Abstract
A thermodynamic description of the Cu-Ni-Si ternary system has been carried out by using the calculation of phase diagrams method. Among the three binary sub-systems, Cu-Ni, Cu-Si and Ni-Si, are taken from earlier assessments and the Cu-Ni-Si ternary system are optimized in this study by using the reported experimental data. Six isothermal sections and four vertical sections with the experimental data are presented, along with the liquidus projection and the reaction scheme. Comprehensive comparisons between the calculated and measured phase diagrams show that the latest experimental information can be satisfactorily accounted for by the present thermodynamic description.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that copper-based alloys exhibit excellent electrical conductivity and improved mechanical properties. The Cu-Ni-Si alloys play an important role in the industry field for the application in lead frames and connectors.[1] Up to now, researchers have been concentrating on understanding processing and compositional optimization of these Corson-type alloys due to their excellent combination of high strength, conductivity and thermal stability.[2] Because of its broad potential application prospect, comprehensive information about the thermodynamic description of the Cu-Ni-Si ternary system is of great value.
Sun et al. investigated the isothermal section at 700 °C recently.[3] Afterwards, Liu et al.[4] established comprehensive isothermal sections in the system at 800, 900 and 1000 °C. The binary phase diagrams of the Cu-Ni,[5] Cu-Si[6] and Ni-Si[7] were calculated earlier. A thermodynamic description in the copper-rich corner of the Cu-Ni-Si ternary system has been published by Miettinen.[8] However, the set of parameters does not apply in the whole system considering the latest experimental data.[3,4] Thus a thermodynamic assessment of the Cu-Ni-Si ternary system is essential.
The present work aims at a critical evaluation of the Cu-Ni-Si ternary system using the CALPHAD method, as well as developing a set of self-consistent thermodynamic parameters for this ternary system.
Experimental Information
The Cu-Ni-Si ternary system was comprehensively studied by Okamoto,[9–11] several isothermal and vertical sections as well as the liquidus projection were reported. Review of the experimental determination of the ternary phase equilibria was given by Kumar et al.[12] However, according to his review, the data are questionable due to the differences of the homogeneity ranges of some phases.
Recently, Liu et al.[4] have investigated the system at 800, 900, 1000 °C, which provides sufficient data combined with Sun et al.’s[5] work at 700 °C for the present assessment. In their work, the γ (Cu56Si11), θ (Ni2Si), γ′ (Ni5Si2) and δ (Ni2Si) phases show a noticeable solubility of a third element in the ternary system. The γ (Cu56Si11) and θ (Ni2Si) phases can be stabilized by substituting Ni and Cu respectively. The ternary compound τ1 has previously observed by Okamoto[10] below 850 °C around Cu60.9-66.3Ni9.9-11.2Si23.4-28.3, which has also been reported to be formed via the peritectic reaction, liquid + γ (Cu56Si11) + θ (Ni2Si) ↔ τ1, at 859 °C.[3,10] It was corroborated by the experimental data of electron probe microanalysis (EPMA) and x-ray diffraction (XRD): the γ (Cu56Si11) can dissolve a large amount of Ni up to 21.9 at.% mainly by substituting Ni for Cu at 700 °C. The solubility of Ni in the γ (Cu56Si11) phase varies from 5.67 to 21.37 at.% at 800 °C, and there still exists a homogeneous region with the solubility of Ni ranging from 16.09 to 22.71 at.% at 900 °C, however, not observed at 1000 °C. The τ2 phase is only observed at 700 °C and the τ1 disappears at 1000 °C. The present work takes Liu et al.[4] and Sun et al.’s experimental data for the assessment.
Thermodynamic Models
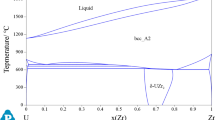
Figure 1 shows the calculated phase diagrams for binaries Cu-Ni,[5] Cu-Si[6] and Ni-Si.[7] They agree well with the experimental phase equilibrium data as presented in these studies.
As there are many studies concerning about the optimization of the Ni-Si system, the optimization of the ternary Cu-Ni-Si system was begun with checking the three binary Ni-Si descriptions.[7,13,14] In both Lindholm[14] and Du[13]’s works, the fcc solubility was too high in regard to the experimental observations made by Okamoto.[9–11] In Tokunaga’s work,[7] the agreement was better through integrated into account. In this work, we treat the ordered β1 (Ni3Si) phase as a simple stoichiometric phase.
The phase equilibria of the Cu-Ni-Si system in the copper-rich corner above 700 °C have been reviewed by Miettinen.[8] Due to the numerous silicides of binaries Cu-Si and Ni-Si, the ternary system is quite complicated. The following phases and their phase equilibria are present: liquid, fcc, bcc, Cu19Si6 (η), Cu56Si11 (γ), Ni5Si2 (γ′), Ni2Si (δ), Ni2Si (θ), Ni3Si_β1, Ni3Si_β2, Ni3Si_β3, NiSi, NiSi2 (α), τ1 and τ2. The disordered solution phases, liquid, fcc, and bcc are described with the substitutional solution model. The ternary γ (Cu56Si11) phase is a nonstoichiometric compound expanding from Cu56Si11 towards Ni56Si11 but it has a large homogeneity range. The γ phase was treated as a simple stoichiometric phase in the earlier Cu-Mn-Si[15] and Cu-Fe-Si[16] descriptions, which allows an easy extension of the phase in a multicomponent system. However, Novikov and Dautova[17] treated the γ phase as a ternary compound. In the present work, we also treat it as a ternary compound with the sublattice model (Cu,Ni)0.62(Cu,Ni)0.18(Si)0.2, which gives a better agreement with the experimental data. The τ1 and τ2 phases are modeled as (Cu,Ni)0.63(Ni)0.1(Si)0.27 and (Cu,Ni)0.46(Ni)0.25(Si)0.29, respectively.
Liquid and Solid Solution Phases
By applying the substitutional solution model to the solution phases of the Cu-Ni-Si system, the molar Gibbs energy of the phases becomes
where G ϕ i is the molar Gibbs free energy of pure element i in the structure ϕ phase, and the term ex G ϕ is the excess free energy, which is expressed by the Redlich-Kister polynomials[18] as:
The binary and the ternary interaction parameters n L ϕ i,j and n L ϕ i,j,k take the following form:
where a, b and c are the coefficients to be optimized.
In the Cu-Ni-Si ternary system, there is a magnetic contribution to Gibbs free energy for the (Cu, Ni, Si) phase due to the present of Ni. The term mag G ϕ is expressed as follows.[19]
where βϕ is a quantity related to the total magnetic entropy, which in most cases is set to Bohr magnetic moment per mole of atoms; f(τϕ) is a polynomial function of τ, and T ϕc is the critical temperature for magnetic ordering, and f(τϕ) represents the polynomials obtained by Hillert and Jarl[20] as follows:
where D = (518/1125) + (11692 + 15975)((1/p) − 1) and p depends on the structure, 0.4 for the bcc structure and 0.28 for others.
The Binary Phases with Ternary Solubilities
Some phases in the Cu-Ni-Si system are formed from the terminal binary phases extending into the ternary system. The phases NiSi, NiSi2 are extended from the binary phases NiSi, NiSi2 respectively with partial Si substituted by Cu.
The Gibbs free energy of the phase ϕ \( (\upphi = {\text{NiSi or }}\upalpha ) \) with the sublattices (Ni)a(Cu,Si)c is expressed as follows:[21]
The phases δ, γ′, β1, β2, are extended from the binary phases δ (Ni2Si), γ′ (Ni5Si2), β1 (Ni3Si), β2 (Ni3Si) respectively with partial Ni substituted by Cu. The phase η is extended from the binary phase η (Cu19Si6) with partial Cu substituted by Ni.
The Gibbs free energy of the phase ϕ \( (\upphi = \delta , \, \upgamma^{'} , \, \upbeta_{1} { , }\upbeta_{ 2} {\text{ or }}\upeta ) \) with the sublattices (Ni,Cu)a(Si)c is expressed as follows:
where \( y_{ * }^{\text{I}} \) and \( y_{ * }^{\text{II}} \) are the site fractions of a certain component located on sublattice I and II, respectively, and the parameters 0 G m:n represents the Gibbs free energy of the compound phase when the two sublattices are occupied by element m or n. L Ni:Cu,Si and L Ni,Cu:Si are the interaction parameter between Cu and Si as well as Ni and Cu in the second or first sublattice, when the other sublattice is occupied by element Ni or Si. 0 G m:n, L Ni:Cu,Si and L Ni,Cu:Si are evaluated in the present work.
Ternary Compounds
As mentioned above, the γ (Cu56Si11) phase was treated as a ternary compound and the θ phase was modeled as (Ni)(Cu,Ni,Va)(Si), the τ1 and τ2 phases were modeled as (Cu,Ni)0.63(Ni)0.1(Si)0.27 and (Cu,Ni)0.46(Ni)0.25(Si)0.29 respectively. The Gibbs free energy of the phase \( \upphi \,(\upphi = \upgamma , \, \theta , \, \uptau_{1} {\text{ or }}\uptau_{2} ) \) with the sublattices (A 1, A 2) a (B 1, B 2) b C is expressed as follows:
where \( y_{ * }^{\text{I}} \) and \( y_{ * }^{\text{II}} \) are the site fractions of a certain component located on sublattice I and II, respectively, and the parameters 0 G A:B:C represents the Gibbs free energy of the compound phase when the two sublattices are occupied by element A, B or C. \( L_{{A_{1} ,A_{2} :B_{1} :C}} \)and \( L_{{A_{1} ,A_{2} :B_{2} :C}} \) are the interaction parameter between Cu and Ni in the first sublattice, when the second sublattice is occupied by element Cu or Ni, the third sublattice is occupied by element Si. \( L_{{A_{1} :B_{1} ,B_{2} :C}} \) and \( L_{{A_{2} :B_{1} ,B_{2} :C}} \) are the interaction parameter between Cu and Ni in the second sublattice, when the first sublattice is occupied by element Cu or Ni, the third sublattice is occupied by element Si. 0 G A:B:C , \( L_{{A_{1} ,A_{2} :B_{1} :C}} ,\,L_{{A_{1} ,A_{2} :B_{2} :C}} ,\,L_{{A_{1} :B_{1} ,B_{2} :C}} \) and \( L_{{A_{2} :B_{1} ,B_{2} :C}} \) are evaluated in the present work.
All the thermodynamic models adopted in this work are listed in Table 1.
Results and Discussions
Based on the recent experimental data, the thermodynamic parameters of each phase in the Cu-Ni-Si system are evaluated using the CALPHAD method. Four calculated isothermal sections of this system at 700, 800, 900 and 1000 °C are presented in Fig. 2-5. At 900 °C, the Liquid + NiSi + α NiSi2 region shows a little discrepancy with the experimental data. We attribute it to the fact that the composition of the liquid phase is not that precise with the poor measuring techniques. Apart from that, the consistency between the experimental data and the calculated results is a good indicator of the accuracy of the proposed assessment. It was found that the γ (Cu56Si11) and θ (Ni2Si) phases can be stabilized by the increasing temperature, which is also confirmed by the present calculation.[4] Then a prediction of the isothermal sections in the Cu-Ni-Si system at 600 and 1200 °C using the present parameters are presented in Fig. 6 and 7, respectively. It can be seen from Fig. 6 that both the τ1 and τ2 phases are stable, there exists an area of phase separation FCC1 + FCC2 at 600 °C and the area becomes larger with the temperature decreasing, the δ (Ni2Si) phase which has been identified responsible for the strengthening effect[22] has a solubility of Cu up to 11.2 at.%. At 1200 °C, the solubility of Cu is predicted to be about 5.1 at.% in the δ (Ni2Si) phase. Meanwhile, the Ni3Si_β2 phase will be stable instead of the Ni3Si_β1 phase. Figure 8 shows the calculated liquidus projection for the entire ternary system and also invariant reactions are presented in Table 2. The reaction scheme is presented in Table 3. Among all the seventeen invariant reactions, there are five eutectic transitions: liquid ↔ γ′ (Ni5Si2) + δ (Ni2Si) + β1 (Ni3Si) (1303.31 °C), liquid ↔ γ′ (Ni5Si2) + FCC + β2 (Ni3Si) (1143.95 °C), liquid ↔ γ (Ni56Si11) + η (Cu19Si6) + τ1 (862.19 °C), liquid ↔ γ (Ni56Si11) + η (Cu19Si6) + FCC (856.51 °C), liquid ↔ α (NiSi2) + η (Cu19Si6) + (Si) (771.20 °C). The calculated temperature of the peritectic reaction (liquid + γ (Cu56Si11) + θ (Ni2Si) ↔ τ1) is 878 °C, which agrees reasonablely well with the experimental data. We have also calculated the enthalpy of mixing in liquid Cu-Ni-Si alloys at 1575 K, at different composition ratios. As can be seen in Fig. 9, expect for the calculated Ni:Si = 4:1 curve shows more negative enthalpy values at high copper contents than the experimental data, the agreement with the experimental data is good on the whole.[23] The enthalpy of mixing in liquid Cu-Ni-Si alloys at 1576 K with Ni:Si = 1:1, at 1577 K with Ni:Si = 4:1, at 1579 K with Ni:Si = 1:4 are shown in Fig. 10. The Ni:Si = 4:1 curve shows a little more negative enthalpy values at high copper contents than the experimental data, the results are in good consistent with the experimental data.[23]
The thermodynamic description of the Cu-Ni-Si system is presented in Table 4. Figure 11-14 show four vertical sections with constant nickel contents of 1, 2, 3 wt.% and silicon content of 0.5 wt.%, respectively. In Fig. 13, the FCC + γ′ area shows a little discrepancy with the experimental data taken from Okamoto’s work.[10] As that experimental work was done long before, the only discrepancy is acceptable. On the whole, the calculated results agree reasonably well with the experimental data.[9–11]
Conclusion
On the basis of the latest literature data, a set of self-consistent parameters is obtained in the frame work of CALPHAD method. This set of parameters takes into account the newly found ternary compound τ2 and reproduces it very satisfactorily; comparisons between the isothermal sections, vertical sections, liquidus projection and invariant reactions show a good agreement with the present description; the liquidus projection and the invariant reaction scheme for the whole system can be used as predictive tool for future experimental studies and materials design.
Reference
S. Suzuki, N. Shibutani, K. Mimura, M. Isshiki, and Y. Waseda, Improvement in Strength and Electrical Conductivity of Cu-Ni-Si Alloy by Aging and Cold Rolling, J. Alloys Compd., 2006, 417, p 116-120
H.-A. Kuhn, I. Altenberger, A. Käufler, H. Hölzl, M. Fünfer, Properties of High Performance Alloys for Electromechanical Connectors, Copper Alloys-Early Applications and Current Performance-Enhancing Processes, L. Collini Ed., InTech, Rijeka, 2012, pp. 51-68
W.H. Sun, H.H. Xu, S.H. Liu, Y. Du, and B.Y. Huang, Phase Equilibria of the Cu-Ni-Si System at 700 °C, J. Alloys Compd., 2011, 509, p 9776-9781
X.J. Liu, S.L. Xiang, S.Y. Yang, R.P. Shi, and C.P. Wang, Experimental Investigation of Phase Equilibria in the Cu-Ni-Si Ternary System, J. Alloys Compd., 2013, 578, p 439-447
A. Jansson, TRITA-MAC-0340, Thermodynamic Evaluation of Zn-Ni System, Materials Research Centre, Royal Institute of Technology, Stockholm, 1987
X. Yan and Y.A. Chang, A Thermodynamic Analysis of the Cu-Si System, J. Alloys Compd., 2000, 308, p 221-229
T. Tokunaga, K. Nishio, H. Ohtani, and M. Hasebe, Development of Thermodynamic Modeling of Oxygen-Doped GaN Semiconductor, CALPHAD, 2003, 27, p 161-168
J. Miettinen, Thermodynamic Description of the Cu-Ni-Si System in the Copper-Rich Corner Above 700 °C, CALPHAD, 2005, 29, p 212-221
M. Okamoto, The Investigation of the Equilibrium State of the Whole System Copper-Nickel-Silicon, J. Jpn. Inst. Met., 1938, 2, p 211-232
M. Okamoto, The Investigation of the Equilibrium State of the Whole System Copper-Nickel-Silicon, J. Jpn. Inst. Met., 1939, 3, p 365-402
M. Okamoto, The Investigation of the Equilibrium State of the Whole System Copper-Nickel-Silicon, J. Jpn. Inst. Met., 1939, 3, p 411-420
K.C.H. Kumar, A. Kussmaul, H.L. Lukas, G. Effenberg, Cu-Ni-Si (Copper-Nickel-Silicon), Non-Ferrous Metal Ternary Systems. Selected Copper Systems: Phase Diagrams, Crystallographic and Thermodynamic Data, Materials Science International Team MSIT, Stuttgart, Germany, 2005, p 374-381
Y. Du and J.C. Schuster, Experimental Investigations and Thermodynamic Descriptions of the Ni-Si and C-Ni-Si Systems, Metall. Mater. Trans., 1999, 30, p 2409-2418
M. Lindholm and B. Sundman, A Thermodynamic Evaluation of the Nickel-Silicon System, Metall. Mater. Trans., 1996, 27, p 2897-2902
J. Miettinen, Thermodynamic Description of the Cu-Mn-Sn System in the Copper-Rich Corner, CALPHAD, 2004, 27, p 395-401
J. Miettinen, Thermodynamic Description of the Cu-Mn-Zn System in the Copper-Rich Corner, CALPHAD, 2004, 27, p 389-394
L.L. Novikov and L.L. Dautova, Study of the Copper Corner of the Cu-Ni-Si System, Russ. J. Inorg. Chem., 1957, 2, p 135
B. Sundman, B. Jansson, and J.-O. Andersson, The Thermo-Calc Databank System, CALPHAD, 1985, 9, p 153-190
N. Saunders and A.P. Miodownik, CALPHAD (Calculation of Phase Diagrams): A Comprehensive Guide: A Comprehensive Guide, Pergamon Press, Oxford, 1998
J. Miettinen, Thermodynamic Description of the Cu-Ni-Sn System at the Cu-Ni Side, CALPHAD, 2003, 27, p 147-152
M. Hillert and L.I. Stafansson, Regular-Solution Model for Stoichiometric Phases and Ionic Melts, Acta. Chem. Scand., 1970, 24, p 3618-3626
S.A. Lockyer and F.W. Noble, Precipitate Structure in a Cu-Ni-Si Alloy, J. Mater. Sci., 1994, 29, p 218-226
V. Witusiewicz, I. Arpshofen, H.-J. Seifert, F. Sommer, and F. Aldinger, Thermodynamics of Liquid and Undercooled Liquid Al-Cu-Ni-Si Alloys, Z. Metallkd., 2000, 91, p 128-142
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51031003 and 51171159), the Ministry of Education of China (Grant Nos. 20120120130004) and the National Key Basic Research Program of China (973 Program) (No. 2012CB825700). The supports from the China Aviation Industry Group are also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Zhu, J., Lu, Y. et al. Thermodynamic Description of the Cu-Ni-Si System. J. Phase Equilib. Diffus. 35, 93–104 (2014). https://doi.org/10.1007/s11669-013-0277-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-013-0277-3