Abstract
Microstructural characterization using scanning electron microscopy and scanning transmission electron microscopy (TEM/STEM) was carried out near the interface between the two AA6061 alloys that were hot isostatically pressed (HIP) to clad Zr laminated U-10 wt.% Mo metallic nuclear fuel. The HIP-bonded AA6061–AA6061 interface consisted of discontinuous layer of Mg2Si along with traces of fine MgO dispersoids and small precipitates of Al19(Fe, Cr, Cu)4MnSi2. To examine the presence of statistical variation, quantitative microscopy was also conducted, using several HIP’ed samples, to measure the relative linear density of the Mg2Si precipitates at the HIP bonded AA6061–AA6061 interface. In order to better understand the formation of discontinuous Mg2Si layer, solid-to-solid diffusion couple experiments were carried out using temperature and time relevant to HIP. The discontinuous Mg2Si layer was not observed in diffusion couples that were rapidly water-quenched, but those slowly cooled in air and in furnace developed the discontinuous Mg2Si. Presence of oxygen, confirmed by electron energy loss spectroscopy via STEM, at the interface would be the potential driving force for the migration of Mg and Si atoms, where Mg would preferentially react with oxygen to form MgO, and excess Mg would react with Si to form Mg2Si during cooling. Faster cooling after HIP may minimize the formation of excessive Mg2Si.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
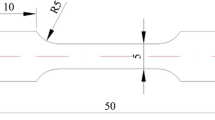
Materials management and minimization (M3) reactor conversion program, previously known as reduced enrichment for research in test reactors (RERTR) program, aims to replace high-enriched nuclear fuels with low-enriched ones in research and test reactors around the world.[1,2,3] For research and test reactors with very high uranium density, monolithic fuel design has been proposed, where in, a monolithic U-10 wt.% Mo (U10Mo) alloy fuel is laminated with Zr diffusion barrier by co-rolling, which is then cladded in AA6061 alloy using hot isostatic press (HIP).[4] Figure 1 shows a schematic design of the monolithic fuel system.
Several investigations[5,6,7,8] have examined the interdiffusion and reactions between the U10Mo fuel and Zr barrier, as well as the Zr barrier and AA6061 cladding alloy. During co-rolling, diffusional interaction between the U10Mo fuel and Zr barrier leads to the formation of α-Zr, δ-UZr2 and Mo2Zr at fuel/Zr interface. Park et al.[6] studied the effect of HIP parameters on the growth kinetics of these phases, and observed negligible change at the U10Mo/Zr interface during HIP. However, Park et al.[6] reported on a significant growth of (Al,Si)3Zr layer during HIP process at the Zr/AA6061 interface. Another investigation by Park et al.[9] on the decomposition of bcc γ-phase in the U10Mo fuel alloy during HIP has reported a more severe decomposition (i.e. γU → αU + γ′-U2Mo) with a slower cooling rate.
The HIP interface between the two pieces of AA6061 alloys (i.e. HIP bonded AA6061–AA6061) has garnered attention, because it was documented to be susceptible to cracking with applied shearing forces.[10] In this paper, we report on the results obtained from extensive microstructural characterization of HIP bonded AA6061–AA6061 (typically referred to as cladding–cladding) interfaces in eight different samples using electron microscopy and quantitative microscopy. Additional solid-to-solid diffusion couple investigation with varying anneal time and cooling rate was carried out to better understand microstructural development at the HIP bonded AA6061-AA6061 interface.
2 Experimental Procedure
2.1 HIP Bonded AA6061-AA6061 Interface
HIP bonding of AA6061–T6 to AA6061–T6 to clad Zr-laminated U10Mo fuel alloy was carried out at 560 °C for 90 min with a pressure of 15 ksi (103.42 MPa). Prior to the HIP, AA6061 surfaces were brushed using stainless steel bristle brush, and chemically cleaned using sodium hydroxide followed by nitric acid. Finally, AA6061 surfaces were cleaned with DI water. Details of the HIP process can be found elsewhere.[5,10,11,12] Figure 1 presents a schematic diagram of the monolithic fuel plate samples examined in this study. As indicated in Fig. 1, each sample was sectioned in two directions, i.e. transverse and longitudinal, along the HIP bonded cladding–cladding interface. These directions were identified based on the co-rolling step to produce Zr laminated U10Mo fuel prior to the HIP bonding. Direction along which the co-rolling was performed is defined as the longitudinal direction. In total 8 samples were analyzed in this study to examine any statistical variation.
2.2 AA6061 Versus AA6061 Diffusion Couples
In order to better understand the microstructural development at the HIP bonded AA6061–AA6061 interface, five AA6061–T6 versus AA6061–T6 diffusion couples were assembled and isothermally annealed at 560 °C with varying annealing time and cooling rate. Prior to assembling diffusion couples, all AA6061–T6 alloys were metallographically polished down to 1 µm finish, and then assembled in stainless steel jigs under the torque of approximately 2.5 N-m. Thin alumina discs were placed between AA6061 and stainless steel jigs to avoid any diffusional interaction. Then the assembly was placed in a quartz tube, evacuated to a pressure of 8.0 × 10−6 torr or better, and flushed with high purity argon and hydrogen gas. Evacuation and flushing was repeated 3 times, and the quartz tube was finally backfilled with high purity Ar before sealing the quartz tube. High purity Argon was backfilled to a pressure of 272 torr to provide atmospheric pressure at elevated temperature (560 °C). All samples were isothermally annealed at 560 °C (e.g., typical HIP temperature) using Lindberg blue three zone tube furnace. Three samples were annealed for 1.5, 24 and 240 h and subsequently water quenched to understand the effect of holding time on formation of Mg2Si. Other two samples were isothermally annealed at 560 °C for 1.5 h and then subsequently cooled in air and in furnace to examine the effect of cooling rate on the development of Mg2Si. Diffusion couples were sectioned and mounted in epoxy for metallographic examination.
2.3 Electron Microscopy and Quantitative Image Analysis
Microstructural features at the HIP bonded AA6061–AA6061 interface were initially examined using Zeiss™ Ultra-55 field emission scanning electron microscope (FE-SEM) equipped with x-ray energy dispersive spectroscopy (XEDS). In order to further investigate the phase constituent present at the HIP bonded AA6061–AA6061 interface, transmission electron microscopy (FEI/Tecnai™ F30 TEM) equipped with Fischione™ high angle annular dark-field (HAADF) detector was employed operating at 300 kV accelerating voltage. Elemental quantification via TEM was performed using x-ray energy dispersive spectroscopy (XEDS) and quantified phases were further corroborated using selected area electron diffraction (SAED). Presence of low atomic number elements (e.g., carbon, oxygen etc.) were examined using electron energy loss spectroscopy (EELS) on TEM. All TEM samples were prepared via in situ lift out (INLO) method using focused ion beam (FEI™ TEM 200-FIB).
Relative linear density of discontinuous precipitate layer of Mg2Si at the HIP bonded AA6061–AA6061 interface was examined using intercept method. The statistical variation was examined in 8 different samples, and over five randomly selected areas of HIP bonded AA6061–AA6061 interface for transverse and longitudinal directions in each sample. In this method, vertical lines with 10, 5, and 3 µm spacing, normal to the HIP interface, were superimposed over the backscatter electron (BSE) micrograph taken at 500X magnification. The 10, 5, and 3 µm spacing of the vertical lines were chosen given the major axis range (0.2-16 µm) of the Mg2Si precipitates along the interface. Image processing and analysis software, ImageJ™, was used to place the vertical lines, and the number of intercepts was counted to determine the percentage of intercept counts for each micrograph. To further ensure the statistical consistency, the image analysis was carried out at two more random depths (e.g., repeated metallographic grinding and polishing). Figure 2(a) shows a schematic illustration of the image analysis, and Fig. 2(b), (c) and (d) presents backscatter electron micrographs with superimposed vertical lines placed 10, 5, and 3 µm apart.
3 Results and Discussion
Figure 3 represents the typical backscatter electron micrograph from HIP bonded AA6061–AA6061 interface. Two types of precipitates were observed: white and dark precipitates. At the AA6061–AA6061 interface, the dark precipitates, labeled as Mg2Si were observed to be aligned along the HIP bond line. In fact, no distinct interfacial bond line could be observed between the two AA6061 alloys, except for the demarcation by the dark precipitates. White precipitates were present in both bulk alloy and at the interface, including within the dark precipitates, and were mostly fine, except for a few larger ones as presented in Fig. 3. They were rich in Al, Fe and Si, according the XEDS via SEM as reported in Table 1. Dark precipitates were heavily concentrated along the HIP bond line and their compositions corresponded to Mg2Si. Similar observations have been made by Jue et al.[10]
Figure 4 represents a HAADF TEM micrograph from the HIP bonded AA6061 versus AA6061 interface. A large precipitate of Mg2Si along with small oxide particles, labeled as MgO were observed at the interface. Elemental map at a higher resolution shown in Fig. 5 demonstrates that the particles at the interface is depleted of Al, rich in Mg and O, and suggests that the fine oxide particles may correspond to MgO. HAADF micrograph, shown in Fig. 6(a), shows the region selected for EELS elemental mapping and corresponding elemental maps of O and Mg are presented in Fig. 6(b) and (c) respectively. Presence of O and Mg was confirmed using the characteristic k-edge at 532 and 1305 eV, respectively. Figure 6(d) represents the characteristic energy loss k-edge after background subtraction. It should be noted that the elemental map of Mg in Fig. 6(c) shows relative depletion of Mg relative to the matrix, which is Mg2Si with higher Mg concentration than MgO. Presence of oxygen would promote the enrichment of Mg and Si near the interface, since they have high affinity for oxygen. Thermodynamically, Mg would preferentially form MgO with available oxygen, and the remaining Mg would form Mg2Si at the HIP interface upon cooling. Additional findings through extensive TEM/EELS analyses include: (1) no carbides and nitrides were found at and near the interface; (2) white precipitates rich in Al, Fe, and Si, did not contain any oxygen.
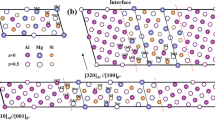
Phase identification was carried out using SAED pattern. Figure 7(a) represents bright field TEM micrograph of Mg2Si containing interfacial region where bulk Al matrix, Pearson—cF4; space group—Fm-3 m (225), was identified by SAED in Fig. 7(b) along [\( \bar{1} \)12] zone axis, Al lattice parameter was measured to be 4.05 Å. Figure 7(c) and (d) presents SAED patterns from Mg2Si precipitates along two zone axes i.e. [01\( \bar{3} \)] and [001], respectively. Diffraction pattern of Mg2Si phase was identified as cF12 (Pearson symbol) with Fm-3 m (225) space group symmetry and lattice parameter was measured to be 6.35 Å. Figure 8 presents SAED pattern from Al, Fe, Si rich, white precipitate along four zone axes i.e. [1\( \bar{1} \)1], [1\( \bar{1} \)3], [\( \bar{1} \)12] and [0\( \bar{2} \)5]. Indexed diffraction patterns corresponded to the Al19Fe4MnSi2 type intermetallic compound with cubic structure symmetry which belongs to space group Im \( \bar{3} \) (space group # 204) with Pearson symbol cI276. Lattice parameter of Al, Fe, Si rich white precipitates was measured to be approximately 12.49 Å, slightly lower than the reported value of 12.56 Å.[13] This discrepancy can arise from the presence of Cr and Cu, which based on Hume-Rothery rule, can substitute for Fe as Al19(Fe,Cr,Cu)4MnSi2. Composition determined for the white precipitates via TEM-XEDS is outlined in Table 1.
(a) Bright-field TEM micrograph of AA6061 vs. AA6061 cladding interface from sample 1 (transverse direction) used for diffraction studies. Selected area electron diffraction patterns obtained from (b) Al matrix in [\( \bar{1} \)12] zone axis. SAED pattern of Mg2Si precipitate in two different beam orientation (c) [01\( \bar{3} \)] and (d) [001]
Figure 9 presents relative linear density of Mg2Si precipitates along the HIP bonded AA6061–AA6061 interface determined by intercepts method described above. Average values ranged from 35 to 55 percent, and standard deviation, in general, decreased with a decrease in vertical line spacing. There was no significant difference between results obtained from transverse and longitudinal directions. One of the sample (sample no. 3) yielded a higher relative linear density, and this result was correlated to insufficient bonding and/or improper metallographic preparation (i.e. gaps), and not necessarily the development of Mg2Si precipitates, as shown in Fig. 10.
In order to better understand the formation of discontinuous layer of Mg2Si precipitates along the interface, diffusion couple experiments were carried out. Figure 11(a), (b) and (c) presents the backscatter electron micrographs of AA6061 versus AA6061 diffusion couples that were water quenched after annealing at 560 °C for (a) 1.5, (b) 24, and (c) 240 h. The bond line was almost indistinguishable from the bulk, and no significant difference was observed as a function of anneal time. In all three diffusion couples, Mg2Si precipitates were absent because the anneal temperature of 560 °C is higher than the solution heat treatment temperature for AA6061,[14] and the water-quench prevented any observable formation.
Microstructure of AA6061 vs. AA6061 diffusion couple obtained by water-quenching after isothermal annealing at 560 °C for (a) 1.5 h, (b) 24 h, (c) 240 h. Microstructure of AA6061 vs. AA6061 diffusion couple obtained by (d) air-cooling and (e) furnace-cooling after isothermal annealing at 560 °C for 1.5 h. (f) Cooling curves for water-quench, air-cooling and furnace-cooling measured in present diffusion couple study
Figure 11(d) and (e) presents the backscatter electron micrographs of AA6061 versus AA6061 diffusion couples that were air cooled and furnace cooled, respectively, after annealing at 560 °C for 1.5 h. Inset in Fig. 11(d) presents a higher magnification micrograph of Mg2Si and arrows in Fig. 11(e) corresponds to the Mg2Si discontinuous layer. Measured cooling rate of diffusion couples are presented in Fig. 11(f). Discontinuous layer of Mg2Si, similar to those observed at the HIP bonded AA6061–AA6061 interface, was observed in both air and furnace cooled diffusion couples. The formation of Mg2Si at the initial contact interface is more pronounced for the furnace cooled diffusion couple.
Schmidt et al.[15] studied the room-temperature mechanical behavior of Mg2Si and observed the slow crack growth, chipping and spalling tendencies with indentation. Average Young’s modulus, hardness and fracture toughness was reported to be 117.3 GPa, 5.3 GPa and 1.3 MPa \( \sqrt m \), respectively. MgO has a high hardness and is susceptible to cracking under micro-indentation.[16] Presence of Mg2Si and MgO at the HIP bonded AA6061–AA6061 interface may affect the mechanical behavior of monolithic fuel system. Both phases would form due to the presence of excessive oxygen at the initial contact interface. Also in this study, using diffusion couples, we demonstrate that the formation of Mg2Si is independent of anneal duration, and could be prevented by high cooling rate. Park et al.[9] also recommended faster cooling rate due to decomposition of the γ phase into γ′ and α phases in the U10Mo fuel alloy.
4 Summary
Microstructural constituents at the HIP bonded AA6061–AA6061 interfaces in longitudinal and transverse directions, were characterized using SEM and TEM. Interfaces mainly consisted of micro-scale Mg2Si, nanoscale MgO dispersoids and minor amount of Al19(Fe,Cr,Cu)4MnSi2 precipitates. A good bond quality was observed in general for all the samples where the interfaces were identified by locating Mg2Si precipitates along the cladding–cladding interface. Linear density measurement for Mg2Si at the AA6061–AA6061 interface demonstrated no significant difference in transverse and longitudinal direction.
Presence of oxygen would have led to diffusion of Mg and Si atoms towards the AA6061–AA6061 interface, where Mg atoms would preferentially react with oxygen to form MgO and remaining of Mg and Si atoms would combine to forms Mg2Si precipitates. Presence of MgO and Mg2Si may influence the quality of the AA6061–AA6061 interface. It was demonstrated by a diffusion couple investigation that the Mg2Si formation at the AA6061–AA6061 interface could be minimized by adopting higher cooling rates after HIP.
References
J.L. Snelgrove, G.L. Hofman, M.K. Meyer, C.L. Trybus, and T.C. Wiencek, Development of Very-High-Density Low-Enriched-Uranium Fuels, Nucl. Eng. Des., 1997, 178(1), p 119-126
J.L. Snelgrove, G.L. Hofman, C.L. Trybus, and T.C. Wiencek, Development of Very-High-Density Fuels by the RERTR Program, 19th International Meeting on Reduced Enrichment for Research and Test Reactors (RERTR); Seoul (Korea, Republic of), (1996)
D.D. Keiser, S.L. Hayes, M.K. Meyer, and C.R. Clark, High-Density, Low-Enriched Uranium Fuel for Nuclear Research Reactors, JOM, 2003, 55(9), p 55-58
G.A. Moore, F.J. Rice, N.E. Woolstenhulme, W.D. Swank, D.C. Haggard, J.-F. Jue, B.H. Park, S.E. Steffler, N.P. Hallinan, M.D. Chapple, and D.E. Burkes, Monolithic Fuel Fabrication Process Development at the Idaho National Laboratory, RERTR 2008-30th International Meeting on Reduced Enrichment for Research and Test Reactors, 2008
Y. Park, N. Eriksson, D. Keiser, J. Jue, B. Rabin, G. Moore, and Y. Sohn, Microstructural Anomalies in Hot-Isostatic Pressed U–10 wt.% Mo Fuel Plates with Zr Diffusion Barrier, Mater. Charact., 2015, 103, p 50-57
Y. Park, J. Yoo, K. Huang, D. Keiser, J. Jue, B. Rabin, G. Moore, and Y. Sohn, Growth Kinetics and Microstructural Evolution During Hot Isostatic Pressing of U-10 wt.% Mo Monolithic Fuel Plate in AA6061 Cladding with Zr Diffusion Barrier, J. Nucl. Mater., 2014, 447(1), p 215-224
K. Huang, Y. Park, D. Keiser, Jr., and Y. Sohn, Interdiffusion Between Zr Diffusion Barrier and U-Mo Alloy, J. Phase Equilib. Diffus., 2012, 33(6), p 443-449
J. Dickson, L. Zhou, A.P.Y. Puente, M. Fu, D. Keiser, and Y. Sohn, Interdiffusion and Reaction Between Zr and Al Alloys from 425 to 625 °C, Intermetallics, 2014, 49, p 154-162
Y. Park, N. Eriksson, R. Newell, D. Keiser, and Y. Sohn, Phase Decomposition of γ-U (BCC) in U-10 wt% Mo Fuel Alloy During Hot Isostatic Pressing of Monolithic Fuel Plate, J. Nucl. Mater., 2016, 480, p 271-280
J.-F. Jue, D.D. Keiser, C.R. Breckenridge, G.A. Moore, and M.K. Meyer, Microstructural Characteristics of HIP-Bonded Monolithic Nuclear Fuels with a Diffusion Barrier, J. Nucl. Mater., 2014, 448(1), p 250-258
J. Crapps, K. Clarke, J. Katz, D.J. Alexander, B. Aikin, V.D. Vargas, J.D. Montalvo, D.E. Dombrowski, and B. Mihaila, Development of the Hot Isostatic Press Manufacturing Process For Monolithic Nuclear Fuel, Nucl. Eng. Des., 2013, 254, p 43-52
G. Moore, J. Jue, B. Rabin, and M. Nilles, Full Size U-10Mo Monolithic Fuel Foil and Fuel Plate Fabrication-Technology Development, Proceedings of the Research Reactor Fuel Management Conference, 2010
P. Villars and L. Calvert, Pearson’s Handbook of Crystallographic Data for Intermetallic Phases, Second Edition, 1991, 1, p 825
H. Chandler, Heat Treater’s Guide: Practices and Procedures for Nonferrous Alloys, ASM international, 1996, p 201–205
R.D. Schmidt, E.D. Case, J. Giles, III, J.E. Ni, and T.P. Hogan, Room-Temperature Mechanical Properties and Slow Crack Growth Behavior of Mg2Si Thermoelectric Materials, J. Electron. Mater., 2012, 41(6), p 1210-1216
R.W. Armstrong, Dislocation Pile-Ups: From 110 Cracking in MgO to Model Strength Evaluations, Mater. Sci. Eng. A, 2005, 409(1), p 24-31
Acknowledgments
This work was supported by the US Department of Energy, Office of Nuclear Materials Threat Reduction (NA-212), National Nuclear Security Administration, under DOE-NE Idaho Operations Office Contract DE-AC07-05ID14517. Accordingly, the US Government retains a non-exclusive, royalty-free license to publish or reproduce the published form of this contribution, or allow others to do so, for US Government purposes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehta, A., Zhou, L., Schulz, E.A. et al. Microstructural Characterization of AA6061 Versus AA6061 HIP Bonded Cladding–Cladding Interface. J. Phase Equilib. Diffus. 39, 246–254 (2018). https://doi.org/10.1007/s11669-018-0629-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-018-0629-0