Abstract
We explored the discoloration of rattan cane using X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR). XPS analysis showed that after the cane was stained by Lasiodiplodia theobromae, carbon and oxygen elements and the ratio of oxygen to carbon decreased. Considering atomic binding, C1 and C4 contents increased, while C2 and C3 contents decreased, and the ratio of O2 to O1 decreased sharply. The relative contents of lignin, cellulose and polysaccharides increased and new substances with low O2/O1 ratio occurred. FTIR analysis showed that the absorption peaks of O–H at 3346 cm−1, aliphatic C–H at 2921, 2853 and 1464 cm−1, and C=O at 1723 cm−1, were characteristic peaks of fungal melanin intensified, indicating that cane discoloration was primarily caused by fungal melanin. The absorption peaks characterizing cellulose and lignin like polysaccharides at 800 cm−1, C–H at 1374 cm−1, C–O at 1058 and 1038 cm−1, phenolic hydroxyl at 1245 cm−1, aromatic ether bonds at 1270 cm−1, carbon skeleton at 1608 cm−1 and benzene ring at 1500 cm−1 were enhanced since the fungus mainly consumed the extractives in cane cell lumens and the main composition content increased relatively. Regardless of the discoloration caused by natural fungi or inoculated fungi, the discoloring feature and composition changes were identical except that the fungus-inoculated cane had more melanin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rattan is an important forest product and several species have been planted on a large scale in many areas of Southeast Asia (Peters et al. 2013). In southern China, Daemonorops margaritae is widespread in tropical and subtropical regions; the most important product of rattan is cane which is used in many forms (Jiang 2007). Discoloration of cane is a serious problem from the viewpoint of rattan cane’s commercial worth (Ed et al. 1997; Widayati and Samantha 2010). Fresh canes are an attractive yellowish white or cream but easily discolor to yellowish red, blue or brown during the course of transportation, storage, processing and utilization which degrades their quality and limits their utilization. Therefore, it is necessary to study the main causes of discoloration, and ways to prevent and remove it to improve cane value and save rattan resources (Jiang 2007; Jiang et al. 2007).
Similarly with wood, rattan cane mainly consists of cellulose, hemicellulose, and lignin (Abasolo et al. 2003; Lü et al. 2009). Whether the discoloration is caused by microorganisms or not, rattan cane components will change accordingly. Cane discoloration induced by microorganisms is primarily attributed to the color of mycelia or reaction products of mycelia secretions and cane composition (Lü et al. 2013). According to the range, size, depth and feature of discoloration and treatment cost, discoloration may be removed by mechanical, physical or chemical methods such as extraction, decomposition or modification. In this study, a typical stain fungus was first isolated from stained cane material, and then inoculated into clear, unstained cane. The changes in chemical composition of fungus-stained cane were investigated by Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS), and the internal discoloration mechanism was explored to provide a theoretical basis for cane discoloration control, color protection and efficient utilization.
Materials and methods
Materials
Daemonorops margaritae cane, approximately 15-year-old and climbing on Chinese fir, was collected from a local farm in Guangdong Province, China. Canes with diameters of 10–20 mm, 15–25 m long, and internode lengths of 15–25 cm were selected. The stain fungus was first isolated from typical blue-stained canes and then inoculated into non-node and defect-free canes with 12% moisture content and 50 mm long × cane stem diameter (about 10–20 mm) wide × 2 mm high.
Isolation and inoculation of the stain fungus
The blue-stained cane surface was first disinfected with 75% alcohol, and then rinsed three times with sterile water. The outer layer was gently removed, small stained pieces cut out and placed on a potato-dextrose-agar (PDA) plate medium, and cultivated at 26–28 °C and 75% relative humidity. When the mycelia were obvious, hyphae tips were picked out to be further cultured and purified. The fungus which was most frequently isolated from the blue-stained canes was selected and its hyphae fragments and spores made into a suspension solution. The defect-free canes were dried at 103 °C and weighed, and their colors measured. They were then moist–heat sterilized at 121 °C for 30 min. The fungal suspension solution was spread on the sterilized cane surface to cultivate the fungus in an incubator for periodical examination.
Cane color measurement
Color was characterized with the CIE L*a*b* (1976) color system, and color indexes of light (L*), green–red (a*) and blue–yellow (a*) measured at three points by the Chromatic Aberration Meter CR-300 (Minolta, Japan), standard light source D65, 0/d vertical irradiation. The color differences in ΔL*, Δa*, Δb* and ΔE* were calculated from averaged color index values ΔE* = [(ΔL*)2 + (Δa*)2 + (Δb*)]2 (Wang et al. 2009).
XPS analysis
XPS analysis was performed by AXIS Ultra X-ray Photoelectron Spectroscopy (Kratos, England) on a sample 5 mm long × 5 mm wide × 1 mm high. The X-ray source was a monochromatic Al Ka (hν = 1486.71 eV); power was 225 W (accelerating voltage: 15 kV, emission current: 40 mA); the C1s level was set at 284.8 eV; the minimum energy resolution of the silver (Ag 3d5/2) line was 0.48 eV; the minimal scan area was 15 μm. Data analysis and processing were performed with Vision (PR 2.1.3) and Casa XPS (2.3.12 Dev 7) programs. Analysis conditions were hybrid Lens Mode, resolution of pass energy 20 eV, step size 100.0 meV, dwell time 500 ms, 1 sweeps, and the acquisition times of 181 for C1s and 80 for O1s.
FTIR analysis
This was conducted by Magna-IR 750 Fourier Transform Infrared Spectroscopy (Nicolet, America). Approximately, 2 mg powder of the sample surface was scrapped off and mixed with KBr powder, and compressed into a disc to collect spectra. FTIR analysis conditions were: scan range between 4000 and 400 cm−1, scan repetition 32 times and a spectral resolution of 4 cm−1.
Results and discussion
Color changes of fungus-stained cane
Unstained canes were inoculated with Lasiodiplodia theobromae, a fungus which is most frequently isolated from blue-stained canes. The chromatic indexes of the inoculated canes were regularly measured. As shown in Table 1, the total color difference (ΔE*) of the cane inoculated for 2 weeks reached 19.75 NBS. When the inoculation time had been extended to 4 weeks, the ΔE* value was up to 28.64 NBS. According to the ΔE* value, visual feeling of color differences may be divided into six grades: trace for 0–0.5 NBS, slight for 0.5–1.5 NBS, noticeable for 1.5–3.0 NBS, appreciable for 3.0–6.0 NBS, much for 6.0–12.0 NBS, and very much for above 12.0 NBS (Shi and Wang 1992). Therefore, the color change caused by the fungus was rapid and very evident. It was observed that the discoloration of fungus-inoculated cane was coincident with that of the fungus-isolated cane. Based on the isolating and cultivating characteristics of the fungus (Lü et al. 2002), the stain was primarily from the color of mycelia or fungal secretions. The weight loss of D. margaritae canes inoculated with the fungus occurred mainly within the first 2 weeks, and it was only 2.5% after 4 weeks inoculation. This indicates that the fungus invasion of rattan cane was rapid and its ability to decompose or degrade the main cane composites was weak.
XPS analysis
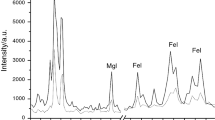
As XPS can be used to determine surface composition by either O/C ratios or high resolution C1s spectra (Ju et al. 2013), both methods were employed to determine the differences in surface composition between unstained and fungus-stained canes. The chemical structure and properties of cane surfaces were determined by combining forms and states of its carbon elements. The electron binding energy of C1s depends on its combined states. According to C1s chemical shift, information about the surface chemical environment can be inferred. The C1s spectra of the unstained (a) and fungus-stained (b) surfaces could be fitted to four different carbon environments (Fig. 1): C1 (–C–H– and –C–C–, 284.8 eV), C2 (–C–O–, 286.4 eV), C3 (–O–C–O– or –C=O, 288.0 eV) and C4 (–O–C=O, 289.0 eV). In general, C1 was mainly attributed to lignin and to extractives like fatty acid, fats, waxes; C2 largely attributed to hemicellulose and cellulose hydroxyls; C3 attributed to hemicellulose and lignin carbonyls; and C4 mainly attributed to esters and carboxyls of hemicellulose and extractives (Sernek et al. 2004; Ozen et al. 2014).
The percentages of C1, C2, C3 and C4 of unstained cane surface were 59.4, 33.5, 4.6 and 2.5%, respectively (Table 2), indicating that the main chemical composition of the cane surfaces was cellulose, hemicellulose and lignin. The C/O ratios of cellulose, lignin and extractives were 1.2, 3.0 and 10.0, respectively (Barry et al. 1990). The higher C1 content of 59.4% and C/O ratio of 3.3 indicates that D. margaritae cane had more lignin and extractives than that of wood (Sinn et al. 2004; Jiang 2007). As shown in Table 2, the surface elemental composition of fungus-stained cane changed greatly. The decrease in carbon and oxygen elements suggests that some substances containing these two elements were digested and metabolized, and finally released as carbon dioxide and water. The increase of C1 and C4 and the decrease of C2 and C3 might be due to: first, cell contents like starch and saccharides in cell lumens were consumed by the fungus; second, a small amount of hemicellulose and cellulose oligomers but no lignin was decomposed or degraded, which agrees with the results of quantitative chemical composition analysis (Lü et al. 2011); and/or third, new substances with different C/O ratios occurred with the fungal invasion. The C/O ratio of fungus-stained cane slightly increased, suggesting that either cane components with low C/O ratios were consumed or new substances with high C/O ratios occurred. In the stained cane surface, nitrogen and silicon increased, and new inorganic elements like calcium and zinc were found. Since the fungus generally did not consume inorganic components, these increased relative to the consumption of other components. Storage condition changes, extractive migration and external substance invasion could all cause the changes of cane element composition and result in cane discoloration.
The composition change of fungus-stained cane surfaces could be further understood through the O1s change. The O1s spectra of unstained (c) and fungus-stained (d) cane surfaces could be due to two different oxygen environments (Fig. 1): oxygen doubly bonded with carbon as C=O (O1), and oxygen singly bonded with carbon as C–O (O2). As shown in Table 2, the percentage of O1 and O2 of unstained cane were 4.03 and 95.97% respectively, showing that most of the oxygen was singly bonded with carbon. Compared with unstained cane, the O2/O1 ratio of fungus-stained cane dropped greatly from 23.72 to 4.25, which indicates remarkable C=O increase and C–O decrease. It was analyzed that the significant decrease in O2/O1 ratio probably related to fungal secretions such as melanin.
FTIR analysis
To further understand the changes of cane chemical composition during the fungus invasion, the fungus-isolated and fungus-inoculated canes were also studied by FTIR and their difference spectra analyzed. As shown in Fig. 2, the absorption peaks of fungus-isolated cane (a) and fungus-inoculated cane (b) changed greatly within fingerprint region from 800 to 2000 cm−1. As for the fungus-isolated cane (a), absorption peaks of hydroxyl and amine (3297 cm−1), C–H (2926, 1456 and 1375 cm−1), carbonyl (1733 and 1653 cm−1) and benzene-ring (1592 and 1513 cm−1) intensified remarkably. All these absorption peaks were characteristics of fungal melanin (Amardeep and Jose 2013), as fungal melanin is a class of polyphenolic polymers with a basic indole ring structure (Zheng and Wu 2007). The quinone polymer, generated from enzymes secreted by microorganisms, could combine with proteins to produce biological melanin which causes a black or blue stain (Lü et al. 2002). According to FTIR spectra, considerable melanin occurred in the fungus-isolated canes. Moreover, the absorption intensity of hydroxyl at 3608 cm−1 and C–O at 1241 cm−1 increased. All this supports the belief that the relative contents of cellulose and lignin increased significantly, and was identical with the result of quantitative chemical composition analysis (Lü et al. 2011).
As shown in Fig. 2b, the absorption peaks, including O–H (3346 cm−1), aliphatic C–H (2921, 2853 and 1464 cm−1) and C=O (1723 cm−1) of fungus-inoculated cane all intensified. They were characteristic peaks of fungal melanin (Amardeep and Jose 2013) and proves that the fungal melanin secreted by L. theobromae mainly caused the cane discoloration. The absorption peak of polysaccharide at 800 cm−1 greatly weakened, and the absorption intensity of C–H (1374 cm−1), C–O (1058 and 1038 cm−1) and anomeric carbon (897 cm−1) all increased. This indicates that more cellulose and polysaccharides existed in fungus-stained cane. The absorption peaks of phenolic hydroxyl (1245 cm−1), aromatic ether bonds (1270 cm−1), carbon skeleton (1608 cm−1) and benzene rings (1500 cm−1) were intensified owing to increased lignin (Abasolo et al. 2003). Since the fungus mainly consumed the extractives, all the main cane composites were relatively increased.
According to FTIR analysis results, cane discolorations caused by the natural fungus and the inoculated fungus were consistent, but there still existed some differences. Compared with naturally stained cane, the fungus-inoculated cane presented a higher absorptivity of OH and –NH2 (3430 cm−1), C–H (2921, 2853 and 1463 cm−1), C=O (1722 cm−1) and benzene ring (1611 cm−1), indicating that the fungus-inoculated cane had more fungal melanin. The stronger absorption peaks at 1463, 1281, 1166 and 1037 cm−1 also suggests that the degree of discoloration of fungus-inoculated cane was more serious than that of fungus-isolated cane. Since nutrition in artificial inoculation was richer, the fungus strains grew more vigorously and secreted more melanin, finally leading to more obvious discoloration. Regardless whether the discoloration was caused by natural or inoculated fungus, the discoloring features and composition changes were identical. Hence, a fungal inoculation test could reflect cane fungal discoloration in practice.
Conclusion
XPS analysis of fungus-stained canes showed that their C, O and C–O content decreased, and C=O and C/O ratios, lignin, cellulose and polysaccharides increased. It indicated that cane components with low C/O ratios were consumed or that new substances with high C/O ratios were present. FTIR analysis of fungus-stained canes showed that absorption peaks characterizing fungal melanin intensified significantly, and those characterizing lignin and cellulose were also enhanced. It suggests that the fungus mainly consumed cane cell lumen extractives, and discoloration was caused by the fungal melanin. Therefore, discoloration could be removed by decomposing or degrading the melanin. The cane discoloration caused by natural and inoculated fungus was similar and a fungal inoculation test could reflect actual cane discoloration.
References
Abasolo WP, Yoshida M, Yamamoto H (2003) Thermal softening of rattan canes: influence of the hemi cellulose-lignin matrix. World Bamboo Rattan 1(4):32–36
Amardeep SS, Jose SM (2013) Biosorption of uranium by melanin: kinetic, equilibrium and thermodynamic studies. J Bioresour Technol 149:155–162
Barry AO, Koran Z, Kaliaguine S (1990) Surface analysis by ESCA of sulfite post-treated CTMP. J Appl Polym Sci 39(1):31–42
Ed DJ, Richard PC, John NS (1997) Effects of a fungal treatment on the brightness and strength properties of a mechanical pulp from Douglas-fir. Bioresour Technol 61(1):61–68
Jiang ZH (2007) Bamboo and rattan in the world. China Forestry Publishing House, Beijing
Jiang ZH, Lv WH, Ren HQ, Fei BH, Wu YZ (2007) Review on rattan cane properties. World For Res 20(4):35–40
Ju XH, Mark E, Zhang X (2013) An advanced understanding of the specific effects of xylan and surface lignin contents on enzymatic hydrolysis of lignocellulosic biomass. Bioresour Technol 132:137–145
Lü WH, Xiao SQ, Mu QY (2002) Main causes of Betula alnoides wood’s discoloration. J Beijing For Univ 24(4):107–110
Lü WH, Jiang ZH, Wu YZ (2009) Basic components and chemical properties of the cane of Daemonorops margaritae. Sci Silvae Sin 45(7):96–100
Lü WH, Liu XE, Liu JL (2011) Fungal staining of Daemonorops margaritae canes. Sci Silvae Sin 47(8):196–200
Lü WH, Jiang ZH, Liu XE, Liu JL (2013) Causes and removal of Daemonorops margaritae cane’s discoloration. Adv Mater Res 634–638:909–912
Ozen E, Yeniocak M, Goktas O, Alma HM, Yilmaz F (2014) Antimicrobial and antifungal properties of madder root (Rubia tinctorum) colorant used as an environmentally-friendly wood preservative. Bioresources 9(2):1998–2009
Peters CM, Thammavong B, Mekaloun B, Phearoom N, Ratanak O, Ledecq T (2013) Growth of wild rattans in Cambodia and Laos: implications for management. For Ecol Manag 306:23–30
Sernek M, Kamke FA, Glasser WG (2004) Comparative analysis of inactivated wood surfaces. Holzforschung 58(1):22–31
Shi BZ, Wang WH (1992) ESR analysis of fungus stained-wood. Sci Silvae Sin 28(4):330–335
Sinn G, Gindl M, Reiterer A, Tschegg SS (2004) Changes in the surface properties of wood due to sanding. Holzforschung 58(3):246–251
Wang XQ, Fei BH, Ren HQ (2009) FTIR spectroscopic studies of the photo-discoloration of Chinese Fir. Spectrosc Spectr Anal 29(5):1272–1275
Widayati AJ, Samantha CB (2010) Accessibility factors and conservation forest designation affecting rattan cane harvesting in Lambusango Forest, Buton, Indonesia. Hum Ecol 38(6):731–746
Zheng L, Wu XQ (2007) Advances on infection structures of plant pathogenic fungi. J Nanjing For Univ Nat Sci 31(1):90–94
Author information
Authors and Affiliations
Corresponding author
Additional information
Project Funding: This study was supported by the National Key Project of S & T Supporting Programs Funded by MOST of China during the 12th Five-year Plan (No. 2012BAD23B0104).
The online version is available at http://www.springerlink.com
Corresponding editor: Yu Lei.
Rights and permissions
About this article
Cite this article
Lyu, W., Shi, Y., Zheng, Y. et al. XPS and FTIR studies of fungus-stained Daemonorops margaritae. J. For. Res. 30, 739–743 (2019). https://doi.org/10.1007/s11676-018-0598-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-018-0598-5