; Lefevre et al. 1995; Meyerhoff 2001; Molina et al. 2006; Pfefferbaum et al. 2002; Winsauer et al. 2002). Each disorder exerts adversive effects on cognitive function and brain structure, some different and some overlapping. Although both disorders affect attention, each can selectively disrupt different component processes of this complex cognitive process. Chronic alcoholism adversely disrupts executive control mechanisms relying on prefrontal and parietal attentional brain systems (Nixon et al. 1988; Sullivan et al. 2000; Tapert et al. 2001). Lack of executive function control over attentional systems has also been reported in patients infected with human immunodeficiency virus (HIV) (Chao et al. 2004; Hinkin et al. 1999; McArthur 2004; Sahakian et al. 1995).
The Stroop Color Word Test (Stroop 1935) is a widely used paradigm to study selective attentional processes that require conflict resolution and response inhibition between competing stimulus attributes (Bush et al. 2003; Carter et al. 1999; Fan et al. 2003; MacDonald et al. 2000; Salo et al. 2001). The Stroop phenomenon describes slower responses when subjects are asked to name the ink color of a word printed in a color incongruent with the word’s meaning (e.g., the word “red” written in blue ink) relative to when the word’s meaning and ink color are the same (e.g., the word “red” written in red ink). When the relevant color-attribute and the irrelevant word-attribute are in conflict because of an inability to inhibit the faster, more automatic process of word reading while naming the word’s color, demands on attentional selection are greater in incongruent than in congruent trials. Thus, resolution of conflict between competing stimulus attributes requires inhibition of irrelevant information while attending to the intended attribute.
To measure component attentional processes of conflict resolution and attentional allocation, we devised a computerized Stroop Match-to-Sample task to test for effects of HIV infection and alcohol on the fronto-parietal attention system. This Stroop Match-to-Sample task requires a decision based on a color cue that directs attention to a specific color and primes the color processing of the Stroop stimulus. In particular, subjects saw either a valid or invalid color cue prior to a target word, printed in a color that was either congruent or incongruent with the word’s meaning. We presented the two stimuli sequentially in order to address attentional allocation with the color patch acting as a cue (Posner and Peterson 1990). The paradoxical finding that greater Stroop effects occur when the cue color correctly predicts the color of the Stroop word (match) than when the color cue misdirects attention in non-match trials (Schulte et al. 2005) is a stable phenomenon that we observed in different neuropsychiatric conditions and with different subjects (Schulte et al. 2006, 2007). It is further consistent with another study by Chen (2003), who found that the Stroop interference was greater when the cue was valid than when it was invalid. There are different possible explanations for this effect. One is derived from the conflict hypothesis, which poses that conflict (e.g., incongruent) preceded by conflict (e.g., nonmatch) will be processed faster than conflict preceded by non-conflict (e.g., match) because a different set of cognitive control mechanisms is involved when cognitive conflict occurs consecutively compared with when non-conflicting precedes conflicting information (Kerns et al. 2004). Alternatively, the result of greater Stroop effects in match than nonmatch trials can also be explained with the perceptual load hypothesis (Lavie 1995), which assumes that the degree of interference from distracting information (like the meaning of the word in the Stroop color-word task) is inversely related to the level of perceptual load, and perceptual load is further negatively related to the amount of processing resources available to process irrelevant information (Lavie and Fox 2000). Accordingly, valid trials afford more resource availability to process the meaning of the Stroop word than invalid trials (see also, Chen 2003).
The Stroop effect can be conceptually considered as a lateralized process with greater interference from the left hemisphere (LH) processing of verbal semantic information (Luo et al. 1999; Weekes and Zaidel 1996) than right hemisphere (RH) visual processing of color (MacLeod 1991). Using the Match-to-Sample Stroop task, we observed that individuals with HIV performed at a comparable level to controls in conflict processing and attentional allocation, whereas alcoholics showed prolonged reaction times (RT) on this task, and those with HIV+alcoholism were even slower. A greater Stroop effect in alcoholics was related to a smaller size of the corpus callosum suggesting involvement of callosal interhemispheric pathways in word-color Stroop processes that becomes apparent when callosal integrity is compromised (Schulte et al. 2006).
Diffusion tensor imaging (DTI) derived measures of microstructure in alcoholism (Pfefferbaum et al. 2002, 2006a) and HIV (Pfefferbaum et al. 2006b, 2007) may reveal even more robust effects than MRI-derived measures of callosal size in identifying disruptions in regional callosal systems that contribute to lateralized components of attentional allocation and conflict processing (Pfefferbaum et al. 1997; Pfefferbaum and Sullivan 2002). DTI permits examination of the integrity of the microstructure of cerebral white matter by measuring the orientational displacement and distribution of water molecules in vivo across tissue components (Basser and Pierpaoli 1996). Fractional anisotropy (FA) is a measure of orientational coherence, commonly applied to white matter (Pierpaoli and Basser 1996), and is typically higher in fibers with a homogeneous or linear structure than in tissue with an inhomogeneous structure, such as areas with pathology (Lansberg et al. 2001; Neumann-Haefelin et al. 2000) or crossing fibers (Virta et al. 1999; Xu et al. 2002). The bulk mean diffusivity (MD) of the diffusion tensor matrix provides a measure of the amount of diffusion and higher values indicate more diffusion, commonly due to larger presence of mobile fluid in a tissue sample (Kubicki et al. 2005; Pfefferbaum et al. 2003; Pfefferbaum and Sullivan 2003; Pierpaoli et al. 2001).
Recent studies of HIV infection and alcohol use disorders report microstructural abnormalities in the white matter of the corpus callosum (Pfefferbaum et al. 2006b, 2007; Pomara et al. 2001). In patients with alcoholism, DTI revealed low FA and high MD in the anterior callosal microstructure and centrum semiovale, the extent of which was related to working memory deficits (Pfefferbaum et al. 2000, 2006a; Pfefferbaum and Sullivan 2002). There is also postmortem evidence of structural disruption of frontal and parietal cortical sites and connections between cortices relevant to processes of selective attention in patients with long-term alcohol abuse (Kril et al. 1997) and in vivo evidence of such disruption in those with immunodeficiency virus infection (Hardy and Hinkin 2002). Despite the efficiency of highly active antiretroviral therapies (HAART) in reducing viral burden, alcohol can impair immune responses that would otherwise reduce neuroinflammation and neurodegeneration (Brailoiu et al. 2006; Chao et al. 2003; Hammoud et al. 2005; Potula et al. 2006).
To test whether abnormalities in microstructural connectivity between hemispheres in patients with HIV and alcoholism affect lateralized brain functions, we used the Stroop Match-to-Sample task invoking left-hemispheric verbal processing and right-hemispheric color processing in combination with measures of callosal microstructure using DTI. Accordingly, we focused on the relationship between Stroop effects and microstructural integrity (FA and MD), assuming that callosal compromise in patients with ALC, HIV and H+A, expressed as low FA and high MD, would be related to greater Stroop effects, i.e., more interference resulting from a decline in inhibitory control. However, because we had observed earlier (Schulte et al. 2005) that performance on our cued-Stroop task differed between patient groups as a function of color priming and cue validity, we hypothesized that these different effects for Stroop-match and Stroop-nonmatch conditions would be related to function of callosal integrity in these patients. Specifically, because HIV did not differ from control subjects in Stroop performance, but alcoholic subjects showed greater interference than control subjects, with further exacerbation resulting from the combined diseases, we expected relationships between the amount of callosal compromise and extent of Stroop interference, especially in ALC and H+A groups. The Stroop data (Schulte et al. 2005) and the DTI data (Pfefferbaum et al. 2007) were taken from our previously published reports, but the relations between these cognitive and brain data have not been published.
Methods
Subjects
The study subjects were 16 alcoholics (ALC), 19 patients with HIV infection, 20 patients with both HIV infection and alcoholism (H+A), and 17 controls (CTL). The same sample reported herein reflects the exclusion of four subjects from the behavioral study (Schulte et al. 2005): two control subjects (1 woman, 1 man) who had ventricle sizes that were more than four standard deviations (SD) from the normal average, and two alcoholic patients whose DTI scans were technically unusable. ALC and HIV patients were recruited from local rehabilitation programs and clinics, and controls were volunteers from the local community. Subjects gave written informed consent prior to participation.
All volunteers were screened by trained clinicians using Structured Clinical Interview for DSM-IV diagnosis (American Psychiatric Association 1994) and medical examination to exclude any with other lifetime Axis I diagnoses. Lifetime alcohol consumption (kg) was assessed using a semi-structured interview (Pfefferbaum et al. 1988) (Table 1). The study groups did not differ in age, handedness, or sex but did differ in lifetime alcohol consumption. Although ALC and H+A did not differ in their lifetime alcohol consumption, alcoholic subjects in ALC and H+A groups had consumed significantly more alcohol than control or HIV subjects. The HIV and H+A groups did not differ in disease severity as indexed by CD4+ and viral load. Six subjects in the HIV and eight subjects in the H+A group had had an AIDS defining event, which was defined as CD4+ count lower than 200 or opportunistic infection (Pfefferbaum et al. 2007). Regarding HIV medication, 68.4% in the HIV group and 55% in the H+A group were taking highly active anti-retroviral treatment (HAART); this difference was not significant (χ 2 = 0.39, n.s.).
Stroop match-to-sample task
The Stroop Match-to-Sample task was developed to test sensory and attentional processes of Stroop conflict. Subjects matched the color of a cue stimulus (4 colored Xs), displayed for 800 ms in the lower visual field, to the color of a target stimulus, appearing 300 ms later, for 1000 ms above the fixation point. Target stimuli were color words written in an ink (1) congruent or incongruent with the word’s meaning and were either (2) single (left or right visual field) or paired (identical stimuli appearing simultaneously in both fields, i.e., redundant targets), and either (3) matched or nonmatched to the cue color.
Single and paired targets in the Stroop Match-to-Sample task were presented to study bottom-up processes of perceptual load, i.e., whether single and paired stimulus inputs would differentially affect Stroop processing. However, no significant effects for stimulation (single/paired) were found, nor were there significant interactions with Stroop effects (Schulte et al. 2005). Because we did not expect relationships between stimulation type and callosal integrity, stimulation effects were not included in the analysis of callosal functions.
Subjects were instructed first to say aloud the color of the target stimulus, then to say either “Yes” or “No,” depending on whether the target color matched the cue color, and finally to press the corresponding YES or NO key with their right hand. Naming the target color was required because neuroimaging data provide evidence that executive control in the Stroop task is associated with vocal control regions (Paus et al. 1993; Picard and Strick 1996) and manual responses are known to produce reduced Stroop effects (MacLeod 1991). Thus, in addition to manual responses subjects were asked to vocalize responses to ensure that verbal processes were invoked. The Stroop effect was examined by comparing congruent and incongruent conditions (Koch and Brown 1994).
Each block included 192 stimuli randomly presented on a 21-in. monitor of a Macintosh computer using PsyScope 1.2.5 (http://psyscope.psy.cmu.edu) for stimulus delivery and RT acquisition. Subjects fixated on a point displayed in the middle of the computer screen at the beginning of each trial. Stimuli were presented at 4° visual eccentricity (distance of about 50 cm) radially distributed around the fixation point, positioned in the visual field quadrants.
Diffusion tensor imaging (DTI)
MRI data were acquired on a 1.5 T General Electric (Milwaukee, WI) Signa human MRI scanner (gradient strength = 40 mT/m; slew rate = 150 T/m/s). FA and diffusivity (MD) data were taken from our published report (Pfefferbaum et al. 2007) and were measured on midsagittal corpus callosum substructures, the genu, body, and splenium. FA, a measure of intravoxel coherence, ranges from 0 (perfect isotropy, lowest FA) to 1 (perfectly coherent anisotropy). FA expresses the fraction of orientational coherence at an intravoxel level and is not affected by the intracranial volume of the supratentorium and thus, does not necessitate head size correction (e.g., Greenberg et al. 2006). MD is expressed as area/time (e.g., 10−6 mm2/s). As previously described (Pfefferbaum et al. 2007) two coronal structural sequences were acquired: (1) a dual-echo fast spin echo (FSE) sequence (47 contiguous, 4 mm thick slices; TR/TE1/TE2 = 7500/14/98 ms; matrix = 256 × 192); and (2) a SPoiled Gradient Recalled Echo (SPGR) sequence (94 2 mm thick slices; TR/TE = 25/5 ms, flip angle = 30°, matrix = 256 × 192). All images were zero-filled to 256 × 256 pixels in-plane by the scanner reconstruction software.
DTI was acquired in the coronal plane with the same slice location parameters as the dual-echo FSE, using a single shot spin-echo echo-planar imaging technique with a 24 cm field of view (47 contiguous, 4 mm thick slices, TR/TE = 10,000/103 ms, matrix = 128 × 128, in-plane resolution = 1.875 mm2). The amplitude of the diffusion-sensitizing gradients was 1.46 Gauss/cm with 32 ms duration and 38 ms separation, resulting in a b-value of 860 s/mm2. Diffusion was measured along six non-collinear directions with alternating signs to minimize the need to account for cross-terms between imaging and diffusion gradients (Neeman et al. 1991). For each gradient direction, six images were acquired and averaged as were six images with no diffusion weighting (b = 0 s/mm2). We chose to cycle through b = 0 with each cycle of b = 1,2,3,4,5,6 for 1) simplicity of the acquisition psd, 2) so that each set of acquisitions (there were six in total) could be treated as a unique entity in the event that a whole NEX needed to be discarded due to artifact, and 3) to have a b = 0 in temporal proximity to each set of the b = 1,2,3,4,5,6 for registration. The coronal MRI and DTI acquisitions produced either 2 or 4 mm thick slices and were prescribed for consistent slice locations so that each 4 mm slice encompassed a pair of 2 mm thick slices.
Image processing
The dual-echo FSE images were passed through the FSL Brain Extraction Tool (BET) (Smith 2002) to extract the brain and exclude dura, skull, scalp and other non-brain tissue, and the mask was also used to extract the brain from the SPGR data.
Eddy-current induced image distortions due to the large diffusion encoding gradients cause spatial distortions in the diffusion-weighted DTI images that vary from one diffusion direction to the next. These artifacts were minimized by alignment with an average made of all 12 diffusion-weighted images with a 2D six-parameter affine correction on a slice-by-slice basis to unwarp the eddy-current distortions in the diffusion-weighted DTI images for each direction. After eddy-current correction and averaging of the multiple acquisitions, the DTI data were aligned with the FSE data with a non-linear 3D warp (third-order polynomial), which provided a 6 orientation (plus and minus direction for each orientation) in-plane and through-plane alignment.
Using the averaged images with b = 0 and b = 860 s/mm2, six maps of the apparent diffusion coefficient (ADC) were calculated, each being a sum of three elements of the diffusion tensor. Based on the eigenvalues from the tensor, FA and MD (the mean of the tensor matrix eigenvalues) were calculated on a voxel-by-voxel basis. Thus, each diffusion-weighted study was reduced to a set of three images for each slice (FA, MD, and b = 0) to be used for analysis in conjunction with the anatomical images.
Warping to common coordinates
To place the images for all subjects into a coordinate system with a common origin and a standardized anatomical orientation, the anterior commissure (AC) and posterior commissure (PC) were manually identified on the native 2 mm-thick SPGR images and rotated into a common orientation. The parameters required to accomplish this transformation for each scan session were applied to all of the structural, FA, and diffusion images. All of the datasets were resliced to isotropic 1 mm3 voxels and the field-of-view set to 20 cm for each axis. Each subject’s SPGR data were then aligned to a grand average laboratory SPGR template with a 12-parameter affine model, followed by creation of a new grand average used as a template for a higher-order (3rd to 5th polynomial) nonlinear warp. The early echo, dual-echo FSE data for each subject were also aligned to the SPGR grand average with AIR5.2.5 (3rd to 5th polynomial) nonlinear warp (Woods et al. 1998a, b). The b = 0 images were warped to the native late echo, dual-echo FSE images in 3-D, first with a 12 parameter affine, followed by stepwise 2nd and 3rd order polynomial functions. Finally, for each subject all the registration transformation matrices were then combined into one function so they could be applied only once to native data. This process allowed for anatomical identification of the corpus callosum in a common space for each subject from structural or FA images.
Identification of the corpus callosum and its sectors
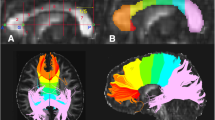
The corpus callosum was identified on the midsagittal slice extracted from the aligned FA data with a semi-automated edge identification procedure with high interrater reliability. Regional callosal areas were defined geometrically, as follows. The corpus callosum silhouette was rotated to a plane parallel to the inferior extremes of the rostrum anteriorly and splenium posteriorly. The midpoint along this plane between the anterior extreme of the genu and posterior extreme of the splenium was used as the center of a circle, and radii were projected at +30° and +150° angles relative to the x-axis from the plane, thus dividing the corpus callosum into genu+rostrum, body, and splenium (Schulte et al. 2004; Sullivan et al. 2002). FA and MD were expressed as the average values for five, 1 mm thick, mid- and para-sagittal slices for each of the three callosal sectors (Fig. 1).
Data analysis
A series of analyses of variance (ANOVAs) for repeated measures were conducted to test for the effects of group (CTL, ALC, HIV, H+A), on (1) performance in Match (cue-target match vs. nonmatch) and Stroop (incongruent vs. congruent) conditions, (2) regional FA and (3) regional MD of the corpus callosum (genu, body, splenium). A multivariate ANOVA was conducted to study group effects on Stroop-match and Stroop-nonmatch trials separately, and Least Significant Difference (LSD) tests were used for post-hoc comparison of groups. Pearson correlations tested the relationships between DTI measures (FA and MD) and Stroop-Match performance separately in each group (CTL, ALC, HIV, H+A). For significant correlations, we then conducted simple regressions to estimate the amount of variance explained by callosal regions (genu, body, and splenium) as predictors of performance. Additionally, we tested for differences between correlations of two groups (Walker and Lev 1953). The alpha level was set to 0.05 for all statistical tests.
To estimate the increasing risk of Type 1 errors with multiple comparisons, we performed 1,000 simulations using our data set, where within each group Stroop data were randomized and then used to calculate correlations with callosal FA and MD measures. We randomized the Stroop RT measurements as blocks among subjects, thus maintaining the internal correlation structure of the Stroop RT measurements (Stroop-match, Stroop-nonmatch) and leaving the FA and MD measurements with the original subjects, thereby maintaining the correlation structure of FA and MD callosal area measurements. Thus, in these simulations we negated the cross-correlational structure between RT and callosal measurements, so that any statistically significant correlations between Stroop RT and FA or MD measurements would be chance occurrences.
Results
Error analysis and overall RT
Mean overall error rate was less than 5% in all groups (2.27% in CTL; 2.67% in ALC; 4.41% in HIV; 7.71% in H+A). A positive correlation between overall RTs and error rates in all groups (CTL: r = 0.46, p = 0.07; ALC: r = 0.76, p < 0.001; HIV: r = 0.69, p < 0.001; H+A: r = 0.53, p = 0.017) indicated a lack of speed–accuracy tradeoff, which would have been indicated by a negative correlation (Salo et al. 2001; Schulte et al. 2005).
Stroop performance
There were age-related effects on Stroop performance in controls (CTL) with longer RTs and greater Stroop effects with advancing age in match and nonmatch trials (overall RT, r = 0.45, p = 0.034; Stroop match, r = 0.64, p < 0.003; Stroop nonmatch, r = 0.50, p = 0.02; one-tailed). Thus, Stroop RTs were adjusted for the age-RT relationship from CTL using the following equation: RTadjusted = RT − beta(Age − Age mean). Overall error rate did not correlate with age in any study group (CTL: r = 0.31, ns; ALC: r = 0.32, ns; HIV: r = 0.26, ns; H+A: r = 0.16, ns; one-tailed).
Even with age-correction of RT data, the samples of subjects analyzed herein showed the same behavioral patterns of group differences observed in the full samples (Schulte et al. 2005). An overall ANOVA on age-corrected RT data with Stroop and Match conditions as within subject factors and group (CTL, HIV, ALC, H+A) as the between subject factor revealed significant Stroop (F(1,68) = 169.95, p < 0.0001) and Match (F(1,68) = 142.8, p < 0.0001) effects and a Stroop-by-Match interaction (F(1,68) = 14.31, p < 0.0001). Furthermore, Stroop effects differed between groups (group-by-Stroop interaction: F(3,68) = 2.87, p = 0.04). Although we did not detect a statistically significant 3-way interaction, the p-value was sufficiently low with p = 0.12, that in the light of the modest power available with our moderate sample sizes, we decided to conduct a MANOVA to test for group-by-Stroop interactions separately for match and nonmatch trials. Stroop effects (difference RT: incongruent-congruent) for match and nonmatch trials were entered as dependent variables and group as fixed factor in this exploratory analysis. Group differences were found for Stroop nonmatch trials (F(3,68) = 4.31, p < 0.008) but not for match trials F(3,68) = 1.82, ns) (MANOVA; Pilai’s Trace F(6,136) = 2.89, p = 0.01). When attention was misdirected by an incorrect color cue (nonmatch), H+A showed greater Stroop effects than CTL (p < 0.0015) and HIV (p < 0.0015; post-hoc analysis; one-tailed), and ALC showed a trend in the impaired direction (p = 0.07; post-hoc analysis; one-tailed) (Table 2, Fig. 2).
Callosal microstructure
Because white matter FA decreased and MD increased with advancing age, we adjusted these DTI metrics in all callosal regions for age based on 120 controls previously published (Pfefferbaum et al. 2007) and expressed values as age-adjusted Z-scores (mean of the 120 control subjects = 0 ± 1 S.D.).
A repeated measures ANOVA for FA of the genu, body, and splenium revealed a significant effect for group, with higher FA in controls than in ALC (p < 0.004) and H+A (p < 0.0001) but not in HIV (p = 0.29; LSD post-hoc comparison), and no group-by-region interaction (p = 0.4). The same analysis for MD yielded a trend for a region effect and significant effects for group, with lower MD in controls than in ALC (p = 0.029) and H+A (p = 0.012; LSD post-hoc comparison), particularly in the callosal body area (Table 3, Fig. 3). Thus, this sample of 72 subjects showed the same pattern of group differences as the full sample of 269 subjects, from which the present sample was drawn (Pfefferbaum et al. 2007).
FA and MD of the total corpus callosum were negatively correlated with each other. However, correlation coefficients in healthy subjects (CTL: r = −0.51) were lower than in the three patient groups (HIV: r = −0.80; ALC: r = −0.86; H+A: r = −0.93).
Callosal microstructure and Stroop-match performance
Relationships between Stroop effects (incongruent–congruent conditions) and measures of callosal integrity were tested with one-tailed Pearson correlations, assuming that poorer microstructural integrity (low FA and high MD) would be related to greater Stroop effects (Table 4). Analyses of Stroop effects for match and nonmatch trials revealed significant correlations between performance on Stroop-match (Table 4), but not Stroop-nonmatch (Table 4) conditions, and lower FA (Fig. 4a–c) and higher mean diffusivity (MD) in each callosal region in ALC.
To estimate the increasing risk of Type 1 errors with multiple comparisons we calculated from our data set the probabilities of observing 8 or more significant correlations at 0.05 individual levels (one-tailed) simply by chance, which was 6%. We also calculated the probability of obtaining 6 significant correlations at 0.05 individual levels in a row for Stroop-match, a pattern that we observed for ALC, and found that the probability to find this pattern by chance is less than 3%: P(6 or more significant correlations) = 2.6%.
Linear regression for each callosal area revealed that genu FA alone explained 27%, body FA alone 23% and splenium FA alone 22% of Stroop-match variance in ALC (genu: F(1,14) = 5.1, p = 0.02; body: F(1,14) = 4.25, p = 0.029; splenium: F(1,14) = 3.9, p = 0.034; one-tailed). FA of the total corpus callosum explained 26% (F(1,14) = 4.82, p = 0.023, one-tailed) of the total variance.
To test whether the Stroop-callosal microstructure correlations differed significantly between alcoholics and controls we used r to z r transformation (z r = 0.5loge(1 + r)∕(1 − r)) (Walker and Lev 1953). We found trends for an ALC-specific relationship between Stroop-match and callosal microstructure (genu FA: z = −1.45, p = 0.07; genu MD: z = 1.34, p = 0.09; body MD: z = 1.32, p = 0.09; total corpus callosum MD: z = 1.34, p = 0.09).
In nonmatch trials, moderate correlations were found between greater Stroop effects and lower genu FA in H+A patients (Fig. 4d) and higher genu MD in HIV patients (Fig. 4e). Testing whether these correlations in H+A and HIV patients differed from those in controls revealed trends for a H+A-specific relationship between genu FA and Stroop-nonmatch performance (z = −1.61, p = 0.055) and for a HIV-specific relationship between genu MD and Stroop-nonmatch performance (z = 1.44, p = 0.075).
Exploratory analyses examined whether a history of having an AIDS-defining event contributed to the Stroop-DTI correlations observed in either HIV-infected group. The correlation between Stroop-nonmatch performance and genu MD was not significant in the six AIDS patients in the HIV alone group (r = 0.31, p = 0.28). By contrast, the correlation between Stroop-nonmatch performance and genu FA was substantially higher in the eight AIDS patients in the H+A group than in the non-AIDS groups (r = −0.73, p = 0.020, one-tailed).
Effects of viral load and CD4+ count on Stroop performance and regional callosal FA and MD
Higher viral loads correlated moderately with greater Stroop effects on nonmatch trials in H+A (r = 0.37, p = 0.053; one-tailed) but less so in HIV patients (r = 0.25, p = 0.15; one-tailed). Similarly, in H+A but not in HIV, lower CD4+ count correlated with greater MD in the genu (r = −0.42, p = 0.034; one-tailed) and lower FA in the splenium (r = 47, p = 0.019; one-tailed).
Discussion
This analysis revealed that alcoholic and HIV-infected patients displayed different patterns of relations between components of our cued Stroop test and regional microstructural integrity of the corpus callosum. In particular, low anisotropy and high diffusivity measures throughout the corpus callosum in patients with chronic alcoholism were correlated with poor Stroop-match performance, i.e., when the cue color correctly predicted the color of the Stroop stimulus. By contrast, low anisotropy and high diffusivity measures in patients with HIV infection, irrespective of alcoholism comorbidity, were restricted to the genu and poor performance in the Stroop-nonmatch condition, i.e., when the cue color was in conflict with the color of the Stroop stimulus.
Alcoholics as a group performed similarly to the control group on Stroop-match trials, despite the negative relation between low callosal integrity and greater susceptibility to Stroop-interference in the alcoholics. Thus, we speculate that callosal integrity may have enabled collaboration between the two hemispheres that contributed to good performance levels in alcoholics with higher callosal integrity than those with lower callosal integrity.
In contrast to alcoholics, HIV patients did not differ significantly in FA or MD of the corpus collosum from normal controls. The HIV patients in the present study were relatively healthy and showed no evidence of clinical dementia (Schulte et al. 2005). Nonetheless, the correlation between genu microstructure and Stroop-nonmatch performance in HIV suggests that such relationships may also become apparent with conflicting cognitive demands: cue-target nonmatch and color-word incongruency. Stroop-match performance, invoking cognitive conflict from color-word incongruency only, was not correlated to the callosal microstructure. When incongruent and nonmatch conflict processes are following each other attentional control mechanisms are engaged, one of the type served by parietal systems activated to disengage from the incorrectly cued color (nonmatch) and another of the type served by frontal systems activated to resolve and monitor Stroop conflict (Carter et al. 2000; Pardo et al. 1990) and to exert executive control (MacDonald et al. 2000). The engagement of fronto-parietal attention systems may be different for Stroop-match trials. Evidence derives from a recent functional MRI study on Stroop Match-to-Sample processing showing involvement of a fronto-temporo-parietal attention network in cued-Stroop processing in healthy participants that differed for Stroop-match and Stroop-nonmatch performance (Schulte et al. 2007). Thus, HIV patients with smaller genu were less able to resolve Stroop conflict when preceded by another conflict from nonmatching information, indicating limited processing resources available to process irrelevant (incongruent and nonmatching) information (Lavie and Fox 2000).
The specificity of genu relations in the HIV group is compatible with an earlier report by Chang et al. (2002) showing that deficits in HIV patients on a Stroop task were associated with markers of glial proliferation, specifically, elevated myo-inositol and choline compounds in the frontal white matter. In other recent studies, HIV patients exhibited diffusion abnormalities in the genu that were associated with deficits in executive functions (Thurnher et al. 2005) and in the splenium that were associated with dementia severity and motor slowing (Wu et al. 2006). One possible mechanism of callosal axonal damage was identified in a non-human primate model of HIV; Macaques infected with simian immunodeficiency virus (SIV) showed compromised performance on a bimanual motor task, the extent of which was associated with the accumulation of beta-amyloid precursor protein in the corpus callosum (Weed et al. 2003). Although others also reported a relationship between Stroop performance and frontal white matter in HIV (Chang et al. 2002), correlations between genu integrity measures (FA, MD) and Stroop-nonmatch performance in both HIV groups were moderate and require replication.
Despite their large Stroop effect, the HIV-alcoholism comorbid patients had only moderate relation between FA in the genu and Stroop-nonmatch performance, that is, conflict resolution, which may reflect compromise of interhemispheric and intrahemispheric attentional networks. Behavioral studies showing abnormally high interference effects in Stroop paradigms by patients with HIV-alcoholism comorbidity (Schulte et al. 2005) or late-life depression (Murphy et al. 2006) provide inferential evidence for degraded integrity of the intrahemispheric frontoparietal attention network. Our current findings extend this functional network to include interhemispheric effects of HIV-alcoholism comorbidity on Stroop interference effects. It could be speculated that other regional structural changes, for example, striatal deficits in HIV patients (Chiang et al. 2007) and alcoholics (Sullivan et al. 2005; Tapert et al. 2003) can compromise executive functions as well as motor functions as observed in other diseases affecting the striatum (e.g. Huntington’s disease: Peinemann et al. 2005; Parkinson’s disease: Cools et al. 2006). Animal models have demonstrated that chronic alcohol consumption induces neuroinflammation and oxidative stress that can exacerbate the progress of the HIV infection (Potula et al. 2006). Evidence for a functional effect of immunocompromise progression in our comorbid patients emerged from the correlation between high viral loads and poor Stroop-nonmatch performance, indicating that severity of HIV infection is related to the degree of disability to disengage attention from an incorrectly cued color to the correct color to resolve a Stroop conflict. However, given the small samples and multiple statistical comparisons, this study has limitations for generalizability and for determination of brain structure-function relations.
In conclusion, our data indicate the following dissociation: indices of nonfocal callosal microstructural compromise were associated with attentional allocation (Stroop-match performance) in alcoholics, whereas focal genu indices were associated with conflict resolution (Stroop-nonmatch performance) in the two HIV infected groups. Thus, disruption of callosal integrity indexed by DTI adversely and differentially affects component processes of selective attention and conflict processing in patients with HIV infection and alcoholism. Together these results provide evidence that alcoholism- and HIV-related degradation of the callosum curtails the opportunity to enable collaboration between the two hemispheres that contributes to normal performance in those with higher callosal integrity.
References
American Psychiatric Association (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Press.
Basser, P. J., & Pierpaoli, C. (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance. Series B, 111, 209–219.
Brailoiu, E., Brailoiu, G. C., Mameli, G., Dolei, A., Sawaya, B. E., & Dun, N. J. (2006). Acute exposure to ethanol potentiates human immunodeficiency virus type 1 Tat-induced Ca(2+) overload and neuronal death in cultured rat cortical neurons. Journal of Neurovirology, 12, 17–24.
Bush, G., Shin, L. M., Holmes, J., Rosen, B. R., & Vogt, B. A. (2003). The multi-source interference task: Validation study with fMRI in individual subjects. Molecular Psychiatry, 8, 60–70.
Carter, C. S., Botvinick, M. M., & Cohen, J. D. (1999). The contribution of the anterior cingulate cortex to executive processes in cognition. Reviews in the Neurosciences, 10, 49–57.
Carter, C. S., MacDonald, A. M., Botvinick, M., Ross, L. L., Stenger, V. A., Noll, D., et al. (2000). Parsing executive processes: Strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America, 97, 1944–1948.
Chang, L., Ernst, T., Witt, M. D., Ames, N., Gaiefsky, M., & Miller, E. (2002). Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. NeuroImage, 17, 1638–1648.
Chao, L. L., Cardenas, V. A., Meyerhoff, D. J., Rothlind, J. C., Flenniken, D. L., Lindgren, J. A., et al. (2003). Abnormal contingent negative variation in HIV patients receiving antiretroviral therapy. Neuroreport, 14, 2111–2115.
Chao, L. L., Lindgren, J. A., Flenniken, D. L., & Weiner, M. W. (2004). ERP evidence of impaired central nervous system function in virally suppressed HIV patients on antiretroviral therapy. Clinical Neurophysiology, 115, 1583–1591.
Chen, Z. (2003). Attentional focus, processing load, and Stroop interference. Perception & Psychophysics, 65, 888–900.
Chiang, M. C., Dutton, R. A., Hayashi, K. M., Lopez, O. L., Aizenstein, H. J., Toga, A. W., et al. (2007). 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. NeuroImage, 34, 44–60.
Cools, R., Ivry, R. B., & D’Esposito, M. (2006). The human striatum is necessary for responding to changes in stimulus relevance. Journal of Cognitive Neuroscience, 18, 1973–1983.
Crovitz, H. F., & Zener, K. (1962). A group test for assessing hand- and eye-dominance. American Journal of Psychology, 75, 271–276.
Fan, J., Flombaum, J. I., McCandliss, B. D., Thomas, K. M., & Posner, M. I. (2003). Cognitive and brain consequences of conflict. NeuroImage, 18, 42–57.
Greenberg, D. L., Messer, D. F., Payne, M. E., Macfall, J. R., Provenzale, J. M., Steffens, D. C., et al. (2006). Aging, gender, and the elderly adult brain: An examination of analytical strategies. Neurobiology of Aging, Oct 13.
Hammoud, D. A., Endres, C. J., Chander, A. R., Guilarte, T. R., Wong, D. F., Sacktor, N. C., et al. (2005). Imaging glial cell activation with [11C]-R-PK11195 in patients with AIDS. Journal of Neurovirology, 11, 346–355.
Hardy, D. J., & Hinkin, C. H. (2002). Reaction time performance in adults with HIV/AIDS. Journal of Clinical and Experimental Neuropsychology, 24, 912–929.
Hinkin, C. H., Castellon, S. A., Hardy, D. J., Granholm, E., & Siegle, G. (1999). Computerized and traditional Stroop task dysfunction in HIV-1 infection. Neuropsychology, 13, 306–316.
Justice, A. C., Lasky, E., McGinnis, K. A., Skanderson, M., Conigliaro, J., Fultz, S. L., et al. (2006). Medical disease and alcohol use among veterans with human immunodeficiency infection: A comparison of disease measurement strategies. Medical Care, 44, S52–S60.
Kerns, J. G., Cohen, J. D., MacDonald, A. W., 3rd, Cho, R. Y., Stenger, V. A., & Carter, C. S. (2004). Anterior cingulate conflict monitoring and adjustments in control. Science, 303, 1023–1026.
Koch, C., & Brown, J. M. (1994). Examining the time course of prime effects on Stroop processing. Perceptual and Motor Skills, 79, 675–687.
Kril, J. J., Halliday, G. M., Svoboda, M. D., & Cartwright, H. (1997). The cerebral cortex is damaged in chronic alcoholics. Neuroscience, 79, 983–998.
Kubicki, M., Westin, C. F., McCarley, R. W., & Shenton, M. E. (2005). The application of DTI to investigate white matter abnormalities in schizophrenia. Annals of the New York Academy of Sciences, 1064, 134–148.
Lansberg, M. G., Thijs, V. N., O’Brien, M. W., Ali, J. O., de Crespigny, A. J., Tong, D. C., et al. (2001). Evolution of apparent diffusion coefficient, diffusion-weighted, and T2-weighted signal intensity of acute stroke. American Journal of Neuroradiology, 22, 637–644.
Lavie, N. (1995). Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception and Performance, 21, 451–468.
Lavie, N., & Fox, E. (2000). The role of perceptual load in negative priming. Journal of Experimental Psychology: Human Perception and Performance, 26, 1038–1052.
Lefevre, F., O’Leary, B., Moran, M., Mossar, M., Yarnold, P. R., Martin, G. J., et al. (1995). Alcohol consumption among HIV-infected patients. Journal of General Internal Medicine, 10, 458–460.
Luo, Y. J., Hu, S., Weng, X. C., & Wei, J. H. (1999). Effects of semantic discrimination of Chinese words on N400 component of event-related potentials. Perceptual and Motor Skills, 89, 185–193.
MacDonald, A. W., 3rd, Cohen, J. D., Stenger, V. A., & Carter, C. S. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288, 1835–1838.
MacLeod, C. M. (1991). Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin, 109, 163–203.
McArthur, J. C. (2004). HIV dementia: An evolving disease. Journal of Neuroimmunology, 157, 3–10.
Meyerhoff, D. J. (2001). Effects of alcohol and HIV infection on the central nervous system. Alcohol Research & Health, 25, 288–298.
Molina, P. E., McNurlan, M., Rathmacher, J., Lang, C. H., Zambell, K. L., Purcell, J., et al. (2006). Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcoholism, Clinical and Experimental Research, 30, 2065–2078.
Murphy, C. F., Gunning-Dixon, F. M., Hoptman, M. J., Lim, K. O., Ardekani, B., Shields JK, et al. (2006). White-matter integrity predicts Stroop performance in patients with geriatric depression. Biological Psychiatry, Nov 20.
Neeman, M., Freyer, J. P., & Sillerud, L. O. (1991). A simple method for obtaining crossterm-free images for diffusion anisotropy studies in NMR microimaging. Magnetic Resonance in Medicine, 21, 138–143.
Neumann-Haefelin, T., Moseley, M. E., & Albers, G. W. (2000). New magnetic resonance imaging methods for cerebrovascular disease: Emerging clinical applications. Annals of Neurology, 47, 559–570.
Nixon, S. J., Tivis, R. D., Jenkins, M. R., & Parsons, O. A. (1988). Effects of cues on memory in alcoholics and controls. Alcoholism, Clinical and Experimental Research, 22, 1065–1069.
Pardo, J. V., Pardo, P. J., Janer, K. W., & Raichle, M. E. (1990). The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proceedings of the National Academy of Sciences U S A, 87, 256–259.
Paus, T., Petrides, M., Evans, A. C., & Meyer, E. (1993). Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: A positron emission tomography study. Journal of Neurovirology, 70, 453–469.
Peinemann, A., Schuller, S., Pohl, C., Jahn, T., Weindl, A., & Kassubek, J. (2005). Executive dysfunction in early stages of Huntington’s disease is associated with striatal and insular atrophy: A neuropsychological and voxel-based morphometric study. Journal of the Neurological Sciences, 15, 11–19.
Pfefferbaum, A., Adalsteinsson, E., & Sullivan, E. V. (2003). Replicability of diffusion tensor imaging measurements of fractional anisotropy and trace in brain. Journal of Magnetic Resonance Imaging, 18, 427–433.
Pfefferbaum, A., Adalsteinsson, E., & Sullivan, E. V. (2006a). Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiology of Aging, 27, 994–1009.
Pfefferbaum, A., Rosenbloom, M. J., Adalsteinsson, E., & Sullivan, E. V. (2007). Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: Synergistic white matter damage. Brain, 130, 48–64.
Pfefferbaum, A., Rosenbloom, M., Crusan, K., & Jernigan, T. L. (1988). Brain CT changes in alcoholics: Effects of age and alcohol consumption. Alcoholism, Clinical and Experimental Research, 12, 81–87.
Pfefferbaum, A., Rosenbloom, M. J., Rohlfing, T., Adalsteinsson, E., Kemper, C. A., Deresinski, S., et al. (2006b). Contribution of alcoholism to brain dysmorphology in HIV infection: Effects on the ventricles and corpus callosum. NeuroImage, 33, 239–251.
Pfefferbaum, A., Rosenbloom, M., & Sullivan, E. V. (2002). Alcoholism and AIDS: Magnetic resonance imaging approaches for detecting interactive neuropathology. Alcoholism, Clinical and Experimental Research, 26, 1031–1046.
Pfefferbaum, A., & Sullivan, E. V. (2002). Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. NeuroImage, 15, 708–718.
Pfefferbaum, A., & Sullivan, E. V. (2003). Increased brain white matter diffusivity in normal adult aging: Relationship to anisotropy and partial voluming. Magnetic Resonance in Medicine, 49, 953–961.
Pfefferbaum, A., Sullivan, E. V., Hedehus, M., Adalsteinsson, E., Lim, K. O., & Moseley, M. (2000). In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcoholism, Clinical and Experimental Research, 24, 1214–1221.
Pfefferbaum, A., Sullivan, E. V., Mathalon, D. H., & Lim, K. O. (1997). Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism, Clinical and Experimental Research, 21, 521–529.
Picard, N., & Strick, P. L. (1996). Motor areas of the medial wall: A review of their location and functional activation. Cerebral Cortex, 6, 342–353.
Pierpaoli, C., Barnett, A., Pajevic, S., Chen, R., Penix, L. R., Virta, A., et al. (2001). Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. NeuroImage, 13, 1174–1185.
Pierpaoli, C., & Basser, P. J. (1996). Toward a quantitative assessment of diffusion anisotropy. Magnetic Resonance in Medicine, 36, 893–906.
Pomara, N., Crandall, D. T., Choi, S. J., Johnson, G., & Lim, K. O. (2001). White matter abnormalities in HIV-1 infection: A diffusion tensor imaging study. Psychiatry Research, 106, 15–24.
Posner, M. I., & Petersen, S. E. (1990). The attention system of the human brain. Annual Review of Neuroscience, 13, 25–42.
Potula, R., Haorah, J., Knipe, B., Leibhart, J., Chrastil, J., Heilman, D., et al. (2006). Alcohol abuse enhances neuroinflammation and impairs immune responses in an animal model of human immunodeficiency virus-1 encephalitis. American Journal of Pathology, 168, 1335–1344.
Sahakian, B. J., Elliott, R., Low, N., Mehta, M., Clark, R. T., & Pozniak, A. L. (1995). Neuropsychological deficits in tests of executive function in asymptomatic and symptomatic HIV-1 seropositive men. Psychological Medicine, 25, 1233–1246.
Salo, R., Henik, A., & Robertson, L. C. (2001). Interpreting Stroop interference: An analysis of differences between task versions. Neuropsychology, 15, 462–471.
Schulte, T., Muller-Oehring, E. M., Mayer, D., Vinco, S., Pfefferbaum, A., & Sullivan, E. V. (2007). Neural correlates of attentional control of conflict processing: fMRI evidence from a Stroop Match-to-Sample Task. Joint Annual Meeting ISMRM-ESMRMB, 19–25 May. Berlin, Germany.
Schulte, T., Mueller-Oehring, E. M., Rosenbloom, M. J., Pfefferbaum, A., & Sullivan, E. V. (2005). Differential effect of HIV infection and alcoholism on conflict processing, attentional allocation, and perceptual load: Evidence from a Stroop Match-to-Sample task. Biological Psychiatry, 57, 67–75.
Schulte, T., Muller-Oehring, E. M., Salo, R., Pfefferbaum, A., & Sullivan, E. V. (2006). Callosal involvement in a lateralized Stroop task in alcoholic and healthy subjects. Neuropsychology, 20, 727–736.
Schulte, T., Pfefferbaum, A., & Sullivan, E. V. (2004). Parallel interhemispheric processing in aging and alcoholism: Relation to corpus callosum size. Neuropsychologia, 42, 257–271.
Smith, S. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–155.
Stroop, J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 12, 643–662.
Sullivan, E. V., Deshmukh, A., De Rosa, E., Rosenbloom, M. J., & Pfefferbaum, A. (2005). Striatal and forebrain nuclei volumes: Contribution to motor function and working memory deficits in alcoholism. Biological Psychiatry, 57, 768–776.
Sullivan, E. V., Pfefferbaum, A., Adalsteinsson, E., Swan, G. E., & Carmelli, D. (2002). Differential rates of regional brain change in callosal and ventricular size: A 4-year longitudinal MRI study of elderly men. Cerebral Cortex, 12, 438–445.
Sullivan, E. V., Rosenbloom, M. J., & Pfefferbaum, A. (2000). Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism, Clinical and Experimental Research, 24, 611–621.
Tapert, S. F., Brown, G. G., Kindermann, S. S., Cheung, E. H., Frank, L. R., & Brown, S. A. (2001). fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism, Clinical and Experimental Research, 25, 236–245.
Tapert, S. F., Cheung, E. H., Brown, G. G., Frank, L. R., Paulus, M. P., Schweinsburg, A. D., et al. (2003). Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry, 60, 727–735.
Thurnher, M. M., Castillo, M., Stadler, A., Rieger, A., Schmid, B., & Sundgren, P. C. (2005). Diffusion-tensor MR imaging of the brain in human immunodeficiency virus-positive patients. American Journal of Neuroradiology, 26, 2275–2281.
Virta, A., Barnett, A., & Pierpaoli, C. (1999). Visualizing and characterizing white matter fiber structure and architecture in the human pyramidal tract using diffusion tensor MRI. Magnetic Resonance in Medicine, 17, 1121–1133.
Walker, H., & Lev, J. (1953). Statistical inference. New York: Holt.
Weed, M. R., Hienz, R. D., Brady, J. V., Adams, R. J., Mankowski, J. L., Clements, J. E., et al. (2003). Central nervous system correlates of behavioral deficits following simian immunodeficiency virus infection. Journal of Neurovirology, 9, 452–464.
Weekes, N. Y., & Zaidel, E. (1996). The effects of procedural variations on lateralized Stroop effects. Brain and Cognition, 31, 308–330.
Winsauer, P. J., Moerschbaecher, J. M., Brauner, I. N., Purcell, J. E., Lancaster, J. R., Jr., Bagby, G. J., et al. (2002). Alcohol unmasks simian immunodeficiency virus-induced cognitive impairments in rhesus monkeys. Alcoholism, Clinical and Experimental Research, 26, 1846–1857.
Woods, R. P., Grafton, S. T., Holmes, C. J., Cherry, S. R., & Mazziotta, J. C. (1998a). Automated image registration. I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography, 22, 139–152.
Woods, R. P., Grafton, S. T., Watson, J. D., Sicotte, N. L., & Mazziotta, J. C. (1998b). Automated image registration: II Intersubject validation of linear and nonlinear models. Journal of Computer Assisted Tomography, 22, 153–165.
Wu, Y., Storey, P., Cohen, B. A., Epstein, L. G., Edelman, R. R., & Ragin, A. B. (2006). Diffusion alterations in corpus callosum of patients with HIV. American Journal of Neuroradiology, 27, 656–660.
Xu, D., Mori, S., Solaiyappan, M., van Zijl, P. C., & Davatzikos, C. (2002). A framework for callosal fiber distribution analysis. NeuroImage, 17, 1131–1143.
Acknowledgements
This research was supported by grants from the National Institute on Alcohol and Alcoholism (AA12999, AA12388, AA05965). The authors thank Stephanie Sassoon, Ph.D., Andrea Spadoni, B.A., Marya Schulte, B.A., Carrie McCloskey, B.A., and Shannon Muir, B.A., for help with recruiting study participants and assistance in data collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schulte, T., Müller-Oehring, E.M., Javitz, H. et al. Callosal Compromise Differentially Affects Conflict Processing and Attentional Allocation in Alcoholism, HIV, and Their Comorbidity. Brain Imaging and Behavior 2, 27–38 (2008). https://doi.org/10.1007/s11682-007-9014-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-007-9014-z