Abstract
The effect of introducing Sb2O3 (antimony oxide) nanoparticles in epoxy coatings on mild steel was analyzed by electrochemical impedance spectroscopy (EIS) and scanning electrochemical microscopy (SECM) techniques in 3.5% NaCl. In order to disperse the nanoparticles properly and enable the interactions of nanoparticles chemically with epoxy resin, (3-mercaptopropyl)trimethoxysilane (MPTMS) was used to modify the nanoparticle. The charge transfer resistance (Rct) and the film resistance (Rf) were improved by incorporating Sb2O3 nanoparticles in the epoxy coating. The dissolution of iron at the scratch was detected using proper potential at the tip of the SECM in the Sb2O3-incorporated nanocomposite-coated sample. The presence of concentrated Sb was found in the scratched surface and examined by SEM/EDX analysis. FIB–TEM technique confirmed the elements present in the degradation products. The higher anti-corrosion properties of Sb2O3-grafted epoxy coating were achieved because surface modified Sb2O3 nanoparticles interacted chemically with the epoxy resin, which resulted in the deposition of the degradation products in the nano-level at the scratch.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In all industrial fields, corrosion becomes a big problem. The degradation of metals causes wastage of huge amounts of money. The corrosion causing components can penetrate all polymeric coatings [1,2,3,4]. One of the most common polymers used to protect steel is epoxy resin because it has higher resistive, adhesive, mechanical and protective properties [5,6,7]. An effective protective film between the metal and the corrosive ions is provided by the epoxy coatings to slow down the degradation of metals. The wear and abrasion of metallic surfaces cause the degradation of the epoxy-coated metals [8]. Pure epoxy-coated metals display weak resistance to corrosion because of the complicated cross-linked shape [9]. Therefore, the anti-corrosion properties of epoxy coating are improved by using various nanofillers [10,11,12,13,14,15,16,17,18]. The electrochemical, mechanical and adhesive properties of the nanocomposite coatings were extensively studied [19,20,21,22,23,24,25,26,27]. The increased anti-corrosion properties of nanoparticle-incorporated epoxy coatings have resulted in an increase in the number of investigations.
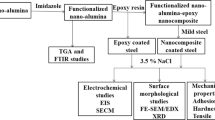
This study is aimed at examining the effect of grafting of Sb2O3 nanoparticles in epoxy resin on electrochemical, mechanical and adhesive properties in 3.5% NaCl. The surface of the nanoparticles was treated with (3-mercaptopropyl)trimethoxysilane (MPTMS) for enhanced dispersion of nanoparticles in the polymer as well as the enhanced chemical interactions of Sb2O3 and epoxy resin. The significance of surface modification of the nanoparticles by silane and subsequent interactions with polymeric component was studied by electrochemical impedance spectroscopy (EIS), scanning electrochemical microscopy (SECM) techniques and adhesive and mechanical testing methods in 3.5% NaCl at different immersion times. The surface morphological studies were carried out using scanning electron microscopy/energy-dispersive X-ray spectroscopy (SEM/EDX) and focused ion beam-transmission electron microscopy/energy-dispersive X-ray spectroscopy (FIB–TEM/EDX) analysis.
Experimental Procedure
Materials
Mild steel was used as the working electrode (composition in mass% was 0.1C, 0.2Si, 0.5Mn, 0.025P, 0.002S, 0.045Al, 0.005 N, 0.004O, balance Fe). Different grits of SiC were utilized for polishing the metal. Then, the sample was coated after rinsing with distilled water and acetone. Epoxy resin, MPTMS (98.8%) and acetone (95%) were obtained from Sigma-Aldrich. The average size of Sb2O3 nanoparticles was 60 nm and was received from Tokyo Chemical Industry Co., Ltd.
Surface Modification of Sb2O3 Nanoparticles
Sb2O3 nanoparticles (10 g) were placed at 140 °C for 2 h in a vacuum container. Then, the nanoparticles were dispersed in the container containing 100 ml of acetone with the help of magnetic stirrer (300 rpm) for 2 h at room temperature. Ultrasonication was carried out for one hour. Secondly, MPTMS (5 g) was mixed with the dispersion, followed by 24-h agitation. Finally, the residue was washed with acetone after centrifuging. After drying, the produced substance was placed at 60 °C for 48 h in an oven. The evaluation of the incorporation of MPTMS in Sb2O3 was conducted by FTIR and TGA analysis. FTIR spectroscopy used KBr pellets for carrying out the sample analysis. Thirty-five scans between the wavelengths of 500–4000 cm−1 at the resolution of 4 cm−1 were produced. TGA was conducted in the temperature range of 25–600 °C for investigating the thermal behavior of antimony as well as MPTMS/Sb2O3 nanoparticles.
Preparation of Nanocomposite
The prepared MPTMS/Sb2O3 nanoparticles were kept at 100 °C for 2 h to drive off absorbed water molecules. The MPTMS/Sb2O3 nanoparticles were dispersed slowly in acetone (75 ml g−1) for 10 min. To the epoxy resin, MPTMS/Sb2O3 nanoparticles were added slowly, followed by constant stirring using the magnetic stirrer (3000 rpm). After adding the curing agent to the dispersed epoxy-MPTMS/Sb2O3 at the ratio of 1:2, the components were thoroughly mixed. Pure epoxy and epoxy-MPTMS/Sb2O3 coatings were applied with the help of a drawdown bar coating system. Coating thickness of 30 μm was achieved.
SECM Studies
SECM was conducted in continuous immersion method to examine the corrosion protective performance of the epoxy coating as well as epoxy-MPTMS/Sb2O3 nanocomposite coating on steel in 3.5% NaCl solution. A continuous immersion method consisted of immersing the sample surfaces continuously in 3.5% NaCl solution. All the experiments were conducted at room temperature in a naturally aerated cell containing 3.5% NaCl solution. A platinum microelectrode (10 μm diameter) acted as the tip of the SECM. The tip of the platinum was utilized for scanning the scratched surface at the fixed height of 20 µm. For keeping the platinum microelectrode above the scratched surface, a microscope fitted with a video screen was used. Silver/silver chloride (reference), a platinum wire (counter) and coated sample (working) electrodes were used. A micro-flat cell was placed above the scratched area of the coating in a horizontal position. The scratched surface was scanned along the x-direction at the rate of 20 μm s−1 to find out the degradation properties of the substrate.
EIS Measurements
Electrochemical studies were carried out in continuous immersion method using a potentiostat/galvanostat (Autolab PGSTAT 30, Eco Chemie, B.V., and Netherlands). A normal three-electrode electrochemical system comprising of the reference electrode (Ag|AgCl), the counter electrode (Pt rod) and the working electrode (coated substrate) was utilized in this experiment. Frequency ranging from 40 kHz to 1 mHz was applied in this experiment. The polarization studies were done at ± 250 mV around the Ecorr with the scan rate of 0.1 mV s−1. Curve fittings were carried out to study the data.
Pull-off Adhesion Test
Pull-off adhesion test was done to investigate the consequences of coating strength on metal (Elcometer 106) per ASTM D 4541 standard. Pull-off adhesion testers measure the force required to pull a specified diameter section of the coating film away from its substrate. Adhesion strength was found by pulling off the coated sample beneath a rounded loop that was loaded in increasing amounts until the coating was removed from the substrate surface. Velocity of 10 mm/min was used to pull coating film from the metal.
Surface Morphological Studies
The enriched components present in the scratched area of the coating were examined by FE-SEM (field emission scanning electron microscope) and FIB-TEM (focused ion beam-transmission electron microscope, JEM-2100F). The coated sample was abraded using SiC paper and diamond paste after the corrosion test. A cross-sectional coated metal was examined by FE-SEM. Voltage of 30 kV was accelerated, and current of 15 μA was irradiated. Iron and antimony available in the degradation product were studied by EDX (energy-dispersive X-ray spectroscopy). The degraded metal complex was investigated by TEM/EDX analysis. FIB helps to retrieve the degraded metal complexes at the scratch, and the amount of iron and antimony in the accumulated product was determined by analyzing the line profile diagram.
Results and Discussion
FTIR Spectroscopy
FTIR spectroscopy of MPTMS, Sb2O3 nanoparticles and MPTMS-modified Sb2O3 nanoparticles is presented in Fig. 1. The peaks at 2935 cm−1 and 2845 cm−1 corresponded to the methyl groups [–(CH2)n–] of MPTMS-treated antimony oxide in the FTIR spectra. The band displayed at 960 cm-1 indicates that the –OCH3 group of MPTMS reacted with hydroxyl group forming Sb–O–Si bonds. The peaks at 515 cm−1 and 475 cm−1 are related to Sb–O–Sb and Si–O stretching because of surface modification of nanoparticle by MPTMS. The peak at 1560 cm−1 due to Si–O bond was also observed. Hence, it is confirmed that the –OH component on Sb2O3 nanoparticles (Sb (OH)3) surface interacted with methoxy component of MPTMS.
Thermogravimetric Analysis (TGA)
Figure 2 depicts thermogravimetric analysis of Sb2O3, as well as MPTMS, treated Sb2O3 nanoparticles. Initially, the removal of water molecules absorbed on the Sb2O3 surface takes place at the temperature of 60–160 °C. The loss of weight for Sb2O3 nanoparticles and surface-treated Sb2O3 nanoparticles was found to be nearly 0.3 and 1.2 wt.%, respectively. This indicates a higher amount of water absorbed by grafting of MPTMS with the nanoparticle. Weight loss also occurs in the temperature range of 250–660 °C. The evolution of H2O molecules by the reaction of hydroxyl component on antimony hydroxide surface resulted in the weight loss of 1.1 wt.% for Sb2O3 nanoparticles. Around 4.6 wt.% loss for the surface-treated Sb2O3 nanoparticles was measured because of the treatment of the Sb2O3 component, thermal degradation of MPTMS and water removal.
Electrochemical Impedance Spectroscopy (EIS) Measurements
The protective performance of pure epoxy and epoxy-MPTMS/Sb2O3 nanocomposite coatings on mild steel was investigated by the EIS technique in 3.5% NaCl. Figures 3 and 4 depict the spectra of EIS for pure epoxy and epoxy-MPTMS/Sb2O3 nanocomposite coatings on mild steel, respectively. The relevant Nyquist graphs are shown in Fig. 5. Figure 6 displays the Nyquist plots consisting of different wt.% of Sb2O3 nanoparticles in the polymeric matrix. It demonstrates the enhanced resistance with a rise in the wt.% of Sb2O3 nanoparticles in the coated surface. The appropriate equivalent circuit given in Fig. 7 is assigned to fit the impedance spectra. The capacitive and resistive characters of the coating are explained by impedance spectra. The resistive nature of the film (Rf) explains the coating properties at the region of high frequency, and the resistive behavior of charge transfer (Rct) demonstrates the electrochemical phenomena taking place on the coatings in the region of low frequency.
Furthermore, the capacitive behavior of the film (Cf) displays a reduced value in the region of high frequency, and the capacitive behavior of the double layer (Cdl) in the region of low frequency shows the enhanced value of 10−5–10−7 F cm−2. It is shown that the Rct value at 1 day is found to be 61 MΩ cm2 and reduced to 18 MΩ cm2 after 80 days for pure epoxy-coated metal sample. However, the Rct value of 218 MΩ cm2 for the epoxy-MPTMS/Sb2O3-coated mild steel is observed at 1 day and gradually decreased to 115 MΩ cm2 at 80 days. The higher Rct of epoxy-MPTMS/Sb2O3 nanocomposite coating (61 MΩ cm2) at 80 days in comparison with pure epoxy coating (18 MΩ cm2) demonstrates enhanced corrosion protection performance of epoxy-MPTMS/Sb2O3 nanocomposite coating.
The Rf demonstrates the movement of ions in the coated surface. The Rf of pure substrate showed 7.4 MΩ cm2 at 1 day and further declined to 1.5 MΩ cm2 at 80 days, whereas Rf value of epoxy–silane/Sb2O3-coated sample dropped from 21.7 MΩ cm2 at 1 day to 17.2 MΩ cm2 at 80 days. Therefore, the Rf value of the epoxy–silane/Sb2O3 coated metal surface exhibits an enhanced value in comparison with the epoxy-coated sample. The reduction in Rf value with extended test time indicates reduced inhibitive properties of the coating film. The rapid reduction of Rf value is hindered because of the appearance of the epoxy–silane/Sb2O3 degradation products at the scratched surface. The degradation product formed by epoxy–silane/Sb2O3 provides better corrosion protection and slows down the corrosion rate of the coated sample.
The value of Cdl was found to be 2.1 × 10−7 F cm−2 at 1 day and 5.4 × 10−6 F cm−2 at 80 days. However, the Cdl of epoxy-MPTMS/Sb2O3 nanocomposite coated substrate displays only 1.7 × 10−6 F cm−2 at 80 days. The corroded area below the film determines the Cdl value. Consequently, the lower Cdl value of epoxy-MPTMS/Sb2O3-coated sample shows little corrosion taking place under the coated film. Thus, a higher Rct, as well as lower Cdl, prevailed in the epoxy-MPTMS/Sb2O3-coated sample in the EIS spectral analysis. The degradation product comprised of Sb, and Si in the epoxy-MPTMS/Sb2O3 coating results in the enhanced resistance to corrosion.
The perforation of corrosive ions through the coating film produces reduced resistance and enhanced conductivity of the coating with a rise in test time. The ion movement in the film creates a conducting pathway at different places of the coating. This initiates reactions at the substrate/metal interface. The enhanced coating resistance and its increased strength demonstrated the usefulness of Sb2O3 nanoparticles for enhanced corrosion protection performance of the coated sample. The reduced ion movement in the epoxy-MPTMS/Sb2O3 coating is obvious in comparison with neat epoxy coating.
Scanning Electrochemical Microscopy (SECM) Analysis
Figures 8 and 9 represent two-dimensional SECM images of scratched epoxy and epoxy-MPTMS/Sb2O3-coated substrates, respectively, at the tip potential of + 0.60 V. The dissimilar color in the images of SECM demonstrates the behavior of corrosion in the coated sample. The scratched area displays enhanced current in comparison with the unscratched area. The current at the scratch of the epoxy-coated sample was found to be 4.2 nA (orange) at 1 day and reached 10.0 nA (pink) at 80 days. This indicates the enhanced dissolution of Fe with a rise in test time. However, the current at the scratched area of the epoxy/Sb2O3-coated surface is found to be 1.5 nA (orange) at 1 day and reaches 3.5 nA (cyan) at 80 days. It is noticeable that the Fe dissolution of the epoxy/Sb2O3-coated substrate is much less in comparison with the epoxy-coated surface.
Figure 10 displays the line scans of SECM across the scratches at Vtip of + 0.60 V on epoxy as well as epoxy-modified Sb2O3 nanocomposite-coated samples, respectively. From the figure, higher current at the scratch was measured in comparison with the unscratched area. Moreover, smaller current was measured at the scratch of the epoxy/Sb2O3 nanocomposite coating compared to the epoxy coating. This was due to the formation of degradation products containing antimony complexes on the metal surface, which hindered the dissolution of Fe in the epoxy/Sb2O3-coated surface.
Pull-Off Adhesion Test
The adhesion strength of epoxy and epoxy-MPTMS/Sb2O3 coatings was evaluated before and after immersion in 3.5% NaCl for various days, and the results are shown in Fig. 11. The adhesive strength of the epoxy coating before immersion was found to be 6.49 MPa, decreased to 5.99 MPa at 1-day immersion and finally reached 1.01 MPa at 80-day immersion. However, the adhesive strength of epoxy-MPTMS/Sb2O3 coating was found to be 7.02 MPa before immersion, lowered to 6.52 MPa at 1-day immersion and further reached 4.03 MPa at 80-day immersion. The higher adhesive strength was observed for the epoxy/Sb2O3 coating to the metal substrate than that of the epoxy coating after 80 days of immersion in the electrolyte. Adherence of antimony oxide nanoparticles/epoxy coating to the metal surface is strong because of the proper dispersion of MPTMS/Sb2O3 in the epoxy matrix. It is noticeable from the graph that the adhesion of the epoxy coating is enhanced due to the dispersion of MPTMS/Sb2O3 nanoparticles in the epoxy matrix. The incorporation of MPTMS/Sb2O3 in epoxy matrix results in vigorous adhesion to the metal substrate and produces strong corrosion protection.
Surface Morphological Studies
The elements present in the degradation products deposited on the scratched area of the coating were identified by SEM/EDX analysis after the electrochemical analysis. Figure 12 displays the analysis of epoxy-MPTMS/Sb2O3-coated substrate by SEM/EDX technique. EDX analysis confirmed the presence of antimony and iron in the degradation product of the epoxy-MPTMS/Sb2O3-coated scratched area. The corrosion products containing complex oxides of Sb and Fe are formed during corrosion testing. This investigation shows that the formation of metal oxide complexes comprised of antimony and iron as the corrosion products further enhances the corrosion protective performance of the epoxy-MPTMS/Sb2O3-coated substrate.
Figure 13 depicts the analysis of corrosion products by TEM. Elements present in the corrosion product were shown in the TEM images. The degradation product consisting of antimony, oxygen and iron was analyzed by EDX. The amount of O, Sb and Fe is found in Fig. 13b, on comparing with a spot in Fig. 13a. It is noticed that spots 1–6 contain a higher Sb and a lower amount of Fe. Thus, these degradation layers consisting of oxides of Sb and Fe magnify the resistance to corrosion of epoxy-MPTMS/Sb2O3-coated surface.
(a) Bright-field image and (b) line profile of EDX according to the spots in Fig. 13a for the epoxy–MPTMS-Sb2O3 coated carbon steel in 3.5% NaCl solution. A small portion of the rust was cut by FIB from the SEM rust part and was analyzed by TEM
Conclusions
The consequence of incorporating MPTMS/Sb2O3 nanoparticles in epoxy matrix on mild steel was evaluated by electrochemical studies. The surface modification of antimony oxide by MPTMS was done to enhance the dispersibility of the Sb2O3 nanoparticles in the epoxy matrix and improve the ability of functional groups present in MPTMS to interact with Sb2O3 nanoparticles and epoxy matrix. The inclusion of MPTMS/Sb2O3 nanoparticles in the epoxy decreased the rate of corrosion drastically in comparison with the blank epoxy matrix. It is obvious from SECM measurements that the lower current detected at scratches demonstrates the capacity of Sb2O3 nanoparticles in safeguarding the metal surface.
Higher film and charge transfer resistances from the EIS measurements imply that the MPTMS/Sb2O3 nanoparticles and the resulting degraded product provide strong resistance to corrosion of the metal and enhance corrosion protection performance of epoxy matrix coatings. The enhanced resistance at the scratch of the epoxy-MPTMS/Sb2O3 coating is due to the repression of dissolution of iron. Therefore, the incorporation of MPTMS/Sb2O3 nanoparticles improved the safeguarding capacity of the epoxy-coated surface. Moreover, the epoxy-MPTMS/Sb2O3-coated substrate remarkably increased the preventive ability of the epoxy matrix to block the movement of H2O and corrosive ions into the epoxy matrix and enhanced the adhesion strength of the coating film.
References
J.H. Potgieter, P.A. Olubambi, L. Cornish, C.N. Machio, E.S.M. Sherif, Influence of nickel additions on the corrosion behavior of low nitrogen 22% Cr series duplex stainless steels. Corros. Sci. 50, 2572–2579 (2008)
M.A.M. Ibrahim, S.S.A. El Rehim, M.M. Hamza, Corrosion behavior of some austenitic stainless steels in chloride environments. Mater. Chem. Phys. 115, 80–85 (2009)
Xiaoyun Ye, Zhaopeng Wang, Lian Ma, Qianting Wang, Anni Chu, Zinc oxide array/polyurethane nanocomposite coating: fabrication, characterization and corrosion resistance. Surf. Coat. Technol. 358, 497–504 (2019). https://doi.org/10.1016/j.surfcoat.2018.11.080
D.J. Mills, S.S. Jamali, K. Paprocka, Investigation into the effect of nano-silica on the protective properties of polyurethane coatings. Surf. Coat. Technol. 209, 137–142 (2012). https://doi.org/10.1016/j.surfcoat.2012.08.056
C.G. Oliveira, M.G.S. Ferreira, Ranking high-quality paint systems using EIS. Part 1: intact coatings. Corros. Sci. 45, 123–138 (2003)
F. Galliano, D. Landolt, Evaluation of corrosion protection properties of additives for water borne epoxy coatings on steel. Prog. Org. Coat. 44, 217–225 (2002)
A. Ghanbari, M.M. Attar, A study on the anticorrosion performance of epoxy nanocomposite coatings containing epoxy-silane treated nano-silica on mild steel substrate. J. Ind. Eng. Chem. 23, 145–153 (2015)
B. Wetzel, F. Haupert, M.Q. Zhang, Epoxy nanocomposites with high mechanical and tribological performance. Compos. Sci. Technol. 63, 2055–2067 (2003)
M. Behzadnasab, S.M. Mirabedini, K. Kabiri, S. Jamali, Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. Corros. Sci. 53, 89–98 (2011)
M. Schem, T. Schmidt, J. Gerwann, M. Wittmar, M. Veith, G.E. Thompson, I.S. Molchan, T. Hashimoto, P. Skeldon, A.R. Phani, S. Santucci, M.L. Zheludkevich, CeO2-filled sol–gel coatings for corrosion protection of AA2024-T3 aluminium alloy. Corros. Sci. 51, 2304–2315 (2009)
G. Bierwagen, D. Tallman, J. Li, L. He, C. Jeffcoate, EIS studies of coated metals in accelerated exposure. Prog. Org. Coat. 46, 149–158 (2003)
Jie Liua, Lunwu Zhang, Mu Xianliang, Peiqing Zhang, Studies of electrochemical corrosion of low alloy steel under epoxy coating exposed to natural seawater using the WBE and EIS techniques. Prog. Org. Coat. 111, 315–321 (2017)
J.T. Zhang, J.M. Hu, J.Q. Zhang, C.N. Cao, Studies of impedance models and water transport behaviors of polypropylene coated metals in NaCl solution. Prog. Org. Coat. 49, 293–301 (2004)
W. Zhang, J. Wang, Y.N. Li, W. Wang, Evaluation of metal corrosion under defective coatings by WBE and EIS technique. Acta Phys. Chim. Sin. 26, 2941–2950 (2010)
M.G. Hosseini, P.Y. Sefidi, Electrochemical impedance spectroscopy evaluation on the protective properties of epoxy/DBSAdoped polyaniline-TiO2 nanocomposite coated mild steel under cathodic polarization. Surf. Coat. Technol. 331, 66–76 (2017)
X. Joseph Raj, T. Nishimura, Evaluation of the corrosion protection performance of epoxy-coated high manganese steel by SECM and EIS techniques. J. Fail. Anal. Prev. 16, 417–426 (2016). https://doi.org/10.1007/s11668-016-0102-5
D. Kong, Y. Wang, W. Zhang, W. Wang, X. Liu, J. Wang, Correlation between electrochemical impedance and current distribution of carbon steel under organic coating. Mater. Corros. 62, 1–6 (2011)
J. Liu, B. Wang, L. Zhu, T.Y. Luo, W. Wang, Correlation between EIS and potential distribution for carbon steel under epoxy coating. Appl. Mech. Mater. 313–314, 249–253 (2013)
J. Liu, W. Wang, J. Wang, Evaluation of the deterioration of epoxy coating by EIS and WBE techniques. Mater. Sci. Tech. 21, 33–39 (2013)
J.R. Xavier, R. Nallaiyan, Application of EIS and SECM studies for investigation of anticorrosion properties of epoxy coatings containing ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. J. Fail. Anal. Prev. 16(6), 1082–1091 (2016). https://doi.org/10.1007/s11668-016-0187-x
J.R. Xavier, Investigation on the effect of nano-ceria on the epoxy coatings for corrosion protection of mild steel in natural seawater. Anti-Corros. Methods Mater. 65(1), 38–45 (2018). https://doi.org/10.1108/ACMM-04-2017-1784
T. Adachi, W. Araki, T. Nakahara, A. Yamaji, M. Gamou, Fracture toughness of silica particulate-filled epoxy composite. J. Appl. Polym. Sci. 86, 2261–2265 (2002)
A. Boonyapookana, K. Nagata, Y. Mutoh, Fatigue crack growth behavior of silica particulate reinforced epoxy resin composite. Compos. Sci. Technol. 71, 1124–1131 (2011)
S.V. Lamaka, M.L. Zheludkevich, K.A. Yasakau, R. Serra, S.K. Poznyak, M.G.S. Ferreira, Nano porous titania interlayer as reservoir of corrosion inhibitors for coatings with self-healing ability. Prog. Org. Coat. 58, 127–135 (2007)
M. Rashvand, Z. Ranjbar, Effect of nano-ZnO particles on the corrosion resistance of polyurethane-based waterborne coatings immersed in sodium chloride solution via EIS technique. Prog. Org. Coat. 76, 1413–1417 (2013)
J. Izquierdo, J.J. Santana, S. González, R.M. Souto, Uses of scanning electrochemical microscopy for the characterization of thin inhibitor films on reactive metals: the protection of copper surfaces by benzotriazole. Electrochim. Acta 55, 8791–8800 (2010). https://doi.org/10.1016/j.electacta.2010.08.020
Xiaoqing Xiao, Dongmei Wang, Yongxin Li, Emily Jackson, Yida Fang, Yan Zhang, Ning Xie, Xianming Shi, Investigation into the synergistic effect of nano-sized materials on the anti-corrosion properties of a waterborne epoxy coating. Int. J. Electrochem. Sci. 11, 6023–6042 (2016). https://doi.org/10.20964/2016.07.66
Acknowledgments
The authors thank Prof. Dr. A. Abudhahir, Prof. Dr. techn.Koteswara Rao Anne, Prof. Dr. P. Sarasu, and the Management of Vel Tech Rangarajan Dr. Sagunthala R&D Institute of Science and Technology, Avadi, Chennai-600 062, Tamil Nadu, India, for their constant encouragement and constructive suggestions regarding this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xavier, J.R. Enhanced Adhesion and Corrosion Protection Properties of Surface Modified Sb2O3–Epoxy Nanocomposite Coatings on Mild Steel. J Fail. Anal. and Preven. 20, 523–531 (2020). https://doi.org/10.1007/s11668-020-00847-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-020-00847-4