Abstract
We prepared spherical microcapsules modified by carboxymethyl cellulose (CMC) with urea-formaldehyde (UF) resin as a shell material with a two-step process by in situ polymerization, and characterized the microcosmic features, chemical structure, and thermal performance of the microcapsules by SEM, FTIR, DSC, and TGA. We studied the effects of different experimental parameters of curing pH, the amounts of the emulsifier and emulsion speed. The CMC-UF microcapsules had good heat resistance and stability. The enthalpy of CMC-UF microcapsules reached 50.33 J g−1. Therefore, CMC-UF resin can be used as a potential wall material of phase change materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phase change materials (PCMs) as novel energy materials have great potential as heat exchangers (Zhao and Zhang 2011; Wang et al. 2006; Lai et al. 2010) and phase change architecture materials (Stritih and Butala 2010; Alawadhi and Alqallaf 2011; Li et al. 2010a, b), as well as in such fields as textiles (Li et al. 2007), refrigeration (Cheng et al. 2011; Fang et al. 2006; Lu and Tassou 2013), and military stealth (Fei et al. 2007; Sun et al. 2009; Wu et al. 2006; Li et al. 2010a, b). Urea-formaldehyde (UF) resin is one of the most popular shell materials of PCMs. Preparation of UF resins require large amounts of formaldehyde, which is highly toxic to the human body and the environment. Thus, it makes great sense to prepare low molar-ratio UF wall materials, whereby the consumption of formaldehyde, the content of free formaldehyde, and the emission of formaldehyde from UF are reduced (Fan and Li 2006).
The shell materials are primarily melamine–formaldehyde (MF) resins (Su et al. 2011; Fallahi et al. 2010) and UF resins (Qin et al. 2012) because of their high mechanical strength and chemical stability (Zhao and Zhang 2011). UF resins are crosslinked products of urea and formaldehyde with mature preparation technology and low cost. These were chosen as shell materials. However, UF resins are brittle, tend to release formaldehyde as they age, and have poor barrier properties as shell materials of PCMs. As a modifier, CMC can increase the degree of crosslinking among the resins and reduce the existence of free formaldehyde. CMC helps UF resin by improving the fragility and barrier properties.
Generally, urea and formaldehyde generate small-molecule monomers in weak base environments for the carboxymethylation reaction. These monomer molecules can be attached to the surface of paraffin droplets and cure to form a large number of insoluble cross-linked resins in acidic environments. Dong (2011) studied curing characteristics and plywood performance UF resins with a 1:1 molar ratio of formaldehyde to urea and using ammonium chloride as the curing agent. The solidified UF resins and plywood performed well. The methods used in the adhesive modification can also be applied in the modification of urea formaldehyde paraffin PCMs to reduce the content of free formaldehyde in shell materials. Thus, it has promising prospects. Jin et al. (2008) used UF resins with a 1:1.5 molar ratio of U/F as wall materials and HSMA as an emulsifier to synthesize UF paraffin PCMs. The product had a higher phase change enthalpy approaching that of pure paraffin. After being used in several heating/cooling cycles, the shape of the product remained unchanged. Hu et al. (2013), using CMC-modified melamine resins as wall materials and OP-10 as an emulsifier, successfully prepared modified melamine capsules. The average particle size of the melamine capsules was 50 nm, and the enthalpy of the capsules was 83.46 J g−1. The content of formaldehyde generally affects the coating situation, barrier properties, and content of free formaldehyde of the products. It is the trend to obtain products yielding low formaldehyde emission or entirely maldehyde-free (Salunkhe and Shembekar 2012). Thus, low-molar ratio UF resins can be used as wall materials. Some modifiers are added to UF resin wall materials to reduce the content of free formaldehyde while improving the usage efficiency of formaldehyde.
We prepared CMC-UF paraffin microcapsules in a two-step process by in situ polymerization. As a modifier, CMC took part in the reaction based on the hydroxyl groups of CMC. We discuss here the preparation conditions and performances of UF paraffin microcapsules modified by CMC. The crystalline structure of CMC increased the compactness of UF wall materials. To date, few studies on core–shell PCMs with UF resins modified by CMC as shell materials have been reported.
Materials and methods
Materials
Urea was purchased from Tianjin Yongda Chemical Reagent Co., Ltd. (Tianjin, China). Formaldehyde (37 %) was purchased from Tianjin Tianli Chemical Reagent Co., Ltd. (Tianjin, China). CMC (Mw = 160.22) and emulsifier OP-10 were obtained from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Triethanolamine was purchased from Tianjin Hongyan Chemical Reagent Co., Ltd. (Tianjin, China). Cyclohexane was purchased from Kermel Chemical Reagents Co., Ltd. (Tianjin, China). Anhydrous ethanol was purchased from Tianjin Fu Yu Fine Chemical Co., Ltd. (Tianjin, China). Paraffin was obtained from Ruhr Energy Technology Co., Ltd. (Hangzhou, China).
Preparation of CMC-UF paraffin microcapsules
Formaldehyde, urea and deionized water were mixed and adjusted to pH of 8–9 with triethanolamine. The mixture was stirred for 60 min at 70 °C. CMC was added to the solution, and mixed for 15 min in an ice bath to obtain the prepolymer solution. OP-10 and paraffin melt were added to the mixture of water and cyclohexane and dispersed at high stirring speed for 15 min to form an oil-in-water dispersion. The paraffin dispersion solution was transferred to a three-necked flask and heated to 80 °C. Then, the prepolymer solution was dropped into the emulsion under stirring, and pH was adjusted to 2.5–3.5 with citric acid. About 2 h later, a CMC-UF paraffin microcapsule solution was obtained. Finally, the solution was filtered, washed with distilled water, and dried at 30 °C for 48 h to obtain the CMC-UF microcapsules. CMC amounts of 0, 0.25, 0.50, and 0.75 g were used to obtain samples A2, A3, A4, and A5, respectively.
Characterization
The Fourier transform-infrared (FTIR) spectra of the CMC-UF microcapsules were recorded with a Thermo Fisher Nicolet 6700 FTIR spectrometer (USA). An FEI QUANTA 200 (USA) scanning electronic microscope was used to observe the surface morphologies of the products. The average diameters of the capsules were measured using a Brookhaven ZetaPlus zeta potential analyzer (USA). The thermal performances of the products were measured using a TA Q20 (USA) differential scanning calorimeter at a heating rate of 5 °C min−1 from 5 to 65 °C under nitrogen atmosphere. Thermogravimetric analysis was performed using a TA Q50 (USA) thermal analyzer from 25 to 500 °C at a heating rate of 10 °C min−1 under nitrogen atmosphere.
Results and discussion
Structural characterization of the CMC-UF microcapsules
The FTIR spectra of CMC and modified UF paraffin microcapsules with differing CMC contents are shown in Fig. 1. The broad bands of stretching vibrations of N–H and –OH groups in the 3,330 cm−1 region were observed for A2, A3, A4, and A5 (Fang et al. 2009). The asymmetric and symmetric –CH2 stretching vibration bands at 2,920 and 2,851 cm−1 increased significantly in A3, A4, and A5, possibly because CMC participated in the polymerization and more –CH2 was formed. The peaks around 1,625 and 1,560 cm−1 for A2, A3, A4, and A5 correspond to absorption peaks of C=O stretching vibration and C–N and N–H deformation vibration (Xie et al. 2008), mostly due to the formation of HN–C=O in the polymerization process. The results indicated that UF paraffin microcapsules were prepared successfully. We attribute the two absorption peaks at 1,440 and 1,389 cm−1 to C–H bending vibration. The band at 1,039 cm−1 was related to C–O stretching vibration, and the band at 1,000 cm−1 was ascribed to O–H stretching vibration (Li et al. 2010a, b). Absorbance was also apparent at 3,330 cm−1, showing that a large number of –OH groups existed in the product; these groups increase with increasing CMC content, possibly due to unreacted OH groups in CMC. The band at 1,039 cm−1 was attributed to asymmetric stretching vibration of the C–O–C (Yuan et al. 2006). The strength of bands at 2,920, 2,851, 1,246, and 1,139 cm−1 increased with increasing amounts of CMC (Hu et al. 2014). The sensitive changes in the FTIR spectra indicated that CMC participated in the poly-condensation reaction.
SEM of the CMC-UF microcapsules
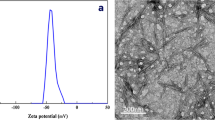
SEM images of UF paraffin microcapsules and CMC-UF paraffin microcapsules are shown in Fig. 2. SEM images of the unmodified UF microcapsules are shown in Fig. 2a, b. In Fig. 2a, b, the particle size distribution of the CMC-UF paraffin microcapsules was heterogeneous. The shapes of the microcapsules were irregular. However, CMC modified UF microcapsules (Fig. 2c, d) were spherical or quasi-spherical with smooth surfaces, and the particle size was uniform. The probable reason for this was that the chemical structures of the resin were changed, and the reaction rate of the prepolymer was reduced due to the addition of CMC to the reaction.
Optical images of paraffin emulsions
To obtain a narrower size distribution of microcapsules, it is necessary to add a suitable amount of emulsifier to the core material and choose a suitable stirring rate because of its ability to form well-dispersed emulsion solutions (Sawada et al. 2003). Optical images of paraffin emulsions resulting from differing stirring rates are shown in Fig. 3. With increasing stirring rate, the diameters of paraffin droplets tended to decrease to form stable emulsion solutions (a > b > c > d). At this time, the mechanical shear stress produced by stirring overcomes the surface tension, and Gb decreases rapidly to a balanced state due to the attachment of OP-10 to the paraffin droplet surfaces.
Effects of the stirring rate and emulsifier dosage on the average particle diameter of CMC-UF microcapsules
Effects of the stirring rate and emulsifier dosage on the average particle diameter of CMC-UF microcapsules are shown in Fig. 4. The stirring rate and emulsifier dosage are important factors affecting paraffin dispersion. These factors play important roles in preparing spherical core–shell PCMs. Generally, the stirring rate and emulsifier dosage have similar functions in the generation of homogeneous paraffin emulsions (Su et al. 2012; Chen et al. 2012). The average particle diameter of CMC-UF microcapsules changed significantly with changing OP-10 content and stirring speed (Fig. 4). At a given stirring speed, the average particle diameter of CMC-UF microcapsules decreased rapidly with an increase in the OP-10 content. When the OP-10 content reached 6–8 %, the average particle diameter of the CMC-UF microcapsules began to stabilize. The core material emulsion was uniform and stable when the core material emulsion was treated by 6–8 % OP-10. Meanwhile, paraffin and water also formed a homogeneous stable paraffin emulsion. As the amount of OP-10 increased to 12 %, this resulted in only limited further reduction in average particle diameter of the CMC-UF microcapsules. Similarly, the average particle diameter of CMC-UF microcapsules decreased rapidly with increasing stirring speed. At stirring speeds of 10,000–16,000 rpm, the particle size of microcapsules tended to stabilize. There were no clear changes with further increases in the stirring rate. This result indicated that paraffin was well dispersed and acceptably stable under these conditions.
Effects of curing pH on the enthalpy of the CMC-UF microcapsules
Effect of the curing pH on the enthalpy of the CMC-UF microcapsules is shown in Fig. 5. Enthalpy initially increased and then decreased slightly after pH reached 3.0. In order to obtain high enthalpy CMC-UF microcapsules, a suitable pH was 3.0. In our study, the enthalpy of CMC-UF microcapsules was low. The main reason was that CMC-UF wall materials had more linear structure rather than body structure. The linear structure of the CMC-UF wall materials caused part of the paraffin to be wrapped in the material. Therefore, the encapsulation efficiency of the CMC-UF microcapsules was low. Usually, it is necessary to adjust the content of formaldehyde to improve the encapsulation efficiency of CMC-UF microcapsules.
Thermal properties of CMC-UF microcapsules
The DSC curves of CMC-modified phase change microcapsules are shown in Fig. 6. The modified UF microcapsule had enthalpy of 50.33 J g−1, lower than that of pure paraffin. And the peaks of the paraffin and microPCMs differed because of the strong interface interactions between shell and core materials (Zeng et al. 2009; Pan et al. 2012), indicating that the modified UF shell materials affected the heat transfer rate of paraffin, and also illustrating that some of the paraffin was entrapped in CMC-UF microcapsules and the core–shell PCMs were obtained successfully.
PCMs should be stable within a given temperature range. They should also be suitable to be mixed or encapsulated with other materials to meet specifications for given practical applications (Salunkhe and Shembekar 2012; Raj and Velraj 2010; Zhang et al. 2010). As a result, the thermal stability of PCMs is very important.
TGA and DTG diagrams of microcapsules prepared with different CMC content are shown in Fig. 7. The curves a, b, and c show UF microcapsules without CMC and UF microcapsules modified with 0.5 and 0.75 g of CMC, respectively. The decomposition temperature of the product was above 120 °C. The initial weight loss temperature of the product changed slightly with increasing amounts of CMC. The results indicated that the chemical structure of the product was changed because CMC participated in the polycondensation reaction. This result is similar to previous findings. Yuan et al. (2006) found that the weight loss temperature (5 %) of poly (urea-formaldehyde) microcapsules was about 122 °C and the capsules were chemically stable below 238 °C. Xu et al. (2012) studied microencapsulated jasmine perfume with UF resin as shell material, and the capsules had good thermal stability. These results imply that our CMC-UF microcapsules were suitable for a range of low-temperature applications.
Conclusion
UF resin was modified by CMC to increase the compactness and to reduce the amount of formaldehyde. CMC-UF can be used as a potential phase change material for further research. The curing pH, amounts of CMC and emulsifier, and stirring rate were adjusted to obtain CMC-UF paraffin microcapsules with suitable properties. The effects of emulsifier content and stirring rate were important determinants of the morphology and particle size distribution of the resulting CMC-UF paraffin microcapsules. CMC-UF paraffin microcapsules had good thermal stability and enthalpy of 50.33 J g−1. This was because the main chemical structure of CMC-UF paraffin microcapsules was linear rather than a body structure. As a result, the paraffin coating was limited. It is necessary to increase the amount of formaldehyde and modifier to form body structure of CMC-UF resin to prepare high-encapsulation efficiency paraffin capsules.
References
Alawadhi EM, Alqallaf HJ (2011) Building roof with conical holes containing PCM to reduce the cooling load: numerical study. Energy Convers Manag 52(8–9):2958–2964
Chen ZH, Yu F, Zeng XR, Zhang ZG (2012) Preparation, characterization and thermal properties of nanocapsules containing phase change material n-dodecanol by miniemulsion polymerization with polymerizable emulsifier. Appl Energy 91:7–12
Cheng WL, Mei BJ, Liu YN, Huang YH, Yuan XD (2011) A novel household refrigerator with shape-stabilized PCM (phase change material) heat storage condensers: an experimental investigation. Energy 36:5797–5804
Dong YH (2011) Study on the curing characteristics and properties of low molar ratio urea-formaldehyde resin. M.S. thesis, Beijing Forestry University, Beijing, China
Fallahi E, Barmar M, Kish MH (2010) Preparation of phase-change material microcapsules with paraffin or camel fat cores: application to fabrics. Iran Polym J 19(4):277–286
Fan DB, Li JZ (2006) The research development of low molar ratio urea-formaldehyde resin adhesive. China Adhes 15(8):33–36
Fang GY, Xing L, Yang F, Li H (2006) Preparation and thermophysical performance investigation on a cool storage phase change material. Cryog Supercond 34(1):68–70
Fang G, Li H, Yang F, Liu X, Wu S (2009) Preparation and characterization of nano-encapsulated n-tetradecane as phase change material for thermal energy storage. Chem Eng J 153:217–221
Fei Y, Li G, Li Z, Tong L, Liang G (2007) Phase change materials and its application in thermal infrared camouflage. Infrared Technol 29(6):328–332
Hu XF, Huang ZH, Yu X, Li BZ (2013) Preparation and thermal energy storage of carboxymethyl cellulose-modified nanocapsules. BioEnergy Res 6:1135–1141
Hu X, Huang Z, Zhang Y (2014) Preparation of CMC-modified melamine resin spherical nano-phase change energy storage materials. Carbohydr Polym 101:83–88
Jin ZG, Wang YD, Liu JG, Yang ZZ (2008) Synthesis and properties of paraffin capsules as phase change materials. Polym 49:2903–2910
Lai CM, Chen RH, Lin CY (2010) Heat transfer and thermal storage behavior of gypsum boards incorporating micro-encapsulated PCM. Energy Build 42(8):1259–1266
Li H, Ma X, Zhang X, Xia S, Tang Z (2007) Composite of phase change materials and automatic temperature conditioning textiles made thereof. J Text Res 28(1):68–80
Li H, Liu X, Fang GY (2010a) Preparation and characteristics of n-nonadecane/cement composites as thermal energy storage materials in buildings. Energy Build 42:1661–1665
Li W, Liu CF, Zhang XX (2010b) Preparation of nanoencapsulated/microencapsulated phase change materials and its application in infrared camouflage. New Chem Mater 38(5):31–33
Lu W, Tassou SA (2013) Characterization and experimental investigation of phase change materials for chilled food refrigerated cabinet applications. Appl Energy 112:1376–1382
Pan L, Tao QH, Zhang SD, Wang SS, Zhang J, Wang SH, Wang ZY, Zhang ZP (2012) Preparation, characterization and thermal properties of micro-encapsulated phase change materials. Sol Energy Mater Sol Cells 98:66–70
Qin R, Xu GY, Guo L, Jiang Y, Ding RY (2012) Preparation and characterization of a novel poly (urea-formaldehyde) microcapsules with similar reflectance spectrum to leaves in the UV–Vis–IR region of 300–2500 nm. Mater Chem Phys 136:737–743
Raj VAA, Velraj R (2010) Review on free cooling of buildings using phase change materials. Renew Sustain Energy Rev 14(9):2819–2829
Salunkhe PB, Shembekar PS (2012) A review on effect of phase change material encapsulation on thethermal performance of a system. Renew Sustain Energy Rev 16:5603–5616
Sawada T, Korenori M, Ito K, Kuwahara Y, Shosenji H, Taketomi Y, Park S (2003) Preparation of melamine resin micro/nanocapsules by using a microreactor and telomeric surfactants. Macromol Mater Eng 288:920–924
Stritih U, Butala V (2010) Experimental investigation of energy saving in buildings with PCM cold storage. Int J Refrig 33:1676–1683
Su JF, Wang SB, Zhang YY, Huang Z (2011) Physico chemical properties and mechanical characters of methanol modified melamine-formaldehyde (MMF) shell microPCMs containing paraffin. Colloid Polym Sci 289:111–119
Su JF, Wang XY, Dong H (2012) Influence of temperature on the deformation behaviors of melamine-formaldehyde microcapsules containing phase change material. Mater Lett 84:158–161
Sun WY, Lv XL, Zheng YH, Chen LF (2009) Preparation of microencapsulated phase change materials and its application in IR stealth paint. J PLA Univ Sci Technol 10(2):156–159
Wang F, Zheng MY, Li ZJ, Lei BW (2006) Application of phase change material in solar assisted ground-source heat pump system. Acta Energ Sol Sin 27(12):1231–1234
Wu XS, Zhang XA, Liu CL, Wu WJ (2006) Primary research of applying phase change material into IR thermal image decoy. Electro Opt Technol Appl 21(3):35–37
Xie JQ, Feng FM, Hu W, Mao HJ, Yan H, Li C (2008) Preparation of a microcapsule containing the paraffin as the core material by in situ polymerization method without prepolymerization and capability of the microcapsule. J Funct Mater 39(2):293–296
Xu C, Cai X, Yang Y, Li Y, Peng M, Lv J (2012) Preparation and characterization of microencapsulated fragrance by in-suit polymerization. Fine Chem Intermed 42(3):58–60
Yuan L, Liang G, Xie JQ, Li L, GUO J (2006) Preparation and characterization of poly (urea-formaldehyde) microcapsules filled with epoxy resins. Polym 47:5338–5349
Zeng R, Wang X, Chen B, Zhang Y, Niu J, Wang X, Di H (2009) Heat transfer characteristics of microencapsulated phase change material slurry in laminar flow under constant heat flux. Appl Energy 86:2661–2670
Zhang H, Wang X, Wu D (2010) Silica encapsulation of n-octadecane via sol-gel process: a novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J Colloid Interface Sci 343:246–255
Zhao CY, Zhang GH (2011) Review on microencapsulated phase change materials (MEPCMs): fabrication, characterization and applications. Renew Sustain Energy Rev 15:3813–3832
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Yu Lei
Project funding: This work was financially supported by the Central University Basic Scientific Research Project of China (No. 2572014DB01).
The online version is available at http://www.springerlink.com
Rights and permissions
About this article
Cite this article
Huang, Zh., Yu, X., Li, W. et al. Preparation of urea-formaldehyde paraffin microcapsules modified by carboxymethyl cellulose as a potential phase change material. J. For. Res. 26, 253–260 (2015). https://doi.org/10.1007/s11676-015-0027-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-015-0027-y