Abstract
We used the cotyledons and cotyledonary nodes of Toona ciliata (Chinese mahogany) as explants to examine callus and adventitious shoot induction when exposed to different ratios of hormones. We also investigated the effects of seedling age, inoculation method, and genotype on the efficient regeneration of T. ciliata. The results showed that different genotypes exhibited significantly different callus induction efficiency. The cotyledons and cotyledonary nodes of 20-day seedlings inoculated onto MS medium with 0.5 mg/L 6-benzylaminopurine (6-BA), 0.5 mg/L kinetin (KT) and 0.05 mg/L 1-naphthylacetic acid (NAA) achieved a greater regeneration rate than did other concentrations of cytokinin and auxin. The numbers of shoots per cotyledon and cotyledonary node explant were 7.33 and 6.67. The optimal inoculation method for cotyledons was that the distal end of the explants was placed in contact with the medium. The optimal adventitious shoot differentiation medium for cotyledon explants was MS medium containing 0.3 mg/L 6-BA and 0.2 mg/L NAA, producing a 3.4 cm height of shoot on average. This study established an efficient regeneration system for T. ciliata with cotyledons and cotyledonary nodes as explants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The economic value of broadleaved trees is acknowledged by the increasing market valuation of their wood products. Research into the use of valuable broadleaved tree species has received unprecedented attention (Ito et al. 2019). Because of their hard wood with its high density, beautiful appearance and bright color, timber from such valuable broadleaved tree species is a scarce resource in the market (Jiang 2013). Toona ciliata, known as “Chinese Mahogany”, is a member of the family Meliaceae and listed as a level II national key protected wild plant in China (Li et al. 2018). T. ciliata grows rapidly when young and its growth exceeds many commonly cultivated broadleaved tree species, suggesting its developmental potential (Li et al. 2015). Because of its straight trunks, red wood and desirable grain, it has become the focus of the development of fast-growing timber species in southern China (Heinrich and Banks 2005; Liu et al. 2019). However, it has been found that the species is susceptible to problems such as insect pests and freezing damage, and it has proved difficult to screen for provenances with strong resistance. Specifically, the tender tips of T. ciliata are highly susceptible to damage caused by Hypsipyla robusta when feeding; this species can cause fatal injuries to the tree or seriously limit its growth and development (Zhang et al. 2016). Genetic engineering can play a huge role in improving resistance (Raza et al. 2017). A stable and efficient genetic transformation system is the basis of genetic engineering, and the establishment of an efficient and consistent regeneration system has always been a difficult problem to be solved to facilitate this (Li et al. 2019).
At present, in most studies, stem segments from mature trees or seedlings have been used as explants for the development of the tissue cultures of T. ciliata. Li et al (2018) first successfully established a regeneration system of T. ciliata using hypocotyls. However, the experimental results were not optimal, because the adventitious shoot induction rate was only 59%, and only four shoots were obtained from each explant (Li et al. 2018). In the cotyledonary node, there are primary germ cells that can directly induce a larger number of shoots through organogenesis and these can be used to culture new plants (Paz et al. 2006). Moreover, the cotyledonary node has many advantages: rapid in vitro growth of adventitious shoots, limited mutation, and they are simple to work with (Behera et al. 2019). They have been used in regenerative systems of other plants, such as Tectona grandis, Cicer arietinum L., peanut and seedless watermelon (Compton et al. 1996; Anuradha et al. 2006; Anwar et al. 2008; Tambarussi et al. 2017). Cotyledons are also a common type of explant used in regeneration systems (Du et al. 2015; Armas et al. 2017; Gambhir et al. 2017; Anandan et al. 2019; Cui et al. 2019; Sivanandhan et al. 2019). In this study, cotyledons and cotyledonary nodes of T. ciliata sterile seedlings were used as explants, and a regeneration system was developed using callus induction with adventitious shoot differentiation (cotyledons as explants) and direct regeneration of adventitious shoots (cotyledonary nodes as explants). At the same time, in view of the varying regenerative ability and response to external induction in the same explants resulting from different inoculation methods and seedling ages, this study also optimized the conditions for the culturing of cotyledons. The high-efficiency regeneration system of the two types of explants is important both theoretically and practically for genetic improvement and genetic modification of T. ciliata.
Materials and methods
Plant materials
We experimented with T. ciliata seeds from Pupiao, Yunnan Province, China (99° 06′ E, 25° 04′ N, elevation 1513 m, annual average temperature 14 °C). All seed capsules were collected from healthy, strong, mature trees within the species’ natural range. Seeds were collected from cracked fruits in sunny conditions, and then stored at 4 °C in the molecular laboratory of the South China Agricultural University in Guangzhou.
Culture medium and conditions
MS medium (PhytoTechnology Laboratories, Kansas, USA) was used as the base medium for callus induction and adventitious shoot differentiation and elongation; it contained 3% sucrose (w/v) and 0.5% agar (w/v) (Murashige and Skoog 1962). The pH of the medium was adjusted to 5.8 with HCl or NaOH (Dingguo Changsheng Biotech Co. Ltd., Beijing, China) before the agar was added, then autoclaved at 121 °C for 20 min. Plant material was cultured under cool white fluorescent light (30 µmol m−2 s−1 photosynthetic photon flux) at 25 °C ± 2 °C under a 12/12-h light/dark cycle.
Seed sterilization
The seeds were sterilized following Li et al. (2018). After soaking the T. ciliata seeds in warm water, they were sterilized with 75% alcohol (Guangzhou Chemical Reagent Factory, Guangzhou, China) for 1 min, followed by 10% NaClO (Guangzhou Chemical Reagent Factory, Guangzhou, China) for 20 min, then washed 3 times with sterile water, and inoculated onto MS medium without addition of hormones to obtain healthy aseptic seedlings.
Callus and adventitious shoot induction from cotyledons
In order to investigate the effects of 6-BA and KT on different explants with respect to callus and adventitious shoot induction, we used healthy cotyledons (about 2–3 cm long) from the aseptic seedlings (Fig. 1B). The cotyledons were inoculated onto the MS medium containing varied concentrations of 6-BA (0.3, 0.5 and 1 mg L−1), KT (0.5, 1 and 2 mg L−1) and NAA (0.1 or 0.05 mg L−1). The plant growth regulators used in the experiment were purchased from MilliporeSigma, Inc., St. Louis, MO, USA.
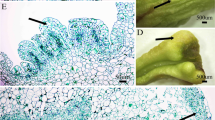
Plant regeneration from cotyledonary and cotyledonary nodes explants of T. ciliata and acclimatization. A 2-week-old seedlings and cotyledonary nodes; B 2-week-old seedlings and cotyledons; C shoots induction from cotyledonary nodes in T. ciliata on MS media containing 0.5 mg/L 6-BA, 0.5 mg/L KT and 0.05 mg/L NAA. a The explant that was cultured for 20 days, buds inducted; b the explant that was cultured for 30 days, shoots elongated continuously, D shoots induction from cotyledons in T. ciliata on MS media containing 0.5 mg/L 6-BA, 0.5 mg/L KT and 0.05 mg/L NAA. a: the explant that was cultured for 10 days, callus inducted; b: the explant that was cultured for 12 days, callus inducted continuously; c: the explant that was cultured for 15 days, shoots elongated from the calli; d: the explant that was cultured for 20 days, shoots elongated continuously; E shoot multiplication and elongation on MS medium containing 0.3 mg/L 6-BA and 0.2 mg/L NAA after15days; F roots formed in half-strength MS with 0.1 mg/L NAA, 1.5% sucrose (w/v) and 0.5% agar (w/v); G acclimatized plantlet after 20 days; H acclimatized plantlet after 50 days. Each treatment consisted of three replicates with 10 culture flasks, each containing five explants
Adventitious shoot induction of cotyledonary nodes
Once the seedlings had developed fully-expanded cotyledons, these were removed and the cotyledonary nodes were inoculated onto growing media containing varied concentrations of 6-BA (0.3, 0.5 and 1 mg L−1) and KT (0.5, 1 and 2 mg L−1) (Fig. 1A). The concentration of NAA in the MS medium was 0.05 mg L−1.
Optimization of culture conditions for cotyledon explants
The cotyledons of 15, 20, and 25 d seedlings were inoculated onto the optimum shoot induction medium to investigate the effect of seedling age on callus and adventitious shoot induction and differentiation. Two inoculation methods were investigated in relation to shoot and callus induction: either the distal or the proximal part of the cotyledons was placed in contact with the medium. The regeneration capacity of cotyledons of different genotypes was recorded. The effect of light was investigated by placing the culture flask with the explants under 12 or 0 h light conditions, and the callus and adventitious shoot induction recorded.
In all experiments, the medium without growth regulators was included as a control. Each treatment consisted of three replicates with 10 culture flasks, each containing five explants. The callus and shoot induction were recorded after 35 days of culturing, enabling calculation of callus and shoot induction rates for the different treatments.
Shoot elongation
The adventitious shoots induced by the cotyledons were transferred to the elongation medium containing different concentrations of 6-BA (0.1, 0.3 and 0.5 mg L−1) and NAA (0.1, 0.2 and 0.3 mg L−1) for elongation culture. Each treatment consisted of three replicates with 10 culture flasks, each containing five explants. Elongation was recorded after 35 days of culturing.
Rooting and field acclimation
The adventitious shoots that were robust were cut, then rooted and cultured according to Li et al. (2018). When the roots were 3-5 cm in length, the culture flask was opened and left for 1 day, then the residual medium was washed off the root, and plantlets were transplanted into plastic cups containing sterilized substrate. The cups were covered with plastic film to ensure that moist conditions were maintained. Watering was gradually reduced as new leaves grew. The plastic film was then removed and the plants were watered as required.
Statistical analysis
Callus and shoot regeneration rates were calculated using the formula: callus and shoot regeneration rate = the number of cotyledon explants producing callus and shoots/total number of cotyledon explants × 100%. Shoot regeneration rates were calculated using the formula: shoot regeneration rate = the number of cotyledonary node explants producing shoots/total number of cotyledonary node explants × 100%. The number of shoots per explant was calculated using the formula: number of shoots per explant = the total number of shoots produced by cotyledon or cotyledonary nodes explants/total number of explants producing callus and shoots. The rooting rate was calculated using the formula: rooting rate = the number of shoots with roots/total number of shoots used for the test × 100%. Range analysis was conducted using Excel 2017. Analysis of variance (ANOVA) was conducted in SAS 10.0, followed by Duncan’s multiple range test at the P ≤ 0.05 level.
Results
Effects of growth regulator combinations on shoot induction in cotyledonary nodes
Because the explants of cotyledonary nodes are intercalary meristems with primary germ cells and strong division and differentiation abilities, adventitious shoots can be produced in the medium without hormone addition, but induction efficiency is poor and few adventitious shoots appear. An auxin concentration of 0.05 mg/L significantly increased the adventitious shoot regeneration rate with the different hormone combinations (Table 1). The combination resulting in the highest induction rate was MS + 0.5 mg/L 6-BA + 0.5 mg/L KT + 0.05 mg/L NAA, which promoted robust, elongated adventitious shoots. When the cotyledonary nodes were cultured in this medium for 30 days, the average number of adventitious shoots was 6.67(Fig. 1Ca, b).
Effects of growth regulator combinations on callus induction in cotyledons
In the absence of exogenous hormones, even if a small amount of callus appeared on the explant, it gradually turned brown, and a large and healthy callus was not produced. Cytokinin (6-BA and KT) in the culture medium had a significant effect on callus induction by cotyledons of T. ciliata. At constant concentrations of NAA, the adventitious shoots of cotyledons responded more to 6-BA than to KT (Table 2). As the concentration of 6-BA increased, the callus induction rate in cotyledons initially increased but then declined. When the concentrations of 6-BA and KT were 0.5 mg L−1 and NAA was 0.05 mg L−1, after 10 days of culture, callus was formed at the incision and gradually enlarged. Thereafter, many green bud spots appeared (Fig. 1D, a), which then differentiated into adventitious shoots (Fig. 1Db, c, d). The differentiation rate reached 77.23%, significantly higher than for any of the other hormone combinations. In other media, explants from cotyledons tended to die immediately, or there were no green bud spots on the callus, and eventually death occurred. Therefore, the optimal medium for callus induction and differentiation of cotyledons in T. ciliata was MS + 0.5 mg/L 6-BA + 0.5 mg/L KT + 0.05 mg/L NAA.
Optimization of regeneration conditions for cotyledons of T. ciliata
Effects of seedling age on callus induction and differentiation in cotyledons
Age of seedling affected the regeneration capacity of cotyledon explants (Table 3). Vigorous calluses were induced in 20-day explants, and the callus induction efficiency exceeded 68.65%. Our results show that it is possible for seedlings to be too young to use in this process, resulting in poor callus production. However, older seedlings are also not ideal: callus induction rates decreased with increasing seedling age (> 20 d) and callus mortality increased accordingly.
Effects of inoculation method on callus induction and differentiation in cotyledons
The inoculation method of explants had a great influence on the callus induction of cotyledons. Inoculation was performed by placing either the abaxial leaf surface close to the medium or the paraxial leaf surface close to the medium. We found that when the abaxial surface was close to the medium, the callus induction rate was only 35.85%, compared to 73.39% when the paraxial surface was near the medium (Table 4).
Effects of genotype on callus induction and differentiation in cotyledons
Genotype affected callus production. Even when different genotypes were cultured under the same conditions in the same optimal medium, we recorded significant differences in callus production. The best induction rate was 100%, but some genotypes produced no calluses at all (Table 5).
Effects of illumination conditions on callus induction and differentiation in cotyledons
When the medium was placed in the dark, the callus induction of the cotyledon explants was slower than when under light, and the induction rate was significantly lower. This indicates that a 12 h light treatment was appropriate (Table 6). Further data on the effects of light intensity are required to optimize conditions.
Effects of combinations of growth regulators on the shoot elongation of cotyledons
Compared with the medium optimized for callus induction and differentiation, the concentration of 6-BA was reduced in the elongation medium and the concentration of NAA, which promotes elongation of plant cells, was increased (Table 7). NAA played a major role at this stage. At a concentration of 0.3 mg/L, the regenerated seedlings exhibited obvious vitrification, the color of seedlings was lighter, and the leaves were small. NAA concentration of 0.1 mg/L yielded slower shoot growth and produced shorter shoots than under optimum conditions. The calluses grew longer adventitious shoots in MS + 0.3 mg L−1 6-BA + 0.2 mg L−1 NAA, with seedlings reaching a maximum height of 3.4 cm.
Rooting of adventitious shoots, acclimation and transplantation
Reducing sucrose concentration and nutrient composition in the growing medium (using 1/2 MS) can reduce the dependence of regenerated seedlings on carbohydrates obtained from the culture medium, stimulate rooting, and achieve an ideal speed of root formation. Adventitious roots formed at the base of shoots after about 5 days. After 10 days, the rooting rate exceeded 98.23%, and the average number of roots was 13.75 (Fig. 1F). After transplanting the seedlings, the survival rate was 88.89% after 20 days (Fig. 1G). The plants grew well with roots developing, leaves expanding and new leaves growing. Two months after transplanting, the average seedling height of the regenerated seedlings exceeded 30 cm (Fig. 1H).
Discussion
Selecting the correct combination of growth regulators was key when attempting to maximize the formation of calluses; the response also varied according to genotype (Xhulaj and Doriana 2019). Plants respond to both endogenous and exogenous hormones, and achieving the correct balance is important (Zhang et al. 2019). In combination with auxin, the cytokinin 6-BA and KT was often used exogenously to promote rapid callus production (Ayala et al. 2019; Lin et al. 2019). Li et al. (2018) used IBA in combination with the two cytokinin to induce adventitious shoot production from hypocotyls, but our preliminary research indicated that this was not the optimum auxin to use for callus production from cotyledons or cotyledonary nodes. Therefore, we used NAA in our experiments.
In our study, the callus induction rate (when the paraxial surface was placed near the medium) was about three times that when the abaxial surface was near the medium (Table 4). This is the opposite to experimental results for Azadirachta indica (Gairi and Rashid 2005), but similar to results for Pongamia pinnata (Sujatha et al. 2008), the reason being that the proximal region of the cotyledons might be the source of highly regenerative cells (Murthy et al. 1995). Simultaneously, seedling age affects the viability, division rate and morphogenesis of explants. Compared with explants of slightly older seedlings, younger explants are generally characterized by a strong capacity for differentiation, which was related to the high regeneration capacity of parenchyma cells (Stavridou et al. 2019).
Among the many factors affecting plant regeneration systems, genotype was important. The main reason that genotypes greatly affected callus induction of explants was that different genotypes exhibit different responses to stimulation resulting in different callus production (Sebastiani and Ficcadenti 2016). Zimik and Arumugam (2017) reported that sesame (Sesamum indicum L.) cotyledons of 10 different varieties showed different induction rates of buds. Similarly, Rathinapriya et al. (2019) reported that different genotypes of millet [Setaria italica (L.) Beauv.] had different bud survival rates in the construction of regeneration systems.
Conclusions
T. ciliata is highly susceptible to damage caused by Hypsipyla robusta, which can be fatal or seriously limit the growth and development of this species. We consider that genetic engineering and genetic improvement are the one method by which the species can be protected. Therefore, an efficient and stable regeneration system is fundamental. In our study, cotyledons and cotyledonary nodes of sterile seedlings of suitable seedling age were used as explants to obtain two efficient regeneration systems of T. ciliata. One method is to produce adventitious buds directly based on cotyledonary nodes; the other is to use cotyledons as explants to obtain many adventitious buds by inducing calluses. The small plants produced using this scheme were uniform, healthy, and had high survival rates after transplanting to soil. The regeneration program reported here might be used for genetic transformation research that could provide technical support for the genetic improvement of T. ciliata.
References
Anandan R, Deenathayalan T, Bhuvaneshwari R, Monisha MM, Prakash M (2019) An efficient protocol for rapid plant regeneration from deembryonated cotyledons of black gram [Vigna mungo (L.) Hepper]. Indian J Agric Res 53:589–593
Anuradha TS, Jami SK, Datla RS, Kirti PB (2006) Genetic transformation of peanut (Arachis hypogaea L.) using cotyledonary node as explant and a promoterless gus:nptII fusion gene based vecto. J Biosci 32:235–246
Anwar F, Sharmila P, Pardha S (2008) An optimal protocol for in vitro regeneration, efficient rooting and stable transplantation of chickpea. Physiol Mol Biol Plants 14:329–335
Armas I, Pogrebnyak N, Raskin I (2017) A rapid and efficient in vitro regeneration system for lettuce (Lactuca sativa L.). Plant Methods 13:58
Ayala PG, Brugnoli EA, Luna CV, Gonzalez AM, Pezzutti R, Sansberro PA (2019) Eucalyptus nitens plant regeneration from seedling explants through direct adventitious shoot bud formation. Trees Struct Funct 33:1667–1678
Behera S, Kar SK, Rout KK, Barik DP, Panda PC, Naik SK (2019) Assessment of genetic and biochemical fidelity of field-established Hedychium coronarium J. Koenig regenerated from axenic cotyledonary node on meta-topolin supplemented medium. Ind Crop Prod 134:206–215
Compton ME, Gray DJ, Elmstrom GW (1996) Identification of tetraploid regenerants from cotyledons of diploid watermelon cultured in vitro. Euphytica 87:165–172
Cui YY, Deng YW, Zheng KY, Hu XM, Zhu ML, Deng XM, Xi RC (2019) An efficient micropropagation protocol for an endangered ornamental tree species (Magnolia sirindhorniae Noot. & Chalermglin) and assessment of genetic uniformity through DNA markers. Sci Rep 9:9634
Du L, Li YP, Yao Y, Hang LW (2015) An efficient protocol for plantlet regeneration via direct organogenesis by using nodal segments from embryo-cultured seedlings of Cinnamomum camphora L. PLoS ONE 10:e0127215
Gairi A, Rashid A (2005) Direct differentiation of somatic embryos on cotyledons of Azadirachta indica. Biol Plant 49:169–173
Gambhir G, Kumar P, Srivastava DK (2017) High frequency regeneration of plants from cotyledon and hypocotyl cultures in Brassica oleracea cv. Pride of India. Biotechnol Rep 15:107–113
Heinrich I, Banks JCG (2005) Dendroclimatological potential of the Australian red cedar. Aust J Bot 53:21–32
Ito S, Shinohara C, Hirata R, Mitsuda Y, Shimizu O, Nomiya H (2019) Factors limiting the distribution of deciduous broadleaved trees in warm-temperate mountainous riparian forests. Landsc Ecol Eng 15:391–400
Jiang XM (2013) Development of the concept, policy, technical problems of valuable broad-leaved tree species in Jiangxi province and countermeasures. For Technol Jiangxi 1:3–8 (in Chinese)
Li JJ, Zhang D, Que QM, Chen XY, Ou Yang KX (2019) Plant regeneration and Agrobacterium-mediated transformation of the miracle tree Neolamarckia cadamba. Ind Crop Prod 130:443–449
Li P, Shang YY, Zhou W, Hu XS, Mao WM, Li JJ, Li JC, Chen XY (2018) Development of an efficient regeneration system for the precious and fast-growing timber tree Toona ciliata. Plant Biotechnol 35:51–58
Li P, Zhan X, Que QM, Qu WT, Liu MQ, Ou Yang KX, Li JC, Deng XM, Zhang JJ, Liao BY, Pian RQ, Chen XY (2015) Genetic diversity and population structure of Toona ciliata Roem. based on sequence-related amplified polymorphism (SRAP) markers. Forests 6:1094–1106
Lin SY, Liu GH, Guo TT, Zhang L, Wang SG, Ding YL (2019) Shoot proliferation and callus regeneration from nodular buds of Drepanostachyum luodianense. J For Res 30:1997–2005
Liu Q, Arnold RJ, Yang SZ, Wu JY, Li ZH, Li Y, Cheng Y (2019) Foliar application of exogenous polyamines to ameliorate drought-induced oxidative damage and physiological inhibition in Toona ciliata seedlings. Aust For 82:139–150
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue culture. Physiol Plant 15:473–497
Murthy BNS, Murch SJ, Saxena P (1995) Thidiazuron-induced somatic embryogenesis in intact seedlings of peanut (Arachis hypogaea): endogenous growth regulator levels and significance of cotyledons. Physiol Plant 94:268–276
Paz MM, Martinez JC, Kalvig AB, Fonger TM, Wang K (2006) Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep 25:206–213
Rathinapriya P, Satish L, Rameshkumar R, Pandian S, Rency AS, Ramesh M (2019) Role of activated charcoal and amino acids in developing an efficient regeneration system for foxtail millet (Setaria italica (L.) Beauv.) using leaf base segments. Physiol Mol Biol Plants 25:533–548
Raza G, Singh MB, Bhalla PL (2017) In vitro plant regeneration from commercial cultivars of soybean. Bio Med Res Int 2017:7379693
Sebastiani MS, Ficcadenti N (2016) In vitro plant regeneration from cotyledonary explants of Cucumis melo L. var. cantalupensis and genetic stability evaluation using RAPD analysis. Plant Cell Tiss Organ Cult 124:69–79
Sivanandhan G, Choi SB, Jiae M, Choi SR, Kim SG, Park YD, Lim YP (2019) High frequency in vitro regeneration of Chinese cabbage (cv. Kenshin) from hypocotyl and cotyledon explants. Hortic Sci Technol 37:640–650
Stavridou E, Tzioutziou NA, Madesis P, Labrou NE, Nianiou-Obeidat I (2019) Effect of different factors on regeneration and transformation efficiency of tomato (Lycopersicum esculentum) hybrids. Czech J Genet Plant 55:120–127
Sujatha K, Panda BM, Hazra S (2008) De novo organogenesis and plant regeneration in Pongamia pinnata, oil producing tree legume. Trees Struct Funct 22:711–716
Tambarussi EV, Rogalski M, Galeano E, Brondani GE, de Martin VD, da Silva LA, Carrer H (2017) Efficient and new method for Tectona grandis in vitro regeneration. Crop Breed Appl Biotechnol 17:124–132
Xhulaj D, Doriana B (2019) Effect of plant growth regulators on in vitro plant regeneration of wheat (Triticum aestivum L.) from embryo explants. J Anim Plant Sci 19:1616–1621
Zhang L, Pan YZ, Liu SL, Chen Y, Shen LJ (2019) Embryonic callus induction and plant regeneration of Lilium leucanthum. Bull Bot Res 39(338–346):357 (in Chinese)
Zhang SN, Ma T, Chen XY, Wen XJ (2016) Bionomics and control of Hypsipyla robusta. For Pest Dis 35:29–33 (in Chinese)
Zimik M, Arumugam N (2017) Induction of shoot regeneration in cotyledon explants of the oilseed crop Sesamum indicum L. J Gene Eng Biotechnol 15:303–308
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: This work was financially supported by the National Key Research Projects, Forestry Resource Cultivation and Utilization Technology Innovation (Grant No. 2016YFD0600606), the Natural Science Foundation of Guangdong Province of China (Grant No. 2018A030313798) and Characteristic innovation projects of department of education of Guangdong province (Grant No. 2019KTSCX017).
The online version is available at http://www.springerlink.com.
Corresponding editor: Yanbo Hu.
Rights and permissions
About this article
Cite this article
Song, H., Mao, W., Shang, Y. et al. A regeneration system using cotyledons and cotyledonary node explants of Toona ciliata. J. For. Res. 32, 967–974 (2021). https://doi.org/10.1007/s11676-020-01189-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-020-01189-5