Abstract
The inhibition effect of Flectofenine on corrosion of mild steel in 1M HCl solution was investigated by using traditional weight loss method and electrochemical techniques at different concentrations and temperatures. Adsorption of the inhibitor follows a Langmuir adsorption isotherm studied at the temperatures of 303–333 K. The change in free energy and change in enthalpy explain the mode of the adsorption. Activation energy values, apparent enthalpy changes and apparent entropy changes explain the corrosion process. The mechanism of inhibition was discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mild steel is an alloy form of iron, which readily undergoes corrosion in acidic media. Acidic solutions are extensively used in chemical laboratories and in several industrial processes, such as acid pickling, acid cleaning, acid de-scaling and oil wet cleaning. This leads to steel corrosion with huge economic losses for potential users [1,2,3,4,5,6,7].
Corrosion inhibition is of great importance in many industries, such as oil and gas extractions, refining and petrochemicals, metallurgy and construction. Among various techniques, use of corrosion inhibitors is the most efficient and economic method. They are adsorbed by the metal surface, form protective films and isolate metals from corrosive media [8,9,10,11,12].
The adsorption of a corrosion inhibitor depends mainly on physicochemical properties of the molecule such as functional groups, steric factor, molecular size, molecular weight, molecular structure, aromaticity, electron density at the donor atoms, p-orbital character of donating electrons and electronic structure of the molecule [2, 13,14,15,16]. This has led to consideration of pharmaceutical ingredients as corrosion inhibitors. Ketoconazole [17], some of the azosulfa drugs [18], meclizine hydrochloride [19] and some antimalarian drugs [20] have been reported as good inhibitors. Thus, drugs with planar structure can be a promising inhibitor which encouraged us to choose a drug Flectofenine [21].
Flectofenine is a class of nonsteroidal anti-inflammatory drugs used for the treatment of inflammatory disorders. The IUPAC name of Flectofenine is 1-[N-[8-(trifluoromethyl)-4-quinolyl] anthranilate]. The presence of electron-rich N, O, F atoms and π-bonds in its structure might be in favor of its adsorption on the metal surface, which gives a scope for its study as a potential corrosion inhibitor. The aim of the present investigation is to study the effect of Flectofenine as a corrosion inhibitor for mild steel in 1M HCl.
Experimental
Material
Steel strips having compositions 0.04% C, 0.35% Mn, 0.022% P, 0.036% S and the rest Fe (99.55%) were used for all experiments. The strips were polished by emery papers from 80 to 1500 grade, washed thoroughly with distilled water, degreased with acetone and dried at room temperature. Weight loss experiments were carried out in 1M HCl media. Strips with an exposed area of 1 cm2 were used for electrochemical measurements. The corrosive 1M HCl solutions were prepared using AR-grade HCl and distilled water.
Test Solution
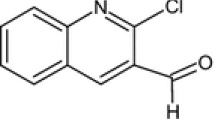
The Flectofenine structure is shown in Fig. 1. It is procured from Hangzhou Zhenghan Biological Technology Co., Ltd. China. It is water-insoluble but soluble in 1M HCl. Different weights of Flectofenine were dissolved in 1M HCl solution. The prepared stock solutions were used for the experiments including a blank or neat solution (1M HCl).
Methods
Weight Loss Measurements
Weight loss measurements were performed by immersing steel plates in a glass beaker containing 100 cm3 of corrosive media (1M HCl) with different concentrations of inhibitor. After an immersion time of 4 h, the steel plates were taken out and washed with plenty of tap water followed by distilled water, dried and weighed accurately using a digital balance (accuracy: ± 0.1 mg). All experiments were carried out in static and aerated conditions. Each measurement was repeated thrice for reproducibility, and an average value was reported.
Electrochemical Measurements
The electrochemical measurements were carried out in a CHI 608D electrochemical work station (manufactured by CH Instruments, Austin, USA) at 303–333 K. The cell consists of three electrodes, namely the working electrode (steel), counter electrode (platinum) and reference electrode (Ag/AgCl electrode). Before each electrochemical measurement, the working electrode was allowed to stand for 30 min in the test solution to establish steady-state open-circuit potential (OCP). All reported potentials were with respect to Ag/AgCl electrode. For Tafel measurements, potential–current curves were recorded at a scan rate of 0.01 V s−1 in the potential range obtained by adding − 0.2 and + 0.2 V to the open-circuit potential (OCP) value. The corrosion parameters such as corrosion potential (Ecorr), corrosion current density (icorr), cathodic Tafel slope (β c ) and anodic Tafel slope (β a ) were calculated from the software installed in the instrument. Impedance measurements were carried out using an AC signal with amplitude of 5 mV at OCP in the frequency range from 0.1 Hz to 1 KHz . The impedance data were fitted to most appropriate equivalent circuit by using ZSimp Win 3.21 software. The impedance parameters were obtained from Nyquist plots.
Adsorption Isotherm and Thermodynamic Considerations
Organic inhibitors are found to protect mild steel corrosion in acid media by adsorbing themselves on the metal surface. In order to gain more information about the mode of adsorption of Flectofenine on mild steel surface in 1M HCl at different temperature, attempts were made to fit the experimental data with several adsorption isotherms (Temkin, Langmuir, Freundlich, Frumkin, Bockris–Swinkels and Flory–Huggins). Thermodynamic parameters were calculated by using standard equations.
Scanning Electron Microscopic Studies
The steel specimens after immersion in experimental solutions were removed and dried. The specimens were coated with film of 50 nm of gold by sputtering and investigated by a JEOL JSM 840A scanning electron microscope. The potential of the accelerating beam employed was 20 kV.
Activation Parameters
Arrhenius graphs and transition-state graphs were plotted by using the corrosion rate data with respect to temperature. Activation parameters were calculated by standard equations.
Result and Discussions
Weight Loss Method
The weight loss method is probably the most widely used method of initial inhibition assessment. In our study, different mild steel samples were used which were immersed in 1M HCl solutions containing different inhibitor concentrations at 303 K temperature for 4 h. The investigations were carried out in aerated solutions. The mass of each steel specimen before and after immersion was determined using an analytical balance.
The value of percentage inhibition efficiency (η w ) was calculated from the following relation:
where Wo and W are corrosion rates of steel in the absence and the presence of inhibitor.
Corrosion parameters for mild steel in 1M HCl in the presence of different concentrations of the Flectofenine are provided in Table 1.
η w is increased with increase in concentration up to 500 ppm, and thereafter (> 500 ppm), the inhibition efficiency (η w ) remains constant. So 500 ppm is considered as optimum concentration for achieving the maximum inhibition efficiency.
Electrochemical Measurements
Polarization Measurements
The polarization behavior of steel immersed in 1M HCl in the presence of different concentration of Flectofenine at different temperatures is shown in Fig. 2.
The electrochemical parameters such as corrosion potential (Ecorr), corrosion current density (icorr), cathodic Tafel slope (β c ), anodic Tafel slope (β a ) and inhibition efficiency according to polarization studies (η p ) are listed in Table 2. The η p was calculated from the following relation.
where icorr and i ocorr are the corrosion current densities in the presence and absence of the corrosion inhibitor, respectively. The presence of Flectofenine shifts both anodic and cathodic branches to the lower values of current densities and thus causes a remarkable decrease in the corrosion rate. Oxygen is reduced in low cathodic overpotentials, but the main cathodic reaction in acidic solution is the discharge of hydrogen ions to produce hydrogen gas. Figure 2 shows that both anodic metal dissolution and cathodic reactions were inhibited after the addition of inhibitor to the aggressive solution. This result is indicative of the adsorption of inhibitor molecules on the active sites of the mild steel surface. The inhibition of both anodic and cathodic reactions is more pronounced with the increase in inhibitor concentration [22]. icorr values are decreased with increase in the concentration of Flectofenine. The inhibition efficiencies of Flectofenine were determined through electrochemical experiments (Table 2). The values of the corrosion current density (icorr) and corrosion potential (Ecorr) as well as the cathodic and anodic Tafel slopes (β c and β a ) were obtained by linear extrapolation of the Tafel slope. The figures show the influence of inhibitor and its concentration. The corrosion current density decreased with increase in inhibitor concentration and it indicates the corrosion rate was mitigated. Inhibition was reached by a blocking mechanism on the anodic and cathodic sites as polarization curves are displaced downward on the whole potential range. These results indicate that the presence of Flectofenine inhibited iron oxidation and to a lower extent the cathodic reduction reaction; consequently, this compound can be treated as mixed type corrosion inhibitor, as electrode potential displacement is lower than 85 mV in any direction [23]. The mixed type inhibition of inhibitor is reported in many literatures [24,25,26].
Electrochemical Impedance Spectroscopy (EIS) Measurements
Electrochemical impedance spectra for steel in 1M HCl with different concentrations of inhibitor at different temperatures are presented as Nyquist plots in Fig. 3.
Nyquist plots were analyzed by fitting the experimental data to a simple circuit model (Fig. 4.). The fitted values of R p and C dl are listed in Table 3. It is clear that by increasing the inhibitor concentration, the C dl values decreases, R p and the inhibition efficiency values increases. The addition of inhibitors provides lower C dl values; this situation was the result of an increase in the thickness of the electrical double layer and decrease in local dielectric constant [27]. The increase in size of the semicircle and R p values with increasing inhibitor concentration indicates a charge transfer process is the main controlling factor of the corrosion of mild steel. Considering the impedance diagrams at different temperatures (Fig. 3), the size of the capacitive loop is increased by increasing the concentration of Flectofenine, indicating that Flectofenine increased the charge transfer resistance. All impedance plots contain a depressed semicircle which can be attributed to the frequency dispersion effect as a result of the roughness and inhomogeneous electrode surface [28].
Popova et al. [29] said that the sum of charge transfer resistance (Rct) and adsorption resistance (Rad) is equivalent to polarization resistance (R p ). Inhibition efficiency (η z ) was calculated using the following equation.
where R p and \(R_{p}^{o}\) are polarization resistance values in the presence and absence of inhibitor. Electrical equivalent circuit model used to fit impedance data is shown in Fig. 4. The variation of η p and η z with temperature in the range 303–333 K is given in Table 3.
Adsorption Isotherm and Thermodynamic Considerations
The adsorption on the corroding surfaces never reaches the real equilibrium and tends to reach an adsorption steady state. However, when the corrosion rate is sufficiently small, the adsorption steady state has a tendency to become a quasi-equilibrium state. In this case, it is reasonable to consider the quasi-equilibrium adsorption in a thermodynamic way using the appropriate equilibrium isotherms [30]. The efficiency of the Flectofenine as a successful corrosion inhibitor mainly depends on its adsorption ability on the metal surface. So, it is essential to know the mode of adsorption and that can give the valuable information on the interaction of the inhibitor with the metal surface.
In order to gain more information about the mode of adsorption of Flectofenine on mild steel surface in 1M HCl at different temperatures, attempts were made to fit experimental data with several adsorption isotherms like Temkin, Langmuir, Freundlich, Frumkin, Bockris–Swinkels and Flory–Huggins. The best fit was obtained with Langmuir’s isotherm which is in good agreement with the following equation.
where C is the inhibitor concentration, θ is the degree of surface coverage defined as η z /100 at different concentration of inhibitor evaluated from AC impedance measurement and Kads is the equilibrium constant of adsorption process which is related to ∆G oads by the following relation
where R is the universal gas constant, T is the absolute temperature and 55.5 is the molar concentration of water in the solution.
The plots of C/θ against C were straight lines with almost unit slopes and are shown in Fig. 5.
It was found that all the regression coefficients were very close to 1 which indicates that the adsorption of Flectofenine on the mild steel surface obeys the Langmuir adsorption isotherm. The thermodynamic parameters for the adsorption process are shown in Fig. 6. It is generally assumed that the adsorption of inhibitor on the metal surface is the essential step in the mechanism of inhibition. The establishment of isotherms that describe the adsorption behavior of corrosion inhibitors is essential because they provide important clues about the nature of metal inhibitor interaction.
The negative values of ΔG oads suggest that the adsorption of Flectofenine on the mild steel surface is spontaneous. Generally, the values of – ΔG oads around or less than 20 kJ mol−1 are associated with the electrostatic interaction between charged molecules and the charged metal surface (physisorption), while those around or higher than 40 kJ mol−1 mean charge sharing or transfer from the inhibitor molecules to the metal surface to form a coordinate type of metal bond (chemisorption) [31].
The values of Kads, ΔG oads , ΔH oads and ΔS oads are listed in Table 4. Imran Naqvi et al. [32] reported the value of ΔG oads is − 33.52 kJ mol−1. T. Poornima et al. [33] reported the value of ΔG oads values in the range of − 30 to – 32 kJ/mol. In the present study, the investigators obtained a value in the range of − 30 to – 33 kJ mol−1, which means that the adsorption of inhibitor on the mild steel surface is predominately chemisorption [23]. In addition, values of the adsorption free energy ΔG oads very close to – 40 kJ mol−1 show that chemisorption makes the major contribution, whereas physisorption makes a minor contribution in the inhibition mechanism of Flectofenine.
The enthalpy of adsorption was deduced from the Gibbs Helmholtz Eq 6
This equation can be rearranged to give the following equation
A plot of ΔG oads /T versus 1000/T is linear (Fig. 6) with the slope equal to ΔH oads and intercept of –ΔS oads . The enthalpy of adsorption ΔH oads is – 58 kJ/mol and the entropy of adsorption ΔS oads is varied from – 82 to – 86 J/mol/K, respectively. Pavithra et al. [34] reported the value of ΔH oads is – 68.28 KJ/Mol. AL-Sawaad et al. [35] reported the value of ΔH oads is – 23 KJ/Mol. In this work, ΔH oads value is – 58 kJ/mol which indicates adsorption of Flectofenine on the steel surface is exothermic process with predominately chemisorption method [36]. The ∆S oads values in the presence of Flectofenine are large and negative values indicate a decrease in entropy is due to decrease in disordering on going from reactants to the metal adsorbed species [37]. The results obtained from thermodynamic study reflect that the adsorption of Flectofenine is comprehensive chemical adsorption.
Activation Parameters
Temperature has a great effect on the rate of metal corrosion. In the case of corrosion in a neutral solution (oxygen depolarization), the increase in temperature has a favorable effect on the overpotential of oxygen depolarization and the rate of oxygen diffusion, but it leads to a decrease in oxygen solubility. In the case of corrosion in acidic media (hydrogen depolarization), the corrosion rate increases exponentially with temperature increase because the hydrogen evolution overpotential decreases [38].
To calculate the activation parameters of the corrosion process and investigate the mechanism of inhibition, electrochemical measurements were performed in the temperature range of 303−333 K in the absence and presence of different concentration of Flectofenine in 1M HCl solution. The dependence of corrosion rate on temperature can be expressed by an Arrhenius equation and transition-state equation as shown in (8) and (9), respectively.
and
where E a is apparent activation energy, A is the pre-exponential factor, ΔH* is the apparent enthalpy of activation, ΔS* is the apparent entropy of activation, h is Planck’s constant and N is the Avogadro number. The apparent activation energy and pre-exponential factors at different concentration of Flectofenine can be calculated by linear regression between ln ϒcorr and 1000/T (Fig. 7); the results are shown in Table 5.
Figure 8 shows a plot of ln (ϒcorr /T) versus 1000/T. Straight lines were obtained with a slope equal to (−ΔH*/(2.303R)) and the intercept was equal to [log(R/Nh) + (ΔS*/(2.303R))], from which the values of ΔH* and ΔS* were calculated and are listed in Table 5.
The increase in activation energy after the addition of the inhibitor to the 1M HCl solution indicated that physical adsorption (electrostatic) occurs in the first stage. At higher concentrations, activation energy gradually decreased. At higher inhibitor concentration, lower activation energy is an indication of chemisorption [39]. The energy barrier was low, facilitating the formation of Fe2+ ions that interacted with the studied inhibitor to form a protective film. Inspection of the data reveals that (ΔH* and ΔS*) dissolution reaction of mild steel in 1M HCl decreases with increase in Flectofenine concentration. The reduction in mild steel corrosion rate is mainly decided by kinetic parameters of activation at lower concentration. When the concentration is increased, the decrease in mild steel corrosion rate is mainly controlled by pre-exponential factor [40] and on comparing the values of the entropy of activation (ΔS*) given in Table 5. It is clear that entropy of activation increased in the presence of Flectofenine at lower concentration, while it decreased at higher concentration.
This indicates that as the concentration increased, disordering increased on going from reactant to activated complex at lower concentration than in the absence of inhibitor, whereas a decrease of ΔS* reveals that an increase in disordering takes place on going from reactant to the activated [41].
Surface Analysis
Figure 9 shows micrographs of the mild steel surface before and after mild steel immersion in 1.0M HCl solution with and without corrosion inhibitor. Figure 9a shows surface of the mild steel specimen after immersion in 1.0M HCl solution for 2 h in the absence of inhibitor.
Figure 9b shows the surface of the mild steel specimen after immersion in the corrosive solution in the presence of 500 ppm Flectofenine inhibitor for the same period of time. The micrographs revealed that the surface morphology was strongly damaged in the absence of the inhibitor, but in the presence of 500 ppm inhibitor damage was considerably diminished, which confirmed the high efficiency of Flectofenine at this concentration.
Mechanism of the Action of Inhibitors
The mechanism of inhibition requires full knowledge of the interaction between the protective compound and the metal surface. Many of the organic corrosion inhibitors have at least one polar unit with atoms of nitrogen, sulfur, oxygen and in some cases phosphorus. It has been reported that the inhibition efficiency decreases in the order O < N < S < P. The polar unit is regarded as the reaction center for the chemisorption process. Further, the size, orientation, shape and electric charge on the molecule determine the degree of adsorption and the effectiveness of the inhibitor. On the other hand, iron is well known for its coordination affinity to heteroatom (N, O, S)-bearing ligands [42].
The results indicate that the inhibition mechanisms involved predominantly a chemisorption process. The presence of a nitrogen atom in the benzene ring, oxygen atoms and whole moiety may be involved in the mechanism. Lone pair electrons of nitrogen and oxygen atoms may donate the electrons to the vacant d orbital of the iron and form the coordinate bond. In addition to this, there may be a chance of physical adsorption of the inhibitor with the metal [43].
Conclusions
-
Flectofenine is a good inhibitor for the corrosion of mild steel in 1M HCl and inhibition efficiency was more pronounced with increase in the inhibitor concentration up to 500 ppm.
-
Inhibition efficiency was increased with increase in temperature up to 323 K.
-
Adsorption of the Flectofenine on the mild steel surface from the HCl solution obeyed Langmuir’s adsorption isotherm.
-
Adsorption of the inhibitor on the steel surface is mainly due to the chemisorption.
-
The inhibition action of the Flectofenine against corrosion is revealed by scanning electron micrographs.
References
M.A. Dahmani, S.S. Et-Touhami, B. Al-Deyab, A. Hammouti, Bouyanzer, corrosion inhibition of C38 steel in 1M HCl: a comparative study of black pepper extract and its isolated pipe rine. Int. J. Electrochem. Sci. 5, 1060–1069 (2010). (in English)
B.M. Praveen, T.V. Venkatesha, Metol as corrosion inhibitor for steel. Int. J. Electrochem. Sci. 4, 267–275 (2009). (in English)
D. Chebabe, Z. Ait Chikh, N. Hajjaji, Corrosion inhibition of Armco iron in 1M HCl solution by alkyltriazoles. Corros. Sci. 45, 309–320 (2003). (in English)
J.M. Bastidas, J.L. Polo, E. Cano, Substitutional inhibition mechanism of mild steel hydrochloric acid corrosion by hexylamine and dodecylamine. J. Electrochem. Soc. 30, 1173–1182 (2000). (in English)
M.A. Migahed, A.M. Abdul-Raheim, A.M. Atta, W. Brostow, Synthesis and evaluation of a new water soluble corrosion inhibitor from recycled poly (ethylene) terphethalate. Mater. Chem. Phys. 195, 3590–3596 (2010). (in English)
D. Asefi, M. Arami, N.M. Mahmoodi, Electrochemical effect of cationic Gemini surfactant and halide salts on corrosion inhibition of low carbon steel in acid medium. Corros. Sci. 52, 1801–1808 (2010). (in English)
G.N. Mu, X.H. Li, Inhibition of cold steel corrosion by Tween-20 in sulfuric acid: weight loss, electrochemical and AFM approaches. J. Colloid Interf. Sci. 289, 184–192 (2005)
N.V. Likhanova, M.A. Domínguez-Aguilar, O. Olivares-Xemetl, N. Nava-Entzana, E.H. Arce, Dorantes, The effect of ionic liquids with imidazolium and pyridinium cations on the corrosion inhibition of mild steel in acidic environment. Corros. Sci. 52, 2088–2097 (2010). (in English)
H.A. Videla, L.K. Herrera, Understanding microbial inhibition of corrosion-A comprehensive overview. Int. Biodeter. Biodegr. 63, 896–900 (2010). (in English)
K.F. Khaled, Experimental and atomistic simulation studies of corrosion inhibition of copper by a new benzotriazole derivative in acid medium. Electrochim. Acta. 54, 4345–4352 (2009). (in English)
T. Arslan, F. Kandemirli, E.E. Ebenso, L. Love, H. Alemu, Quantum chemical studies on the corrosion inhibition of some sulphonamides on mild steel in acidic medium. Corros. Sci. 51, 35–47 (2009). (in English)
R. Solmaz, G. Kardas, B. Yazici, M. Erbil, Adsorption and corrosion inhibitive properties of 2-Amino-5-Mercapto-1,3,4-Thiadizole on mild steel in hydrochloric acid media. Colloid. Surf. 312, 7–17 (2008). (in English)
N. Shankaresha, T.V. Venkatesha, G. Achary, B.M. Praveen, Y. Arthoba Naik, T.V. Venkatesha, Corrosion behaviour of surface modified steel by condensation product. Bull. Electrochem. 23, 123–127 (2007). (in English)
B.S. Shylesha, T.V. Venkatesha, G. Harshini, B.M. Praveen, Veratraldehyde as corrosion inhibitor for mild steel in different acid medium. J. chem. Chem. Eng. 4, 1934–7375 (2010). (in English)
B.S. Shylesha, T.V. Venkatesha, B.M. Praveen, A.V. Shanbhag, Corrosion Inhibition studies of mild steel by new inhibitor in different corrosive medium. Res. J. chem. sci. 1, 46–50 (2011). (in English)
R.A. Prabhu, T.V. Venkatesha, A.V. Shanbhag, B.M. Praveen, G.M. Kulkarni, R.G. Kalkhambkar, Quinol-2-thione compounds as corrosion inhibitors for mild steel in acid solution. Mat. Chem Phy. 108, 283–289 (2008). (in English)
I.B. Obot, N.O. Obi-Egbedi, Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: experimental and theoretical investigation. Corros. Sci. 52, 198–204 (2010). (in English)
I.B. Obot, N.O. Obi-Egbedi, S.A. Umoren, Antifungal drugs as corrosion inhibitors for aluminium in 0.1M HCl. Corros. Sci. 51, 1868–1875 (2009). (in English)
J. Ishwara Bhat, D. Vijaya Alva, Meclizine hydrochloride as a potential non-toxic corrosion inhibitor for mild steel in hydrochloric acid medium. Arch. Appl. Sci. Res. 3, 343–356 (2011). (in English)
A.S. Fouda, F. Al-Sarawy, H.M.El-Abbasy Sh-Ahmed, Corrosion inhibition of aluminum 6063 using some pharmaceutical compounds. Prot. Met. Phy. Chem. Surfaces. 45, 635–643 (2009). (in English)
M.M. Saleh, A.A. Atia, Effects of structure of the ionic head of cationic surfactant on its inhibition of acid corrosion of mild steel. J. Appl. Electrochem. 36, 899–905 (2006). (in English)
H. Jafari, I. Danaee, H. Eskandari, M.R. Avei, Electrochemical and theoretical studies of adsorption and corrosion inhibition of N, N′-Bis (2-hydroxyethoxy acetophenone)-2,2-dimethyl-1,2-propanediimine on low carbon steel (API 5L Grade B) in acidic media. Ind. Eng. Chem. Res. 52, 6617–6632 (2013)
K. Benbouya, I. Forsal, M. Elbakri, T.R. Anik Touir, M. Bennajah, M. Chebab, D. Rochdi, A. Mernari, B. Ebn Touhami, Influence of pyridazine derivative on corrosion inhibition of mild steel in acidic media. Res. Chem. Intermed. https://doi.org/10.1007/s11164-013-1037. (in English)
R.T. Loto, C.A. Loto, T. Fedotova, Electrochemical studies of mild steel corrosion inhibition in sulfuric acid chloride by aniline. Res. Chem. Intermed. https://doi.org/10.1007/s11164-013-1055. (in English)
R.T. Loto, Corrosion inhibition of mild steel in acidic medium by butyl alcohol. Res. Chem. Intermed. https://doi.org/10.1007/s11164-013-1088-1. (in English)
P. Lowmunkhong, D. Ungthararak, P. Sutthivaiyakit, Tryptamine as a corrosion inhibitor of mild steel in hydrochloric acid solution. Corros. Sci. 52, 30–36 (2010). (in English)
A.K. Singh, M.A. Quraishi, Effect of Cefazolin on the corrosion of mild steel in HCl solution. Corros. Sci. 52, 152–160 (2010). (in English)
A. Popova, M. Christov, S. Raicheva, E. Sokolova, Adsorption and inhibitive properties of benzimidazole derivatives in acid mild steel corrosion. Corr. Sci. 46, 1333–1350 (2004). (in English)
C.B. Pradeep Kumar, K.N. Mohana, Adsorption and thermodynamic characteristics of plumeria rubra plant extracts on mild steel corrosion in industrial water medium. Int. Res. J. Pure Appl. Chem. 3, 330–346 (2013). (in English)
A.K. Singh, Inhibition of mild steel corrosion in hydrochloric acid solution by 3-(4-((Z)-Indolin-3-ylideneamino) phenylimino)indolin-2-one. Ind. Eng. Chem. Res. 51, 3215–3223 (2012)
I. Naqvi, A.R. Saleemi, S. Naveed, Cefixime: a drug as efficient corrosion inhibitor for mild steel in acidic media: electrochemical and thermodynamic studies. Int. J. Electrochem. Sci. 6, 146–161 (2011). (in English)
T. Poornima, J. Nayak, N.A. Shetty, 3,4-Dimethoxybenzaldehydethiosemicarbazone as corrosion inhibitor for aged 18 Ni 250 grade maraging steel in 0.5 M sulfuric acid. J. Appl. Electrochem. 41, 223–233 (2011). (in English)
M.K. Pavithra, T.V. Venkatesha, M.K. Punith Kumar, H.C. Tondan, Inhibition of mild steel corrosion by Rabeprazole sulfide. Corr. Sci. 60, 104–111 (2012). (in English)
H.Z. Al-Sawaad, Evaluation of the ceftriaxone as corrosion inhibitor for carbon steel alloy in 0.5 M of hydrochloric acid. Int. J. Electro. Sci. 8, 3105–3120 (2013). (in English)
I.B. Obot, N.O. Obi-Egbedi, A.O. Eseola, Anticorrosion potential of 2-Mesityl-1H-imidazo[4,5-f][1,10] phenanthroline on mild steel in sulfuric acid solution: Experimental and theoretical study. Ind. Eng. Chem. Res. 50, 2098–2110 (2011). (in English)
G.E. Badr, The role of some thiosemicarbazide derivatives as corrosion inhibitors for C-steel in acidic media. Corros. Sci. 51, 2529–2536 (2009)
M.A. Migahed, E.S.M. Azzam, A.M. Al-Sabagh, Corrosion inhibition of mild steel in 1M sulphuric acid solution using anionic surfactant. Mater. Chem. Phys. 85, 273–282 (2004)
I. Dehri, M. Ozcan, The effect of temperature on the corrosion of mild steel in acidic media in the presence of some sulphur-containing organic compounds. Mater. Chem. Phys. 98, 316–323 (2006). (in English)
N. Guan, M.L. Xueming, L. Fei, Synergistic inhibition between o-phenanthroline and chloride ion on cold rolled steel corrosion in phosphoric acid. Mater. Chem. Phys. 86, 59–68 (2004). (in English)
E. Khamis, A. Hosney, S. El-Khodary, Thermodynamics of mild steel corrosioin inhibition in phosphoric acid by ethylene trithiocarbonate. Afinidad 52, 95–106 (1995). (in English)
M.S. Kumar, S. Loganathan, A. Kumar, A. Sreekanth, Anticorrosion potential of 4-amino-3-methyl-1,2,4-triazole-5-thione derivatives (SAMTT and DBAMTT) on mild steel in hydrochloric acid solution. Ind. Eng. Chem. Res 51, 5408–5418 (2012). (in English)
D. Guzman-Lucero, O. Olivares-Xometl, R. Martínez-Palou, N.V. Likhanova, A. Domínguez-Aguilar, C. Garibay-Febles, Synthesis of selected vinylimidazolium ionic liquids and their effectiveness as corrosion inhibitors for carbon steel in aqueous sulfuric acid. Ind. Eng. Chem. Res. 50, 7129–7140 (2011). (in English)
Acknowledgments
The authors are grateful to the authorities of Srinivas School of Engineering, Mukka, Mangalore, Karnataka, India, for providing laboratory facilities. The authors also thank Department of Science and Technology, New Delhi, Government of India, under fast-track scheme for young scientist [DST: Project Sanction No. SERB/F/2231/2012-13 dated 12-07-2012] and All India council for Technical Education, New Delhi, Government of India, under MODROBS scheme [Ref. No 8024/RIFD/MOD 292 /2010-11 dated 31-03-2011 for providing instrumental facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hebbar, N., Praveen, B.M., Prasanna, B.M. et al. Anticorrosion Potential of Flectofenine on Mild Steel in Hydrochloric Acid Media: Experimental and Theoretical Study. J Fail. Anal. and Preven. 18, 371–381 (2018). https://doi.org/10.1007/s11668-018-0416-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-018-0416-6