Abstract
Shelford's law of tolerance is illustrated by a bell-shaped curve depicting the relationship between environmental factor/factors’ intensity and its favorability for species or populations. It is a fundamental basis of ecology when considering the regularities of environment impacts on living systems, and applies in plant biology, agriculture and forestry to manage resistance to environmental limiting factors and to enhance productivity. In recent years, the concept of hormesis has been increasingly used to study the dose–response relationships in living organisms of different complexities, including plants. This requires the need for an analysis of the relationships between the hormetic dose–response model and the classical understanding of plant reactions to environments in terms of Shelford's law of tolerance. This paper analyses various dimensions of the relationships between the hormetic model and Shelford’s tolerance law curve under the influence of natural environmental factors on plants, which are limiting for plants both in deficiency and excess. The analysis has shown that Shelford’s curve and hormetic model do not contradict but instead complement each other. The hormetic response of plants is localized in the stress zone of the Shelford’s curve when adaptive mechanisms are disabled within the ecological optimum. At the same time, in a species range, the ecological optimum is the most favorable combination of all or at least the most important environmental factors, each of which usually deviates slightly from its optimal value. Adaptive mechanisms cannot be completely disabled in the optimum, and hormesis covers optimum and stress zones. Hormesis can modify the plant tolerance range to environmental factors by preconditioning and makes limits of plant tolerance to environmental factors flexible to a certain extent. In turn, as a result of tolerance range evolution, quantitative characteristics of hormesis (width and magnitude of hormetic zone) as well as the range of stimulating doses, may significantly differ in various plant species and even populations and intra-population groups, including plants at different development stages. Using hormetic preconditioning for managing plant resistance to environmental limiting factors provides an important perspective for increasing the productivity of woody plants in forestry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most plant species cannot avoid adverse impacts in a variable environment (Doley 2017). Therefore, adaptation to environmental stressors holds an important role in the survival of both herbaceous (Wu et al. 2007) and woody (Lüttge and Buckeridge 2020) plants. Plant resilience to environment challenges, especially to deviations of abiotic factors (temperature, soil moisture, light, mineral nutrition etc.,) from the optimum, is crucial for successful growth and development as well as productivity (Sanghera et al. 2011; Wani et al. 2016; Waqas et al. 2019); this is all applicable to tree species in forestry (Niinemets 2010).
Shelford's law of tolerance (Shelford 1931) is illustrated by a bell-shaped curve depicting the relationship between environmental factor/factors’ intensity and its favorability for species or populations. It is a fundamental basis of ecology when considering the regularities of environmental impacts on living systems (Odum and Barrett 2004), and applies in plant biology (Hatfield and Prueger 2015), agriculture (Zinn et al. 2010; Badr et al. 2020) and forestry (Greenberg et al. 2015; Tan et al. 2017) to manage plant resistance to environmental limiting factors and to enhance plant productivity.
In recent years, the concept of hormesis has been increasingly used to study the dose–response relationships in living organisms of different complexities (Agathokleous and Calabrese 2020a). The current literature provides sufficient evidence of hormetic responses in plants both with various anthropogenic factors (e.g., ground-level ozone, nanomaterials, pesticides, antibiotics) (Agathokleous et al. 2017, 2020d; Agathokleous and Calabrese 2020a) and natural environmental factors, such as temperature (Agathokleous et al. 2018), soil moisture, and mineral nutrition (Agathokleous et al. 2019a). This suggests the need for an analysis of the relationships between the hormetic dose–response model and the classical understanding of plant reactions to the environment in terms of Shelford's law of tolerance. Some authors have addressed this issue to some extent for temperature- induced hormesis in plants (Agathokleous et al. 2018), but a detailed analysis of this aspect has not been conducted. To this end, this review analyses various dimensions of the relationships between the hormetic model and Shelford’s tolerance law curve under the influence of natural environmental factors on plants, which are limiting for plants both in deficiency and excess. Understanding these patterns provides a perspective for hormesis to increase the resistance of trees to environmental limiting factors in forestry.
Shelford’s tolerance law
In 1840, Justus Liebig suggested the law of the minimum, according to which the environmental limiting factor for the success of a species is one close to the necessary minimum. For example, grain yields were limited by essential elements which were lacking in the soil (Odum and Barrett 2004). The tolerance principle was a further elaboration of Liebig's idea. The law of tolerance or environmental maximum, first developed by Shelford (1913), states that ‘the success of a species, its number, sometimes its size, etc., are determined largely by the degree of deviation of a single factor (or factors) from the range of optimum of the species’. Hence, an environmental limiting factor for any species can be minimal or maximal, the range between which determines the species endurance (tolerance) to this factor. This principle was shown by Shelford in animal studies (1931) and developed by Ronald Good (1931) in plant biology.
Graphically, the tolerance law is illustrated by the Shelford’s curve (Fig. 1), which represents the dependence of the species response (factor favorability for the species) on environmental factor/factors’ intensity and is described by the Gauss function (Lynch and Gabriel 1987; Hatfield and Prueger 2015).
Shelford’s tolerance law curve (Helaouёt and Beaugrand 2009, with changes). (The tolerance range is the range between minimal and maximal values of the environmental factor within which the species is able to survive. In the reproduction, growth, and feeding ranges, respectively, reproduction, growth, and feeding can occur. The critical range is the range in which the death of individuals begins, i.e., the environmental factor varies from a minimal lethal value to a 100% lethal one in this range)
Population size and density (or species abundance) are most often used as indicators of the favorability of environmental factors (Costamagno et al. 2016; Faith and Lyman 2019), as well as growth indicators which are applied to plants (Hatfield and Prueger 2015). Thus, an environmental tolerance curve for a population or species gives its fitness as a function of the environment (Lande 2014).
Several zones are allocated for the tolerance curve (Shelford 1913; Faith and Lyman 2019) (Fig. 1):
-
1.
The zone of ecological optimum is the range of the most favorable values of the factor, where the most optimal growth, survival and reproduction are observed (Lynch and Gabriel 1987). The population size is maximum in this zone (Faith and Lyman 2019). In the optimum zone, adaptive mechanisms are disabled and energy is only consumed on fundamental life processes such as growth and reproduction, amongst others (Kuznetsov et al. 2016; Shilov 2019).
-
2.
Zones of physiological stress are ranges where a species can survive as a result of the activation of adaptation processes to stressful values of the factor (Faith and Lyman 2019). In addition to fundamental life processes, energy is spent on adaptation (Kuznetsov et al. 2016; Shilov 2019). Therefore, there is a decrease in basic biological functions (reproduction, growth) and in population size, which increases as the factor deviates from the optimum (Helaouёt and Beaugrand 2009; Costamagno et al. 2016).
-
3.
Zones of intolerance are ranges of environmental factor values that make it impossible for a species to survive (Faith and Lyman 2019).
The species tolerance range to an environmental factor (or ecological valence) is the range between minimal and maximal values of the environmental factor within which the species is able to survive (Shelford 1913; Faith and Lyman 2019). This range is defined by a set of tolerance ranges for all individuals of the species and is always wider than the individual tolerance (Lynch and Gabriel 1987; Faith and Lyman 2019). Environmental factors whose values are close to the limits of the tolerance range are environmental limiting factors for species. Limiting factors have a crucial role in the geographic distribution of plant species, including woody plants; they determine species ranges as well as their abundance and density, cover, growth rate and biomass. For instance, they can affect the maximum forest stand response (e.g., stand density and percentage tree cover) under a given site’s environmental conditions (Greenberg et al. 2015).
A number of principles were also formulated to complement the tolerance law (Odum 1971): (1) Tolerance ranges to different environmental factors have different widths for the same species; (2) Species with wide tolerance ranges to major environmental factors (referred to as eurytopic species) tend to have larger geographic distributions than those with narrow tolerances (referred as stenotopic species); (3) The suboptimal value of one environmental factor may narrow the tolerance ranges for other factors; (4) If even one factor goes beyond the tolerance range, then despite the optimal values of other factors, individuals still face death; (5) During development, the width of the tolerance range to environmental factors changes. This range is commonly narrower for the reproduction period.
Plant hormesis upon exposure to natural environmental factors
Hormesis is an adaptive response to stress factors, manifesting in a biphasic manner and is characterised by stimulation (trait/traits are higher than in controls) at low doses, and inhibition (trait/traits are worse than in controls) at high doses (Calabrese 2008; Agathokleous and Calabrese 2020a).
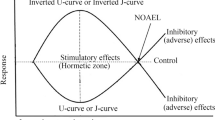
The hormetic dose-response relationship may have two forms (Calabrese and Blain 2009): (1) the most frequently observed inverted U-shaped curve representing low-dose stimulatory and high-dose inhibitory responses (Fig. 2); (2) the U-shaped curve representing a decrease in damage at low doses and an increase in damage at high doses.
Hormetic dose–response relationship (Agathokleous and Calabrese 2020a, with changes)
The hormetic curve was demonstrated to have common quantitative features in different groups of organisms including plants (Calabrese and Blain 2009; Calabrese 2008; Agathokleous and Calabrese 2020a) (Fig. 2). The hormetic zone of the curve is allocated as a range of doses having a stimulating effect relative to the control. This zone is suggested to be characterised by two quantifiable indicators (Calabrese 2008): (1) the width of the stimulating dosage range which is usually less than 100-fold. But for about 2% of the dose responses, this width exceeds 1000 times; (2) the maximum value of the stimulating effect (maximum response), expressed as a percentage of the control, which typically is 130–160% of the control value. But the maximum response can (rarely) reach 200% of the control (Calabrese and Blain 2009; Agathokleous and Calabrese 2020a).
It is considered that the hormetic stimulating effect should be taken into account starting from 110% of the control value (Calabrese and Blain 2009). In addition, the maximal dose which does not have a damaging effect is allocated (no-observed adverse effect level—NOAEL). The hormetic zone is generally below the NOAEL (Fig. 2).
A literature analysis revealed that natural environmental factors subjected to Shelford’s tolerance law can also induce plant hormesis. As early as the nineteenth century and at the beginning of the twentieth century, numerous data were obtained concerning the hormetic effects of some plant macronutrients (Ca, Mg, K, N) and micronutrients (Cu, Zn, Fe, Mn) on growth and a number of other indicators (Calabrese and Baldwin 1999). Findings from subsequent studies (Calabrese and Blain 2009; Erofeeva 2014; Sanchez-Zabala et al. 2015) (Table 1) confirmed the ability of plant macro- and micronutrients to cause hormesis in various plants, including different environmental plant groups such as heavy metal hyperaccumulators (Küpper et al. 1999; Tang et al. 2009) (Table 1). Essential elements increased growth indexes, root/shoot ratios, yield as well as chlorophyll content, while reducing lipid peroxidation rates relative to controls (Table 1). These effects were observed with a mild increase in the content of macro- and microelements in the soil or nutrient solution compared to the control level (Table 1). Analysis of literature data did not reveal plant hormesis under a mild decrease in mineral nutrition. An increase in root/shoot ratios and chlorophyll content are considered as important indicators of hormetic stimulation in plants (Agathokleous et al. 2019a, 2020b).
In fact, the law of tolerance applies to environmental factors that are limiting in both deficit and excess (Shelford 1913), many of which are considered as abiotic factors of plants (air and soil temperature, soil moisture, light, mineral nutrition). Therefore, only these factors are analysed in this review. However, not all environmental factors have this feature. For example, many pollutants (herbicides, nanomaterials, human and veterinary pharmaceuticals, amongst others) are not necessary for the vital activity of an organism, including plants, (Agathokleous and Calabrese 2020a), with the exception of the pollutants required by plants in small doses as essential nutrients (for example, Cu, Zn, Mn, Mo and others) (Tripathi et al. 2015).
Hormetic stimulatory effects on various plant traits (growth, photosynthesis, peroxide homeostasis, yield, etc.,) were also found for elevated soil and air temperatures, carbon dioxide excess in the air, deficit and excess soil moisture and light intensity, as well as for changes in the spectral composition of light (Table 1). The hormetic effects of abiotic factors were also shown for woody plants. For example, a hormetic-like response was found in Betula alnoides Buch. Ham. ex D. Don and Pinus sylvestris L. under elevated soil nitrogen, in Camptotheca acuminate Decne. in response to a deficiency of light intensity, in Eucalyptus tereticornis Sm. with exposure to elevated CO2 in the air and in various species of woody plants in response to higher air temperature (Table 1).
Only soil moisture and light intensity caused hormesis under deviations from the control (control corresponded to the optimum in Table 1, i.e., normal environmental conditions for this species) in both directions (in deficiency and excess) (Table 1). Apparently this is due to the significant interest of most researchers in studying the certain type of factor deviation from the optimum in order to increase plant productivity (excessive mineral nutrition, elevated air and soil temperatures, etc.). At the same time, from the concept of hormesis, it follows that a moderate deviation (any mild stress) in any direction from the control value (i.e., normal environmental conditions for this species) can cause hormetic stimulation in plants (Agathokleous and Calabrese 2020a).
It should be emphasized that the optimal values of plant traits observed in the optimal zone are not the highest. Hormetic stimulation causes an increase in plant traits (for example, the rate of growth and photosynthesis, the content of photosynthetic pigments, etc.) above the optimum (Table 1). This is due to adaptive processes and as a result, energy consumption for this stimulation. This also applies to plant stimulation by abiotic factors to increase productivity. Long-term adaptation costs are energetically unprofitable for a species. Therefore, the value of the environmental factor/factors in the optimum zone seems to be the best environment for the species because in this case, energy is only used for fundamental life processes, such as growth and reproduction, etc., and there are no adaptation costs.
In the majority of cases, the hormetic curves found for abiotic factors had an inverted U-shape. These were observed only for plant traits, in which the decrease relative to the control is considered as a positive influence of environmental factors (fluctuating leaf asymmetry, the rate of lipid peroxidation) (Table 1).
Hormesis location on Shelford’s curve
As shown above, there is ample evidence of the ability of environmental factors subjected to Shelford's law to cause hormesis both in herbaceous and woody plants (Table 1). Hence, a question arises concerning the relationship between the hormetic model and Shelford’s curve. Historically, the phenomenon of animal and plant hormesis was studied in the most detail in fields of toxicology and ecotoxicology (Calabrese and Baldwin 1999; Calabrese 2008), where researchers commonly dealt with toxicant excess and did not consider the whole tolerance range of living organisms to toxic agents. Although some elements are necessary for living organisms in small doses, such as Cu, Zn, Mn, Mo, their deficiency causes stress (Tripathi et al. 2015). On the other hand, studies of environmental limiting factors in ecology did not consider the phenomenon of environmental hormesis (Helaouёt and Beaugrand 2009; Greenberg et al. 2015; Hatfield and Prueger 2015; Tan et al. 2017). As a result, paradoxically, accurate experimental data showing the location of hormesis on Shelford’s curve does not currently exist. Nevertheless, this review analyses this important issue using indirect evidence.
Hormesis is well- established to be closely related to the phenomenon of stress in living things (Calabrese 2008; Agathokleous and Calabrese 2020a). According to the author of the stress concept, Selye (1974), stress is a sum of non-specific biological responses to stimuli or events that are perceived as threatening and tend to disrupt homeostasis. Thus, stress is a set of changes in the body, both adaptive and sometimes maladaptive, that occur when exposed to any environmental factor requiring adaptation and is termed a stressor. Selye (1975) suggested a method to distinguish two types of stress: eustress (a positive stress), which is induced by moderate stressors and characterised by adaptive processes enhancing a resistance to the stressor, and distress (a negative stress), induced by severe stressors and having maladaptive changes along with adaptive ones.
Recent studies in the field of hormesis also consider the hormetic stimulating effect in connection with the concept of eustress (Agathokleous et al. 2019a, 2020c). The stimulating effect of hormesis is currently considered as a non-specific adaptive response to low doses of the stressor. The low-dose stress occurs in the low-dose zone (i.e., in the hormetic zone) of the hormetic dose–response model and accordingly, high-dose stress is in the high-dose area of the hormetic curve. Low-dose stressors are suggested to cause a mild increase in the activity of non-specific protective mechanisms, including those in plants, such as reactive chemical species, stressful hormones and antioxidant defense, as well as synthesis of stress proteins (e.g., heat shock proteins) (Agathokleous et al. 2020c). Hence, the hormetic stimulating effect (i.e., hormetic zone) can correspond to eustress in Selye's terminology, and the inhibiting effect of hormesis can correspond to distress (Jocelyn 2003).
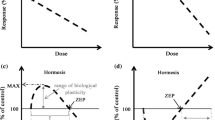
As mentioned above, adaptive mechanisms are assumed to be disabled within the ecological optimum zone of Shelford’s curve (Kuznetsov et al. 2016; Shilov 2019). Given the activation of such processes under hormesis and its relationship with stress, it is highly likely that the hormetic response is localised in the stress zone of Shelford’s curve (Fig. 3a), at least for the conditions of laboratory and open-field experiments that are controlled to a significant extent.
Hormesis location on Shelford’s curve under a disabled and b non-disabled adaptive mechanisms within the ecological optimum. The hormetic response of plants is localised in the stress zone of the Shelford’s curve when adaptive mechanisms are disabled within the ecological optimum (a). At the same time, in a species range, the ecological optimum is the most favorable combination of all or at least the most important environmental factors, each of which usually deviates slightly from its optimal value. Adaptive mechanisms cannot be completely disabled in the optimum, and hormesis covers optimum and stress zones (b)
For natural conditions, the hormesis position within Shelford’s curve is not as clear as in experiments due to the complexity of determining the optimum in a changeable environment. In natural ecosystems, plants are simultaneously exposed to multiple environmental factors and they can never all be in the optimal zone (Chapin et al. 1987; Greenberg et al. 2015; Shilov 2019). In addition, the influence of interacting factors can be observed. For example, plant damage from high light levels increases dramatically due to low soil humidity and high air temperature (Chapin et al. 1987).
Therefore, in a species range, the ecological optimum is suggested to be the most favorable combinations of all or at least the most important environmental factors, each of which usually deviates slightly from its optimal value (Shilov 2019). Another issue in identifying the optimum in natural conditions is the fact that environmental factors are not stable for a long time and have periodic and non-periodic changes. Periodic changes caused by the rotation of the Earth around its axis and its movement around the Sun, include seasonal and diurnal fluctuations perceived by the plant's circadian system (Panter et al. 2019). For example, these include circadian and seasonal fluctuations in light, temperature and humidity. Therefore, some authors distinguish a dynamic (astatic) optimum along with the classic ecological optimum, with constant values of factors. The dynamic optimum is a set of certain dynamic environmental characteristics which are optimum conditions for the life of an organism in natural habitats (Verbitsky and Verbitskaya 2007; Kuznetsov et al. 2016). It has been shown that periodic fluctuations of abiotic factors, which are similar to natural ones, improve the state of organisms, including plants, in relative to constant optimal conditions. For instance, fluctuations in air temperature within the natural range of night and day temperatures accelerated the growth of cucumber compared to that under a constant temperature (Kuznetsov et al 2016). Similarly, natural changes of light stimulated the process of photosynthesis in algae (Walsh and Legendre 1983; Flameling and Kromkamp 1997). In turn, cyclical changes in the environment never strictly have the same pattern. Their cyclical pattern is always accompanied by non-periodic stochastic fluctuations (He et al. 2018). For example, circadian changes of temperature and humidity strongly depend on the weather in the same season. Even greater variability is observed for the seasonal course of environmental factors under climate change (Walker et al. 2019).
Plants, like other organisms, have a more or less permanent hereditary resistance to periodic fluctuations of conditions within their species range because they are predictable. For example, deciduous woody plants in temperate conditions successfully survive low negative temperatures during cold seasons, which is impossible for evergreen tropical tree species which die at non-freezing low temperatures because of their lack of the mechanisms to allow cold acclimation (Sanghera et al. 2011). Some trees in temperate conditions tolerate extremely low temperatures of up to − 80 °C (Betula nigra L., Acer saccharum Marsh., Tilia americana L., Salix nigra Marsh.) and even − 120 °C (Larix sibirica Ledeb.) (Strimbeck et al. 2015). Apparently that is why these changes in environment in the optimal zone do not activate inducible adaptive processes, i.e., hormesis. Moreover, it is possible that periodic fluctuations may be necessary for the optimal level of plant life processes (i.e., the state of the plant organism corresponding to the optimal factor/factors) because plants have adapted to them during evolution.
At the same time, non-periodic changes in the environment cannot be accurately predicted, so plants can adapt to them only through induced hormetic adaptive mechanisms (i.e., using biological plasticity or phenotypic plasticity) which, unlike constant adaptations, are activated by environmental stress factors. Phenotypic plasticity is suggested as one of the major means by which plants can cope with environmental factor variability (Gratani 2014; Agathokleous et al. 2019b).
Therefore it seems that in real environmental conditions, the ecological optimum with ideal values of all factors is never realised or is rarely observed. This means that hormesis is always within the ecological optimum, at least its hormetic zone, if we consider the favorability of the leading environmental factors for the species within its range (Fig. 3b).
The permanent presence of constant and random fluctuations in the environment requires fine-tuning of the regulation of plant hormesis, since long-time excessive adaptation costs are not energetically beneficial to the plant. It is possible that the quantitative characteristics of hormesis (width and magnitude of hormetic zone), as well as the asynchronous manifestation of the stimulating hormetic effect in plants (when it is observed for some traits and absent for others) (Erofeeva 2014; Agathokleous et al. 2019c), are largely determined by this fact.
To clarify the location of hormesis on Shelford’s curve in plants of natural ecosystems, further detailed targeted research is required which takes into account all aspects of the variability of the natural environment.
Hormesis effect on the tolerance range in plants
In terms of the hormesis concept, low doses of an environmental factor having a stimulating hormetic effect can increase the resilience of living organisms to subsequent, more severe stressors. This phenomenon is known as preconditioning or priming (Calabrese 2008; Martinez-Medina et al. 2016; Agathokleous et al. 2020c). Preconditioning is observed in plants under the influence of various abiotic factors (drought, frost, heat and others) subjected to Shelford's law (Walter et al. 2013; Martinez-Medina et al. 2016). Low-dose impacts increase plant resilience to subsequent, more severe exposures to the same abiotic factor or to other environmental stressors. In the latter case, there is cross-tolerance or cross-adaptation, since resistance to one stressor induces tolerance to other stressors (Foyer et al. 2016).
It follows that due to preconditioning, hormesis can affect the width of the tolerance range in plants to a specific environmental factor or even to other factors when they affect after the low dose exposure. Moreover, it has been suggested that the preconditioning effect can be preserved in a number of plant generations via epigenetic processes (Agathokleous and Calabrese 2020a). For example, in Arabidopsis thaliana (L.) Heynh., mild heat and moderate excess of some micronutrients (e.g., Cu and Ni) increased the next generation's resistance to high doses of these factors (accordingly, to high temperature and high salt concentrations of these metals) and even enhanced resilience to another stressor (NaCI) (Whittle et al. 2009; Rahavi et al. 2011). In addition, low-dose stress at the embryonic stage may increase stress tolerance throughout adult life, as shown in animal studies (Costantini et al. 2014; López-Martínez and Hahn 2014; Agathokleous and Calabrese 2020a).
These facts show that preconditioning can cause a shift of the stress zone to the area of higher and/or lower doses relative to the optimum, and hence increases the plant tolerance range to this factor (Fig. 4). Thus, hormetic preconditioning makes the limits of plant tolerance to environmental factors flexible to a certain extent, which enhances the resistance of plants to subsequent severe stressors not only in this generation, but also in a number of subsequent generations (Agathokleous and Calabrese 2020a).
Environmental limiting factors whose values are close to the limits of a species tolerance have a crucial significance for plant productivity, including that of woody species (Greenberg et al. 2015). In this regard, the management of plant resistance to limiting factors using hormetic preconditioning provides an important perspective for increasing the productivity of woody plants in forestry.
Dependence of hormesis quantitative characteristics on plant tolerance range
The tolerance ranges (i.e., ecological valencies) to various environmental factors form during the evolutionary processes of plant adaptation to environments of a species range, that is, as a result of natural selection (Mickelbart et al. 2015), including tree species (Körner et al. 2016). Therefore, the question arises whether the quantitative characteristics of hormesis (width and amplitude of the hormetic zone), as well as the range of stimulating doses, differ in plant species having various tolerance ranges to the same environmental factor, i.e., whether these quantitative features of hormesis are species-specific.
Hormesis is suggested to be a manifestation of biological plasticity (or phenotypic plasticity) which is ‘the ability of a biological organism to modify its functioning at any level (biological, physiological, morphological) via adaptive responses activated in response to environmental stimuli (Agathokleous et al. 2019b). In turn, biological plasticity is part of phenotypic plasticity, i.e., the ability of a genotype to produce different phenotypes (Pigliucci et al. 2006), both adaptive and maladaptive, under different environmental conditions. It is known that the ability of species to use phenotypic plasticity changes during the evolutionary process (Fusco and Minelli 2010).
This indicates that the capacity of plant species to respond by hormetic hyperactivation of defense systems to moderate environmental stress can also change during evolution. Consequently, the quantitative characteristics of hormesis, including hormetic dosage range and the qualitative features of the molecular hormetic mechanisms, may differ in various plant species as well as in populations and subpopulation groups. In a recent review, Agathokleous et al. (2019b), based on data concerned with organic toxicant effects on plants (Belz et al. 2018; Belz and Sinkkonen 2019), suggested that the hormetic response of high-risk subpopulations occurs at dosage levels lower than in normal-risk subpopulations and at higher doses in low-risk subpopulation. They also believe there is no single biological mechanism of hormesis in plants.
Another confirmation of plant hormetic response evolution is that hormesis depends on the state of the genome and epigenome and is defined, in particular, by non-lethal mutations, recombination of genes, i.e., the emergence of new genotypes, as well as epigenetic regulation including epigenetic memory (Agathokelous and Calabrese 2020a). This means that the parameters of hormesis and apparently the probability of its occurrence can change over a number of generations during the evolution of plants and differ in various species.
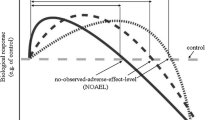
Based on the above, it can be assumed that the width of the hormetic zone, range of stimulating doses and possibly the amplitude of the stimulating effect, will be enhanced with an increase in the tolerance range to the environmental factor in the course of plant evolution (Fig. 5).
Quantitative characteristics of hormesis in plant species with different tolerances to the environmental factor. An increase in the plant tolerance range during evolution can cause a shift of hormesis to the region of higher/lower doses relative to the optimum, as well as an increase in the width and amplitude of the hormetic zone
There is some evidence to support this assumption. For example, in Arabis paniculata Franch., which is a hyperaccumulator of zinc, this microelement excess in the range of 1223–2447 µM caused hormetic stimulation of growth because of a species-specific ability to accumulate the metal without harm to the body (Tang et al. 2009). At the same time, in plants with lower zinc resistance, these concentrations induced severe stress and significantly reduced growth (Pisum sativum L., ryegrass, Populus deltoids W. Bartram ex Marshall, Datura spp. and others) (Tsonev and Lidon 2012). Another zinc hyperaccumulator, Thlaspi caerulescens (Lej.) Lej. and Court. had hormetic stimulation of growth at Zn soil content of 400–2000 µg/g (Küpper et al. 1999), while this concentration range caused a stress-induced growth decrease in more sensitive species (Artemisia annua L., Betula pendula Roth., Betula pubescens Ehrh. and others) (Tsonev and Lidon 2012).
In addition, our calculations have shown that an increase in the plant species tolerance range during evolution occurs due to an increase in two stress zones, or one of the stress zones and the optimum at the same time (Fig. 6). Since hormesis can fully or partially locate in stress zones, an increase in stress zones during plant evolution can enhance plant ability to hormesis (Fig. 5). It can be assumed that the greatest ability for hormesis should be in eurytopic plant species that have wide ranges of tolerance to many environmental factors.

source data are presented as Supplementary material (Table S1, Table S2). The normality of the distribution was checked using the Shapiro–Wilk test (Statistica 10). Using simple regression (Statistica 10) and Spearman correlation (Primer of Biostatistics 4.03), a statistically significant relationship between the width of the tolerance range and the zones of Shelford’s curve was found only for the cases shown in the figure)
Dependence of the tolerance range for the germination soil temperature of seeds in a grain crops and b vegetables on the Shelford’s curve zone width. (*—p-level for the determination coefficient (R2), **—p-level for the correlation coefficient (r). We used data on the temperature tolerance of the germination of seeds of various plant species (Albert 2016). The list of plant species and the
Hormesis and tolerance range during plant development
The analysis of literature data has shown that a hormetic response is observed at different stages of plant development from seedlings to reproductive plants in herbaceous and woody species (Table 1). This raises the question of whether plant capacity for hormetic reactions can change at different stages of development.
As mentioned above, the width of the tolerance range changes throughout development in living organisms (Odum 1971). In higher plants, the lowest tolerance to environmental stress is observed at the juvenile and reproductive stages. For instance, high temperature stress tolerance of many crops (e.g., wheat, maize, rice, and soybean) is greatest during early vegetative stages and decreases progressively during flowering and early seedling stages (Djanaguiraman and Prasad 2014). Similar data was also obtained for low temperature stress (Zinn et al. 2010) and drought (Badr et al. 2020). In woody plants, an increase in stress resistance (and hence the width of the tolerance range) during development was found from a seedling to a mature tree (Kreuzwieser and Rennenberg 2014; Niinemets 2010). This is suggested to be due to the greater pool of non-structural carbohydrates in adult trees covering plant metabolic requirements under stressful environments, which allows them to quickly restore the loss of biomass, as well as to initiate a greater activity of protective systems, in particular antioxidant defense (Niinemets 2010). In addition, different phenophases of woody plants can also differ in their stress tolerance (Stephenson et al. 2003), i.e., they can have different tolerance ranges to environmental factors.
Hence plants’ ability to respond hormetically to mild stressors can also change during development which may be manifested in the following ways. First, the position of hormetic zone on Shelford’s curve may change because of a shift in the optimum and stress zones throughout development. For instance, for most plant species, vegetative stages usually have a higher optimum temperature than for reproductive stages (Hatfield and Prueger 2015). Secondly, it is possible to change the quantitative characteristics of the hormetic curve. Under the narrowing of the tolerance range in more stress-sensitive stages, the width of the stimulating zone may also decrease. In addition, it is possible to observe an amplitude change of the stimulating effect. It can be assumed that this indicator will be higher at the most mature stress-resistant stages due to the most highly formed protective mechanisms. In the future, these issues require detailed experimental analysis in different plant species under the action of various environmental factors.
Conclusion
This review has demonstrated that Shelford’s curve and the hormetic model do not contradict but complement each other. The hormetic response of plants is localised in the stress zone of the Shelford’s curve when adaptive mechanisms are disabled within the ecological optimum. At the same time, in a species range, the ecological optimum is the most favorable combination of all or at least the most important environmental factors, each of which usually deviates slightly from its optimal value. In this case, adaptive mechanisms cannot be completely disabled in the optimum, and hormesis covers the optimum and the stress zone. Quantitative characteristics (width of optimum and stress zones, etc.,) of the tolerance curve are formed during the evolutionary process of plant adaptation to certain environmental conditions in a species range. These characteristics are not absolutely rigid because of the need to adapt also to random changes in the environment that cannot be accurately predicted. Therefore, plants have a changeable component of adaptation, named phenotype plasticity, of which hormesis is a part. This indicates the ability of hormesis to modify the plant tolerance range to environmental factors, in particular due to preconditioning. In turn, hormesis is also affected by the evolutionary process since the protective systems that provide the hormetic stimulating effect are controlled genetically and epigenetically. Hence, the quantitative characteristics of hormesis, as well as the qualitative aspects (for example, the features of the molecular mechanisms of hormesis) may differ in different species and even in populations and intra-population groups, as well as at different stages of plant development. This may affect the ability of hormesis to modify a plant’s tolerance range. In this review, I have highlighted the most important aspects of the relationships between the hormetic model and Shelford’s tolerance law curve. A detailed experimental analysis has yet to be carried out. Undoubtedly, this will be of particular importance to the development of methods for managing plant tolerance to environmental limiting factors, including in forestry, as well as for understanding the fundamental regularities of adaptation processes.
References
Agathokleous E, Belz RG, Calatayud V, De Marco A, Hoshika Y, Kitao M, Saitanis CJ, Sicard P, Paoletti E, Calabrese EJ (2019a) Predicting the effect of ozone on vegetation via linear non-threshold (LNT), threshold and hormetic dose-response models. Sci Total Envir 649:61–74. https://doi.org/10.1016/j.scitotenv.2018.08.264
Agathokleous E, Belz RG, Kitao M, Koike T, Calabrese EJ (2019b) Does the root to shoot ratio show a hormetic response to stress? An ecological and environmental perspective. J For Res 30:1569–1580. https://doi.org/10.1007/s11676-018-0863-7
Agathokleous E, Feng Z, Iavicoli I, Calabrese EJ (2020d) Nano-pesticides: a great challenge for biodiversity? The need for a broader perspective. Nano Today 30:100808. https://doi.org/10.1016/j.nantod.2019.100808
Agathokleous E, Feng ZZ, Peñuelas J (2020b) Chlorophyll hormesis: are chlorophylls major components of stress biology in higher plants? Sci Total Environ 726:138637. https://doi.org/10.1016/j.scitotenv.2020.138637
Agathokleous E, Kitao M, Calabrese EJ (2019c) Hormesis: a compelling platform for sophisticated plant science. Trends Plant Sci 24(4):318–327. https://doi.org/10.1016/j.tplants.2019.01.004
Agathokleous E, Kitao M, Calabrese EJ (2020c) Hormesis: highly generalizable and beyond laboratory. Trends Plant Sci 25(11):1076–1086. https://doi.org/10.1016/j.tplants.2020.05.006
Agathokleous E, Kitao M, Harayama H, Calabrese EJ (2018) Temperature-induced hormesis in plants. J For Res 30:13–20. https://doi.org/10.1007/s11676-018-0790-7
Agathokleous E, Saitanis CJ, Burkey KO, Ntatsi G, Vougeleka V, Mashaheet AM, Pallides A (2017) Application and further characterization of the snap bean S156/R123 ozone biomonitoring system in relation to ambient air temperature. Sci Total Environ 580:1046–1055. https://doi.org/10.1016/j.scitotenv.2016.12.059
Agathokleous E, Calabrese EJ (2020a) A global environmental health perspective and optimisation of stress. Sci Total Environ 704:135263. https://doi.org/10.1016/j.scitotenv.2019.135263
Albert S (2016) Vegetable seed germination temperatures. https://harvesttotable.com/vegetable-seed-germination-temperatures/. Accessed 28 Oct 2020
Badr A, El-Shazly HH, Tarawneh RA, Börner A (2020) Screening for drought tolerance in maize (Zea mays L.) germplasm using germination and seedling traits under simulated drought conditions. Plants 9(5):565. https://doi.org/10.3390/plants9050565
Belz RG, Patama M, Sinkkonen A (2018) Low doses of six toxicants change plant size distribution in dense populations of Lactuca sativa. Sci Total Environ 631–632:510–523. https://doi.org/10.1016/j.scitotenv.2018.02.336
Belz RG, Sinkkonen A (2019) Low toxin doses change plant size distribution in dense populations—glyphosate exposed Hordeum vulgare as a greenhouse case study. Environ Int 132:105072. https://doi.org/10.1016/j.envint.2019.105072
Calabrese EJ (2008) Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem 27(7):1451–1474. https://doi.org/10.1897/07-541
Calabrese EJ, Baldwin LA (1999) Chemical hormesis: its historical foundations as a biological hypothesis. Toxicol Pathol 27(2):195–216
Calabrese EJ, Blain RB (2009) Hormesis and plant biology. Environ Pollut 157(1):42–48. https://doi.org/10.1016/j.envpol.2008.07.028
Campbell CA, Davidson HR, Warder FG (1977) Effects of fertilizer N and soil moisture on yield, yield components, protein content and N accumulation in the aboveground parts of spring wheat. Can J Soil Sci 57(3):311–327
Chapin FS, Bloom AJ, Field CB, Waring RH (1987) Plant responses to multiple environmental factors. Bioscience 37(1):49–57. https://doi.org/10.2307/1310177
Chen L, Wang C, Dell B, Zhao Z, Guo J, Xu D, Zeng J (2018) Growth and nutrient dynamics of Betula alnoides seedlings under exponential fertilization. J For Res 29(1):111–119. https://doi.org/10.1007/s11676-017-0427-2
Costamagno S, Barshay-Szmidt C, Kuntz D, Laroulandie V, Pétillon J, Boudadi-Maligne M, Langlais M, Mallye J, Chevallier A (2016) Reexamining the timing of reindeer disappearance in southwestern France in the larger context of late glacial faunal turnover. Quatern Int 414:34–61. https://doi.org/10.1016/j.quaint.2015.11.103
Costantini D, Monaghan P, Metcalfe NB (2014) Prior hormetic priming is costly under environmental mismatch. Biol Lett 10(2):20131010. https://doi.org/10.1098/rsbl.2013.1010
d’Aquino L, de Pinto MC, Nardi L, Morgana M, Tommasi F (2009) Effect of some light rare earth elements on seed germination, seedling growth and antioxidant metabolism in Triticum durum. Chemosphere 75(7):900–905. https://doi.org/10.1016/j.chemosphere.2009.01.026
Davidson RL (1969a) Effect of root/leaf temperature differentials on root/shoot ratios in some pasture grasses and clover. Ann Bot 33:561–569. https://doi.org/10.1093/oxfordjournals.aob.a084308
Davidson RL (1969b) Effects of soil nutrients and moisture on root/shoot ratios in Lolium perenne L. and Trifolium repens L. Ann Bot 33:571–577
Djanaguiraman M, Vara Prasad PV (2014) High temperature stress. In: Jackson M, Ford-Lloyd B, Parry M (eds) Plant genetic resources and climate change. CAB International, Wallingford, pp 201–220
Doley D (2017) Plants as pollution monitors. In: Thomas B, Murray BG, Murphy DJ, Waltham MA (eds) Encyclopedia of applied plant sciences. Academic Press, United States, pp 341–346
Erofeeva EA (2014) Hormesis and paradoxical effects of wheat seedling (Triticum aestivum L.) parameters upon exposure to different pollutants in a wide range of doses. Dose Response 12(1):121–135. https://doi.org/10.2203/dose-response.13-017.Erofeeva
Faith JT, Lyman RL (2019) Paleozoology and Paleoenvironments: fundamentals, assumptions, techniques. Cambridge University Press, Cambridge. https://doi.org/10.1017/9781108648608
Flameling IA, Kromkamp J (1997) Photoacclimation of Scenedesmus protuberans (Chlorophyceae) to fluctuating irradiances simulating vertical mixing. J Plankton Res 19(8):1011–1024
Foyer CH, Rasool B, Davey JW, Hancock RD (2016) Cross-tolerance to biotic and abiotic stresses in plants: a focus on resistance to aphid infestation. J Exp Bot Adv 67(7):2025–2037. https://doi.org/10.1093/jxb/erw079
Fusco G, Minelli A (2010) Phenotypic plasticity in development and evolution: facts and concepts. Philos Trans R Soc B 365(1540):547–56. https://doi.org/10.1098/rstb.2009.0267
Good R (1931) A theory of plant geography. New Phytol 30:139–171
Gratani L (2014) Plant phenotypic plasticity in response to environmental factors. Adv Bot 4:1–17. https://doi.org/10.1155/2014/208747
Greenberg JA, Santos MJ, Dobrowski SZ, Vanderbilt VC, Ustin SL (2015) Quantifying environmental limiting factors on tree cover using geospatial data. PLOS ONE 10(2):e0114648. https://doi.org/10.1371/journal.pone.0114648
Hatfield JL, Prueger JH (2015) Temperature extremes: effect on plant growth and development. Weather Clim Extrem 10:4–10. https://doi.org/10.1016/j.wace.2015.08.001
He Q, Silliman BR, van de Koppel J, Cui B (2018) Weather fluctuations affect the impact of consumers on vegetation recovery following a catastrophic die–off. Ecology 100(1):e02559. https://doi.org/10.1002/ecy.2559
Heck WW, Dunning JA (1976) Effects of sulfur dioxide and/or ozone on two oat varieties. Corvallis Environmental Research Laboratory, Corvallis, p 60
Helaouёt P, Beaugrand G (2009) Physiology, ecological niches and species distribution. Ecosystem 12(8):1235–1245. https://doi.org/10.1007/s10021-009-9261-5
Högberg P, Fan H, Quist M, Binkley D, Tamm CO (2006) Tree growth and soil acidification in response to 30 years of experimental nitrogen loading on boreal forest. Glob Chang Biol 12(3):489–499. https://doi.org/10.1111/j.1365-2486.2006.01102.x
Holub P, Klem K, Linder S, Urban O (2019) Distinct seasonal dynamics of responses to elevated CO2 in two understory grass species differing in shade-tolerance. Ecology and Evolution 9(24):13663–13677. https://doi.org/10.1002/ece3.5738
Jocelyn K (2003) Sipping from a poisoned chalice. Science 302(5644):376–379. https://doi.org/10.1126/science.302.5644.376
Johkan M, Shoji K, Goto F, Hashida S, Yoshihara T (2010) Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 45(12):1809–1814
Kleiber T, Borowiak K, Schroeter-Zakrzewska A, Budka A, Osiecki S (2017) Effect of ozone treatment and light colour on photosynthesis and yield of lettuce. Sci Hort 217:130–136
Körner C, Basler D, Hoch G, Kollas C, Lenz A, Randin CF, Vitasse Y, Zimmermann NE (2016) Where, why and how? Explaining the low-temperature range limits of temperate tree species. J Ecol 104(4):1076–1088. https://doi.org/10.1111/1365-2745.12574
Kreuzwieser J, Rennenberg H (2014) Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ 37(10):2245–2259. https://doi.org/10.1111/pce.12310
Küpper H, Zhao FJ, McGrath SP (1999) Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiol 119:305–311
Kuznetsov VA, Zdanonich VV, Lobachev EA, Lukiyanov SV (2016) Revisiting the problem of astatic ecological optima. Biol Bull Rev 6(2):164–176. https://doi.org/10.1134/S2079086416020043
Lande R (2014) Evolution of phenotypic plasticity and environmental tolerance of a labile quantitative character in a fluctuating environment. J Evol Biol 5:866–875. https://doi.org/10.1111/jeb.12360
López-Martínez G, Hahn DA (2014) Early life hormetic treatments decrease irradiation-induced oxidative damage, increase longevity, and enhance sexual performance during old age in the Caribbean fruit fly. PLOS ONE 9(1):e88128. https://doi.org/10.1371/journal.pone.0088128e88128
Lüttge U, Buckeridge M (2020) Trees: structure and function and the challenges of urbanization. Trees. https://doi.org/10.1007/s00468-020-01964-1
Lynch M, Gabriel W (1987) Environmental tolerance. Am Nat 129(2):283–303. https://doi.org/10.1086/284635
Ma X, Song L, Yu W, Hu Y, Liu Y, Wu J, Ying Y (2015) Growth, physiological, and biochemical responses of Camptotheca acuminata seedlings to different light environments. Front Plant Sci 6:321. https://doi.org/10.3389/fpls.2015.00321
Martinez-Medina A, Flors V, Heil M, Mauch-Mani B, Pieterse CMJ, Pozo MJ, Ton J, van Dam NM, Conrath U (2016) Recognizing plant defense priming. Trends Plant Sci 21(10):818–822. https://doi.org/10.1016/j.tplants.2016.07.009
Maximov NA (1958) Kratkiy kurs fiziologii rasteniy. In: Short course in plant physiology. W.B. Selhozgiz, Moscow , p 560
Mickelbart MV, Hasegawa PM, Bailey-Serres J (2015) Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat Rev Genet 16(4):237–251. https://doi.org/10.1038/nrg3901
Motai A, Terada Y, Kobayashi A, Saito D, Shimada H, Yamaguchi M, Izuta T (2017) Combined effects of irrigation amount and nitrogen load on growth and needle biochemical traits of Cryptomeria japonica seedlings. Trees 31:1317–1333
Niinemets Ü (2010) Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For Ecol Manage 260(10):1623–1639. https://doi.org/10.1016/j.foreco.2010.07.054
Odum EP (1971) Fundamentals of ecology. W.B. Saunders Company, Philadelphia
Odum EP, Barrett GW (2004) Fundamentals of ecology. Brooks Cole, Belmont, p 624
Pan J, Guo B (2016) Effects of light intensity on the growth, photosynthetic characteristics, and flavonoid content of Epimedium pseudowushanense B.L.Guo. Molecules 21(11):1475. https://doi.org/10.3390/molecules21111475
Panter PE, Muranaka T, Cuitun-Coronado D, Graham CA, Yochikawa A, Kudoh H, Dodd AN (2019) Circadian regulation of the plant transcriptome under natural conditions. Front Genet 10:1239. https://doi.org/10.3389/fgene.2019.01239
Pardo GP, Aguilar CH, Martínez FR, Pacheco AD, Martínez CL, Ortiz EM (2013) High intensity led light in lettuce seed physiology (Lactuca sativa L.). Acta Agrophys 20(4):665–677
Pigliucci M, Murren CJ, Schlichting CD (2006) Phenotypic plasticity and evolution by genetic assimilation. J Exp Biol 209:2362–2367. https://doi.org/10.1242/jeb.02070
Rahavi MR, Migicovsky Z, Titov V, Kovalchuk I (2011) Transgenerational adaptation to heavy metal salts in Arabidopsis. Front Plant Sci 2:91. https://doi.org/10.3389/fpls.2011.00091
Saleem MH, Gohar F, Muhammaf IF, Rehman O, Naseem N, Iqbal M, Tahir S, Yaqoob MT, Aslam R, Hassan A (2019) Effect of different colors of lights on growth and antioxidants capacity in rapeseed (Brassica napus L.) seedlings. Ann Agric Crop Sci 4(2):1045
Sanchez-Zabala J, González-Murua C, Marino D (2015) Mild ammonium stress increases chlorophyll content in Arabidopsis thaliana. Plant Signal Behav 10(3):e991596. https://doi.org/10.4161/15592324.2014.991596
Sanghera GS, Wani SH, Hussain W, Singh NB (2011) Engineering cold stress tolerance in crop plants. Curr Genom 12(1):30–43. https://doi.org/10.2174/138920211794520178
Saxe H, Cannell MGR, Johnsen Ш, Ryan MG, Vourlitis G (2002) Tree and forest functioning in response to global warming. New Phytol 149:369–399
Selye H (1974) Stress without distress. Harper and Row, New York, p 50
Selye H (1975) Confusion and controversy in the stress field. J Hum Stress 1(2):37–44. https://doi.org/10.1080/0097840X.1975.9940406
Shelford VE (1913) Animal communities in a temperate America. University of Chicago Press, Chicago, p 386
Shelford VE (1931) Some concepts of bioecology. Ecology 12:455–467. https://doi.org/10.2307/1928991
Shilov IA (2019) Ekologiya (Ecology). Moscow: Vysshaya Shkola, p 539 (in Russian)
Stephenson RA, Gallagher EC, Doogan VJ (2003) Macadamia responses to mild water stress at different phenological stages. Aust J Agric Res 54:67–75
Strimbeck GR, Schaberg PG, Fossdal CG, Schröder WP, Kjellsen TD (2015) Extreme low temperature tolerance in woody plants. Front Plant Sci 6:884. https://doi.org/10.3389/fpls.2015.00884
Tan ZH, Zeng J, Zhang YJ, Slot M, Gamo M, Hirano T, Kosugi Y, da Rocha HR, Saleska SR, Goulden ML, Wofsy SC, Miller SD, Manzi AO, Nobre AD, de Camargo PB, Restrepo-Coupe N (2017) Optimum air temperature for tropical forest photosynthesis: mechanisms involved and implications for climate warming. Environ Res Lett 12:054022. https://doi.org/10.1088/1748-9326/aa6f97
Tang Y-T, Qiu R-L, Zeng X-W, Ying R-R, Yu F-M, Zhou X-Y (2009) Lead, zinc, cadmium hyperaccumulation and growth stimulation in Arabis paniculata Franch. Environ Exp Bot 66(1):126–134. https://doi.org/10.1016/j.envexpbot.2008.12.016
Toscano S, Ferrante A, Romano D (2019) Response of Mediterranean ornamental plants to drought stress. Horticulturae 5(1):6. https://doi.org/10.3390/horticulturae5010006
Tripathi DK, Singh S, Singh S, Mishra S, Chauhan DK, Dubey NK (2015) Micronutrients and their diverse role in agricultural crops: advances and future prospective. Acta Physiol Plant 37(7):1–14. https://doi.org/10.1007/s11738-015-1870-3
Tsonev T, Cebola Lidon FJ (2012) Zinc in plants. Emir J Food Agric 24(4):322–333
Verbitsky VB, Verbitskaya TI (2007) Ecological optimum of ectothermic organisms: static-dynamical approach. Dokl Akad Nauk 416:830–832
Walker WH, Meléndez-Fernández OH, Nelson RJ, Reiter RJ (2019) Global climate change and invariable photoperiods: a mismatch that jeopardizes animal fitness. Ecol Evol 9:5747. https://doi.org/10.1002/ece3.5537
Walsh P, Legendre L (1983) Photosynthesis of natural phytoplankton under high frequency light fluctuations simulating those induced by sea surface waves. Limnol Oceanogr 28(4):688–697
Walter J, Jentsch A, Beierkuhnlein C, Kreyling J (2013) Ecological stress memory and cross stress tolerance in plants in the face of climate extremes. Environ Exp Bot 94:3–8. https://doi.org/10.1016/j.envexpbot.2012.02.009
Wani SH, Kumar V, Shriram V, Sah SK (2016) Phytohormones and their metabolic engineering fosr abiotic stress tolerance in crop plants. Crop J 4(3):162–176. https://doi.org/10.1016/j.cj.2016.01.010
Waqas MA, Kaya C, Riaz A, Farooq M, Nawaz I, Wilkes A, Li Y (2019) Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Front Plant Sci 10:1336. https://doi.org/10.3389/fpls.2019.01336
Whittle CA, Otto SP, Johnston MO, Krochko JE (2009) Adaptive epigenetic memory of ancestral temperature regime in Arabidopsis thaliana. Botany 87(6):650–657. https://doi.org/10.1139/B09-030
Wu G, Zhang C, Chu LY, Shao HB (2007) Responses of higher plants to abiotic stresses and agricultural sustainable development. J Plant Interact 2:135–147. https://doi.org/10.1080/17429140701586357
Xu Z, Hu T, Zhang Y (2012) Effects of experimental warming on phenology, growth and gas exchange of treeline birch (Betula utilis) saplings, Eastern Tibetan Plateau, China. Eur J For Res 131:811–819. https://doi.org/10.1007/s10342-011-0554-9
Xu Z, Zhou G, Shimizu H (2009) Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J Exp Bot 60(13):3737–3749. https://doi.org/10.1093/jxb/erp216
Yang J, Medlyn BE, De Kauwe MG, Duursma RA, Mingkai J, Kumarathunge D, Crous KY, Gimeno TE, Wujeska-Klause A, Ellsworth DS (2020) Low sensitivity of gross primary production to elevated CO2 in a mature eucalypt woodland. Biogeosciences 17(2):265–279. https://doi.org/10.5194/bg-17-265-2020
Yuan Y, Ge L, Yang H, Ren W (2018) A meta-analysis of experimental warming effects on woody plant growth and photosynthesis in forests. J For Res 29(3):727–733. https://doi.org/10.1007/s11676-017-0499-z
Zinn KE, Tunc-Ozdemir M, Harper JF (2010) Temperature stress and plant sexual reproduction: uncovering the weakest links. J Exp Bot 61(7):1959–1968. https://doi.org/10.1093/jxb/erq053
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This study did not receive any financial support.
The online version is available at http://www.springerlink.com.
Corresponding editor: Zhu Hong
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erofeeva, E.A. Plant hormesis and Shelford’s tolerance law curve. J. For. Res. 32, 1789–1802 (2021). https://doi.org/10.1007/s11676-021-01312-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-021-01312-0