Abstract
Reactive plasma spraying (RPS) is a promising technology for in situ formation of aluminum nitride (AlN) coatings. Recently, AlN-based coatings were fabricated by RPS of alumina (Al2O3) powder in N2/H2 thermal plasma. This study investigated the feasibility of RPS of a fine Al2O3/AlN mixture and the influence of the plasma gases (N2, H2) on the nitriding conversion, and coating microstructure and properties. Thick AlN/Al2O3 coatings with high nitride content were successfully fabricated. The coatings consist of h-AlN, c-AlN, Al5O6N, γ-Al2O3, and a small amount of α-Al2O3. Use of fine particles enhanced the nitriding conversion and the melting tendency by increasing the surface area. Furthermore, the AlN additive improved the AlN content in the coatings. Increasing the N2 gas flow rate improved the nitride content and complete crystal growth to the h-AlN phase, and enhanced the coating thickness. On the other hand, though the H2 gas is required for plasma nitriding of the Al2O3 particles, increasing its flow rate decreased the nitride content and the coating thickness. Remarkable influence of the plasma gases on the coating composition, microstructure, and properties was observed during RPS of the fine particles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thermal spraying is a generic term for a group of processes commonly used to fabricate thick coatings of metallic and nonmetallic materials due to its high deposition rates (several μm/min or more). The process consists of spraying and deposition of molten or semimolten (heat-softened) material that is propelled onto a substrate surface. The powder material is injected into a very high-temperature plasma flame, where it is rapidly heated and accelerated to a high velocity. The molten or semimolten particles collide with the substrate surface and rapidly solidify. Then the accumulated particles form the coating. Owing to this deposition process, fabrication of nitride ceramic coatings including aluminum nitride (AlN) has been considered to be impossible. This inability has been attributed to the thermal decomposition of the AlN particles during spraying without a stable molten phase.
Among nitride ceramics, AlN has attracted much attention from researchers due to its outstanding electrical and mechanical properties. AlN possesses an excellent combination of high thermal conductivity (up to 320 W/m-K for a pure single crystal and 180–220 W/m-K for hot-pressed material), high dielectric resistivity, appropriate thermal expansion coefficient (close to those of silicon and GaAs), high electrical insulation, high mechanical strength, and high hardness (Hv 1,400). In addition, AlN has good chemical/physical stability at fairly high temperatures and high resistance to molten metals, wear, and corrosion (Ref 1, 2). This makes it an ideal material not only for electronic substrates, heat sinks, and packaging for electronic components, but also for semiconductor packaging, as a material for crucibles and vessels for handling corrosive chemicals and molten metals, for parts of semiconductor equipment, and for reaction vessels for etching. Moreover, it has been reported that fabrication of thermally sprayed AlN coatings would enable cost-effective solutions for a number of applications (Ref 3), especially for structural parts and power devices (requiring thick coatings).
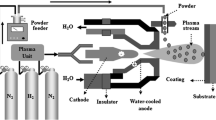
To fabricate nitride and AlN ceramic coatings by thermal spraying, the solution was to use the reactivity of the thermal plasma which is usually used to melt and accelerate the feedstock materials. Reactive plasma spraying (RPS) is a promising technology for in situ formation of AlN thermally sprayed coatings (Ref 4-8). The process is based on reaction of molten or semimolten particles (metallic or nonmetallic materials) with surrounding active species in the plasma such as atoms, ions, and radicals.
In our previous studies (Ref 5-8), it was possible to fabricate AlN-based coatings by RPS of aluminum (Al) powders in N2/H2 plasma. During spraying, the Al particles react with the surrounding active nitrogen plasma, collide with and rapidly solidify on the substrate. However, it was difficult to achieve complete nitridation due to the coagulation and aggregation of the Al particles [which is attributed to the low melting point of Al (660 °C) (Ref 9)]. The remaining metallic Al phase degrades the excellent insulation properties of such AlN coatings.
Recently, we introduced the possibility of fabrication of AlN-based coatings by RPS of alumina (Al2O3) feedstock powder in N2/H2 plasma (Ref 10, 11). The use of Al2O3 feedstock avoids the presence of metallic phases in the fabricated coatings. The fabricated coating consists of cubic AlN (c-AlN), aluminum oxynitride (Al5O6N), and γ-Al2O3 with a high content of α-Al2O3 feedstock.
This study investigates the feasibility of improving the nitriding conversion and nitride content by spraying of fine Al2O3 feedstock powder and using AlN itself as an additive. Furthermore, to optimize this process, the effects of the plasma gases (the nitriding species: N2 and H2 plasma gases) on the fabrication process, coating microstructure, and properties were investigated.
Experimental Procedures
Commercial fine Al2O3 powder (1 μm; Kojundo Chemical Lab Co., Ltd., Saitama, Japan) was spray-dried with fine AlN powder (1.14 μm; Tokuyama Co., Tokyo, Japan) using an L-8i spray dryer (Ohkawara Kakohki Co., Ltd., Yokohama, Japan). A 5 wt.% polyvinyl alcohol (PVA; Wako Pure Chemical Industries, Osaka, Japan) adhesive solution was used for mixture agglomeration. The feedstock powders were spray-dried in mixing ratio of 60 wt.% Al2O3 and 40 wt.% AlN.
All spray experiments were carried out by using an atmospheric DC plasma spray system (APS, 9MB; Sulzer Metco, Switzerland), using N2 as primary gas and H2 as secondary gas. The spray parameters are presented in Table 1. The feedstock mixture was supplied to the plasma with N2 carrier gas. Grit-blasted mild-steel plates (50 × 50 × 5 mm3) were prepared as the substrate material.
To investigate the influence of the N2 plasma gas, its flow rate was changed from 47.2 to 75.5 L/min while keeping its pressure at the standard condition of 330.9 kPa. The H2 (second) plasma gas was kept at the standard conditions of flow rate of 2.4 L/min and pressure of 344.7 kPa. Furthermore, the influence of the H2 plasma gas was investigated to clarify and optimize the fabrication process for high-AlN-content coatings with reasonable thickness by RPS of a fine AlN/Al2O3 mixture. In this part of the work, the flow rate of the H2 gas was varied from 0 to 7 L/min while keeping its pressure at the standard condition of 344.7 kPa. The effect of the H2 gas was investigated under the standard conditions for the N2 plasma gas (flow rate 47.2 L/min, pressure 330.9 kPa).
The phase composition of the fabricated coatings was verified by x-ray diffraction (XRD, RINT-2500; Rigaku, Tokyo, Japan) analysis using Cu Kα radiation. A scanning electron microscope (SEM, JSM-6390; JEOL, Tokyo, Japan) was used to observe the feedstock, its cross-section, and the coating cross-section microstructures. The coating’s hardness was measured by a micro Vickers hardness tester (Shimadzu, Kyoto, Japan). Hardness tests were carried out using a load of 0.98 N and a holding time of 14 s. The coating porosity was estimated by image analysis of SEM cross-sections.
Results and Discussion
Feedstock Mixture Characterization
Figure 1 shows the XRD spectra of the primary Al2O3 powder, the spray-dried Al2O3/AlN mixture, and the primary AlN powder. The feedstock mixture consists of α-Al2O3 and AlN without any impurities as shown in Fig. 1b. The average particle size of the spray-dried mixture is about 55 μm (D 10 = 45.54 μm, D 50 = 54.68 μm, D 90 = 65.12 μm), and the particle size distribution profile is shown in Fig. 2.
Figure 3 shows the SEM morphology of the feedstock mixture and its cross-section. It is clear that the spray-dried particles have the spherical shape which is common for the spray-drying process. This reveals that the amount of PVA is reasonable. The SEM images show that the particle size of the agglomerated mixture is about 55 μm. Furthermore, the primary particle size of the Al2O3 and AlN particles forming the agglomerates are in the submicrometer range (0.4–1 μm), as shown at the higher magnifications.
These results indicate that the powders were homogeneously mixed, agglomerated, and dispersed in the mixture. This structure will enable easy supply and good flow of the feedstock powder to the plasma stream during spraying. It is well known that the size distribution of the mixture should be adapted to that required for spraying. Thus, many fine particles (below 5 μm average diameter) are not suitable for conventional spraying without their agglomeration. Especially for this study, it is necessary to mix rather fine ceramic particles. Furthermore, homogeneity of the mixture is required to assist in-flight interaction between the powders and the surrounding plasma.
Reactive Plasma Spraying of Fine Al2O3/AlN Mixture
Figure 4 shows the XRD spectra of coatings fabricated by spraying the mixture and by spraying only Al2O3 powder (26.6 μm) in N2/H2 plasma at N2 gas flow rate of 47.2 L/min. The plasma condition was kept at the standard condition for the H2 plasma gas of flow rate of 2.4 L/min. It is clear that, for the spray-dried mixture, the cubic AlN (c-AlN) phase appeared in the fabricated coating (Fig. 4a). This is related to the plasma nitriding of the Al2O3 particles in the N2/H2 plasma [as in the case of only Al2O3 powder (26.6 μm) in Fig. 4b]. The XRD diffraction peak at 2θ value of 44° corresponds to the c-AlN phase. Furthermore, the hexagonal AlN phase (h-AlN) also appears in the fabricated coating, which is attributed to AlN deposited from the feedstock mixture. However, the h-AlN content in the fabricated coating is lower than in the feedstock. This is due to decomposition of the AlN at the high temperature in the plasma stream. In addition, the fabricated coating contains Al5O6N, γ-Al2O3, and a small amount of α-Al2O3 phases. This composition agrees with the plasma nitriding of only Al2O3 powder in Fig. 4b. However, the α-Al2O3 content decreased greatly and only a very small amount appeared in the coating when spraying the fine Al2O3/AlN mixture. On the other hand, high contents of γ-Al2O3 and Al5O6N phases appeared in the coating (the main phases) in the case of the fine particles compared with the large particles.
The formation of γ-Al2O3 in the coatings is related to the rapid quenching of the molten Al2O3 and the rapid solidification rate in the plasma spray process. Thus, the α-Al2O3 melted, and then the molten Al2O3 solidified in the form of γ-Al2O3 due to the rapid solidification. This transformation to the transient γ-Al2O3 occurs easily for the high temperature and rapid solidification rate of the plasma spray process, as reported previously (Ref 12). The α-Al2O3 content in the coating is related to the unmelted ingredient in the spray droplets of the feedstock powders. Thus, during the quick solidification after the impact process, γ-Al2O3 will preferentially nucleate if the initial droplet was completely molten. Thus, its energy barrier to nucleate is less than that of α-Al2O3. Furthermore, the crystal growth kinetics is much faster for the metastable phase than for the stable phase. Incompletely molten particles were regarded as due to crystallization of preexisting α-Al2O3 nuclei. This reveals that the fine Al2O3 particles are almost fully melted, as indicated by the low α-Al2O3 intensities in Fig. 4a. On the other hand, the higher content of α-Al2O3 in the case of the larger Al2O3 feedstock (Fig. 4b) reveals its lower melting ability.
The higher content of γ-Al2O3 and the low α-Al2O3 intensities for the coatings suggest a higher proportion of molten powder particles. This reveals that the particle size of the feedstock strongly affects the nitriding process of the Al2O3 particles as well as their melting behavior. This is because, during plasma nitriding of Al2O3 particles, the nitriding reaction starts from the particle surface. Decreasing the particle size of the feedstock powder increased the specific surface area, which increased the possibility of surface nitriding and promoted the nitriding conversion. In other words, use of fine Al2O3 particles increased the nitriding area, which enhanced the formation of AlN phase.
On the other hand, the particle size also affects the melting behavior of the particles. Thus, the heat capacity of the fine particles is smaller than that of the large particles, meaning that their temperature increases faster. Furthermore, the total heat transfer to the fine particles is larger than that to the large particles at constant weight. Therefore, the heat conduction inside the small particles is faster and their decomposition rates are faster than for the large particles. Moreover, for the same plasma temperature, the smaller particles attain a higher temperature than the large particles. This is due to the larger heat absorbed per unit volume for the smaller particles. Furthermore, Remesh et al. (Ref 13) reported that a larger particle requires eight times more heat to attain the same temperature rise as a particle with half the diameter.
Moreover, the surface area of a large particle through which the heat is transferred increases by four times when compared with a particle of half the diameter. Hence, the heat absorbed per unit volume is the inverse of the particle diameter. Therefore, the fine α-Al2O3 particles are almost fully melted and transformed to γ-Al2O3 in the coating due to the rapid quenching of the molten Al2O3. In this work, it was therefore possible to fabricate AlN/Al2O3 coatings with high nitride content by reactive spraying of a fine Al2O3/AlN mixture in N2/H2 plasma. The use of the smaller particle size improved the nitriding process during flight and enhanced the melting of the Al2O3 particles, which agrees with our previous results using Al feedstock. Moreover, the AlN additive improved the nitride content in the coating, as explained in detail in our previous report (Ref 8).
Figure 5 shows the SEM cross-section morphology of a coating fabricated in N2/H2 plasma using N2 gas flow rate of 47.2 L/min. The coating is approximately 150 μm thick, with a uniform and porous structure. Therefore, it was possible to fabricate thick AlN/Al2O3 coatings with uniform structure and high nitride content by RPS of the fine Al2O3/AlN mixture. The fabricated coating consists of h-AlN, c-AlN, γ-Al2O3, Al5O6N, and a very small amount of α-Al2O3. Use of the fine primary powder improved the nitriding process, and the AlN additive improved the nitride content in the coating. Furthermore, the fine α-Al2O3 particles were almost fully melted and mostly completely transformed into γ-Al2O3 in the coating due to the rapid quenching of the molten Al2O3.
To enhance the nitride content and optimize the nitriding process of the fine feedstock powder, the influence of the plasma gases (N2 and H2) was investigated.
N2 Plasma Gas in Spraying of Fine AlN/Al2O3 Mixture
The effect of the N2 plasma gas was investigated to optimize the fabrication process of thick coatings with high AlN content. In this part of the work, the flow rate of the N2 plasma gas was varied from 47.2 to 75.5 L/min while keeping its pressure at the standard condition of 48 psi (330.9 kPa). The H2 plasma gas was kept at the standard conditions of flow rate of 2.4 L/min and pressure of 50 psi (344.7 kPa).
The AlN content was significantly improved by increasing the N2 gas flow. The relative amounts of the assigned phases in the coatings were estimated by comparing the peak intensities from the XRD data using the following equation (Ref 14, 15):
where R X is the concentration ratio, and I (hkl) is the intensity of the diffraction peak for the corresponding plane of the c-AlN, α-Al2O3, Al5O6N, and γ-Al2O3 phases.
Figure 6 shows the relative amounts of the assigned phases in coatings fabricated using different N2 plasma gas flow rates, as estimated by comparing the peak intensities from the XRD spectra using Eq 1.
It is clear that increasing the N2 gas flow rate improved the h-AlN content whereas the c-AlN content gradually decreased. The nitriding reaction was enhanced by the higher N2 gas flow rate due to an increase in the amount of nitriding species surrounding the molten Al2O3 particles. This assists the formation of Al5O6N and its transformation to the c-AlN phase, as reported in detail previously (Ref 11). Moreover, such increase in the nitriding species facilitates complete crystal growth of the c-AlN to the h-AlN phase after deposition (through plasma irradiation) on the substrate. Complete crystal growth of c-AlN to h-AlN was clearly exhibited when using the fine particles compared with the large-size particles. This result is in agreement with previous reports by Cao et al. (Ref 14) that increasing the N2 gas flow improves the nitriding conversion in the plasma spray process.
In other words, increasing the N2 gas flow rate improved the postdeposition nitriding as well as the in-flight nitriding when spraying the fine particles. It also assisted complete crystal growth of the cubic phase to the hexagonal one after deposition on the substrate. Therefore, the increase in the h-AlN content in the coating can be partially attributed to the complete crystal growth of the c-AlN phase after deposition on the substrate due to the increase in the nitriding species in the plasma.
On the other hand, the effect of the N2 plasma gas during RPS of fine powders is also strongly related to the particles’ in-flight diagnostics (velocity and temperature) in the plasma. Thus, increasing the N2 gas flow gradually improved the particles’ in-flight velocity but did not affect the in-flight temperatures, as explained previously in detail for RPS of Al2O3 (Ref 11). The gradual increase in the particle velocity with the N2 gas flow rate is related to the fact that the acceleration of the particles is linked to the flow momentum. Therefore, it strongly depends on the mass flow rate of the plasma gas. As nitrogen ions are much heavier than hydrogen ions, their mass flow rate is much higher than that of hydrogen. Correspondingly, the nitrogen gas is mainly responsible for accelerating the particles.
This increase in the particle velocity (with the N2 gas flow) decreased the residence time of the particles in the plasma and thereby reduced the decomposition of AlN (from the feedstock) during the spraying. This partially contributed to the increased h-AlN content in the coating for the higher N2 gas flow. Furthermore, decreasing the particle residence time by further increase in the N2 gas flow can be considered as one of the reasons for the reduction in the c-AlN content for higher N2 gas flow rates as well as its complete crystal growth.
These results reveal that increasing the N2 gas flow effectively improved the nitride content during RPS of fine particles compared with larger particles. Thus, during spraying of large Al2O3 particles, the effect of the N2 gas flow rate started to appear above 66 L/min (Ref 11). However, during spraying of fine Al2O3 particles, the N2 gas effect was clear after only a slight increase in its flow (starting from 56.6 L/min). This is attributed to the high reactivity of the fine particles, as explained in Sect. 3.2.
Furthermore, the N2 gas flow rate affected the phase transformation of the Al2O3 feedstock during spraying, an effect that was clearer in the case of the fine particles compared with the larger ones for the same spray conditions. Thus, the N2 gas flow rate did not affect the phase transformation of the large particles. On the other hand, during spraying of the fine Al2O3 particles, the phase composition was clearly affected by the N2 flow rate. The γ-Al2O3 and Al5O6N contents decreased slightly with further increase of the N2 gas flow. This is mainly attributed to their nitridation to the AlN phase. At the same time, the α-Al2O3 content slightly increased with further increase of the N2 flow rate, which is related to the particles’ in-flight diagnostics (velocity and temperature). Thus, decreasing the particles’ residence time in the plasma (by increasing their velocity) for the same temperature condition (no change in the temperature with the N2 gas flow) led to worse melting behavior of the particles. Therefore, the transformation of the α-Al2O3 to γ-Al2O3 phase decreased and the α-Al2O3 gradually increased with the N2 flow rate in the case of fine particles. This proves that a shorter in-flight time due to the higher particle velocity (caused by increasing the nitrogen gas flow rate) led to less molten powder, and the α-Al2O3 gradually increased in the case of fine particles.
In this work, it was possible to improve the nitride content in the coatings, and coatings with high AlN content (mainly h-AlN) could be fabricated by spraying the fine Al2O3/AlN mixture and increasing the N2 gas flow, as shown in Fig. 7, which shows the XRD spectrum of a coating fabricated using N2 gas flow rate of 75.5 L/min.
In addition, the N2 gas flow rate also significantly affected the coating microstructure. Figure 8 shows SEM cross-section images of coatings fabricated with increasing N2 gas flow rate. It is clear that thick coatings with uniform and porous structure were fabricated and their thickness gradually increased with the N2 gas flow. The thickness improved from about 150 μm to about 200 μm on increasing the N2 gas flow from 47.2 to 75.5 L/min. This improvement in thickness with increasing N2 gas flow can be attributed to the increased velocity of the particles, i.e., the greater acceleration and driving force of the particles towards the substrate. Furthermore, increasing the particle velocity resulted in a slight decrease in the flight time (the residence time of particles in the plasma jet). This factor significantly affects the particle melting and deposition efficiency on the substrate as well as the tendency for evaporation in the plasma. This reveals that increasing the N2 gas flow rate enhanced the AlN content and the thickness of the fabricated coatings.
Influence of N2 Plasma Gas on Coating Properties
The porosity of the coatings was estimated by image analysis of SEM cross-sections. Figure 9 shows the average porosity of fabricated coatings as a function of the N2 flow rate when spraying the fine Al2O3/AlN mixture as well as only the Al2O3 powder (26.6 μm). It is clear that the porosity of the coating achieved by spraying only Al2O3 (26.6 μm) is higher than when spraying the fine (1 μm) Al2O3 in the Al2O3/AlN mixture. This can be attributed to two factors: firstly, an increase of the melting efficiency of the fine particles in the plasma plume compared with the large particles, as explained above. Secondly, Kulkarni et al. (Ref 16) reported that the morphology of the splats changed from a disk-like to a fragmented shape with increasing particle size. This difference in the splat morphology manifests itself in the coating structure and porosity. Thus, the increasing fragmentation of the splats leads to poor adhesion and porosity.
On the other hand, with increasing N2 gas flow rate, the coating porosity gradually increased as shown in Fig. 9. This can be attributed to the increasing particle velocity with the increasing N2 gas flow rate. Thus, with the increasing particle velocity, the particle residence time in the plasma will decrease. This leads to worse melting behavior of the powder in the plasma, which significantly affects the coating porosity. Furthermore, in the RPS process, the reaction of the particles with the surrounding active plasma must be considered. Thus, increasing the N2 gas flow enhanced the tendency for reaction between the particles and the surrounding plasma, which may lead to a gradual increase in the porosity of the coating. This point is different from normal plasma spray techniques, in which increasing the particle velocity and the driving force leads to a decrease of the porosity of the coating.
Figure 10 shows the average microhardness of the fabricated coatings as a function of the N2 gas flow rate when spraying the fine Al2O3/AlN mixture as well as only the Al2O3 powder (26.6 μm). It is clear that the coating hardness when spraying the Al2O3 powder (26.6 μm) in the N2/H2 plasma is lower than for the coating fabricated in the Ar/H2 plasma using the same feedstock material. This means that the formation of the AlN phase decreases the hardness of the fabricated coating. This is attributed to the lower hardness value of AlN compared with Al2O3: for bulk materials, Al2O3 exhibits Vickers hardness about two times that of AlN. It is also clear that the hardness decreased when spraying the fine Al2O3/AlN mixture in the N2/H2 plasma and further decreased when increasing the N2 gas flow rate. This is attributed to the increasing proportion of AlN in the coatings when using the fine Al2O3/AlN mixture or increasing the N2 gas flow rate (as shown above in the XRD spectra). It is also considered that the increasing porosity of the coating with the N2 gas flow (as shown in Fig. 9) reduced the coating hardness. This is due to a decrease in the solid area carrying the load.
This reveals that increasing the N2 gas flow rate enhanced the AlN content in the fabricated coatings. It also improved the thickness of the coatings due to the increased particle velocity. Furthermore, the N2 gas affected the coating porosity and hardness.
H2 Plasma Gas in Spraying of Fine AlN/Al2O3 Mixture
The effect of the H2 plasma gas flow rate was investigated to optimize the RPS of the fine Al2O3/AlN mixture. The H2 plasma gas flow was varied from 0 to 7 L/min while keeping its pressure at the standard condition of 344.7 kPa. The N2 plasma gas was kept at the standard conditions of flow rate of 47.2 L/min and pressure of 330.9 kPa. Figure 11 shows the XRD spectra of coatings fabricated by spraying without H2 gas (0 L/min, i.e., in only N2 plasma), using H2 gas (N2/H2 plasma), and when increasing its flow rate up to 7 L/min. It is clear that the c-AlN phase did not appear in the coatings fabricated without H2 gas (using only N2 plasma) as shown in Fig. 11a. This reveals that the H2 plasma gas is required for RPS of Al2O3 particles. This result is in complete agreement with the previous result on spraying of only Al2O3 powder (26.6 μm) in the absence of H2 plasma gas. Furthermore, the fabricated coating consists of h-AlN, γ-Al2O3, Al5O6N, and a small amount of α-Al2O3. This indicates that, although the nitriding reaction did not occur, use of the fine Al2O3 particles assisted the transformation to the γ-Al2O3 phase, as explained above.
On the other hand, the c-AlN phase (due to the nitriding reaction) started to appear when using the N2/H2 plasma, as shown in Fig. 11b. However, the AlN content (c-AlN and h-AlN phases) gradually decreased with increasing H2 gas flow. On the other hand, the phase transformation of the α-Al2O3 to γ-Al2O3 phase was improved with increasing H2 gas flow. The gradual increase of the transformation rate of α-Al2O3 (decreasing its content) to γ-Al2O3 (increasing its content) with the H2 gas flow rate is attributed to an increase in the particle in-flight temperature. Thus, increasing the H2 gas flow enhanced the particle in-flight temperature [as confirmed by previous DPV-2000 measurements (Ref 11)]. This assists the melting of the particles, especially the fine particles. Moreover, when spraying the fine particles, the α-Al2O3 phase mainly disappeared with increasing flow rate, as shown in Fig. 11c.
Furthermore, the XRD spectra reveal that the AlN content in the coating decreased with the H2 gas flow rate. It was considered that increasing the particle temperature (with the H2 gas) assists the evaporation of AlN (from the feedstock and/or even the reacted phase), especially when using the fine particles. This led to a gradual decrease in the AlN content (c-AlN and h-AlN phases) with increasing H2 gas flow. However, further studies are required to investigate this evaporation tendency quantitatively. In this work, increasing the particle temperature enhanced the proportion of molten particles, especially when using the fine particles, which assisted the phase transformation to the γ-Al2O3 phase. However, it also assisted the evaporation of the AlN phases and decreased their content. This reveals that, although the H2 plasma gas is required for reactive plasma nitriding of Al2O3 particles, increasing its amount is not recommended to fabricate high-AlN-content coatings.
Figure 12 shows SEM cross-section images of fabricated coatings as a function of the H2 plasma gas flow rate. It is clear that, without the H2 plasma gas, a coating of about 300 μm was fabricated, as shown in Fig. 12a. The coating thickness gradually decreased when using the H2 plasma gas and increasing its flow rate. The coating was suppressed to about 110 μm when using a H2 gas flow rate of 7 L/min, as shown in Fig. 12d.
The decrease in the coating thickness when using the H2 plasma gas or increasing its flow rate is in agreement with our previous results on spraying of Al powder. However, it is different from the case of using the larger (26.6 μm) Al2O3 powder. This is attributed to the melting behavior and particle size of the feedstock particles. Thus, during spraying of the fine Al2O3 powder as well as Al powders (low melting point), the particles can be easily melted even when using only N2 plasma. Therefore, increasing the particle temperature by using the H2 plasma gas or increasing its flow rate leads to evaporation of particles during flight and decrease of their deposition efficiency. Therefore, for fine particles or low-melting-point materials, the coating thickness decreased with increasing H2 gas flow rate. On the other hand, during spraying of large (26.6 μm) Al2O3 particles, the temperature was insufficient to melt all of the particles when using only N2 plasma. Therefore, increasing the particle temperature (by using the H2 plasma gas and increasing its flow rate) assists the probability of particle melting, which improved the coating thickness.
These results reveal that the H2 plasma gas is an essential factor during plasma spraying of Al2O3/AlN powder for fabrication of AlN coatings in the RPS process. Thus, in the absence of H2 plasma gas, the coating contains the h-AlN phase (from the feedstock mixture) whereas the c-AlN phase (due to plasma nitriding of Al2O3 particles) does not appear in the coating. Although, when using the N2/H2 plasma, the c-AlN phase appeared in the coating, increasing the H2 gas flow did not promote the nitriding conversion. The AlN content (h-AlN and c-AlN) decreased with the H2 gas flow, which is attributed to vaporization of the particles in flight due to increase of their temperature. The increase in the particle temperature with the increasing H2 gas flow enhanced the transformation rate of the α-Al2O3 to γ-Al2O3 phase. Furthermore, the H2 gas and its flow rate affect the coating thickness. The thickness decreased with the use of the H2 gas and increasing its flow rate. Thus, as the fine particles were easily melted even in only N2 plasma, increasing the particle temperature by using the H2 gas led to particle evaporation and decreased the coating thickness.
Influence of H2 Plasma Gas on Coating Properties
Figure 13 shows the average porosity of the coatings as a function of the H2 plasma gas flow rate when spraying the fine Al2O3/AlN mixture as well as only the Al2O3 powder (26.6 μm). It is clear that the porosity of the coating decreased for both powders (Al2O3 and Al2O3/AlN mixture) when using the H2 plasma gas. This is attributed to the increase in the particle temperature on use of the H2 gas and increasing its flow rate. Increasing the particle temperature enhanced the melting efficiency of the particles and reduced the porosity of the coating. Furthermore, the porosity of the coating decreased on decreasing the feedstock particle size (primary), as explained above in Sect. 3.4. In addition, the porosity decreased on increasing the H2 gas flow rate. Therefore, the porosity of the coating was significantly affected by the feedstock particle size and particle temperature.
In addition, the H2 plasma gas flow affected the coating hardness. Figure 14 shows the average microhardness of the fabricated coatings as a function of the H2 gas flow when spraying both Al2O3 and the Al2O3/AlN mixture. It is clear that the hardness of the coating fabricated by spraying only Al2O3 powder (26.6 μm) in only N2 plasma is almost the same as the hardness of the pure Al2O3 coating (obtained by spraying Al2O3 powder in Ar/H2 plasma). Thus, spraying only Al2O3 powder (26.6 μm) in only N2 plasma led to a coating consisting mainly of Al2O3 with a small Al5O6N content. However, when spraying Al2O3 powder in N2/H2 plasma, the hardness of the coating decreased. This is related to the formation of the AlN phase as a result of reactive plasma nitriding of some particles (the AlN decreased the hardness due to its lower hardness compared with Al2O3).
Moreover, spraying the Al2O3/AlN mixture in only N2 plasma lowered the hardness to become similar to that of the coating fabricated by spraying Al2O3 powder (26.6 μm) in N2/H2 plasma. Then, further decrease in the hardness was observed when spraying the fine Al2O3/AlN mixture in N2/H2 plasma. This can be attributed to the increase in the nitride content as a result of reactive plasma nitriding of the particles. On the other hand, with increasing H2 gas flow, the hardness gradually increased. This is attributed to the decrease in the AlN content with further increase in the H2 gas flow, as presented above. It is also considered that the decrease in the porosity of the coating with the H2 gas flow rate affected the hardness of the fabricated coatings.
In this work, it was possible to fabricate AlN/Al2O3 composite coatings by reactive plasma nitriding of a fine Al2O3/AlN spray-dried mixture. The fabricated coating consists of h-AlN, c-AlN, Al5O6N, γ-Al2O3, and a small amount of α-Al2O3. Decreasing the particle size of the Al2O3 powder and using the AlN additive are suitable solutions to fabricate thick and uniform AlN/Al2O3 coatings with high nitride content and a very small amount of α-Al2O3. Furthermore, the nitriding species (N2, H2) significantly affect the phase composition, coating microstructure, and properties when using the fine particles in the process.
Conclusions
Thick and uniform AlN/Al2O3 coatings with high nitride content were fabricated by RPS of a spray-dried fine Al2O3/AlN mixture. The fabricated coatings consist of h-AlN, c-AlN, Al5O6N, γ-Al2O3, and a very small amount of raw α-Al2O3. The particle size, AlN additive, and plasma gases (N2, H2) play important roles in this process. The results of this study can be summarized as follows:
-
Spraying of primary particles of small size and use of AlN additive improved the nitriding conversion and the AlN content in the coating. Use of fine particles assisted the nitriding conversion (by increasing the surface area).
-
The fine α-Al2O3 feedstock was almost fully melted and completely transformed to γ-Al2O3 in the coatings due to the rapid quenching of the molten Al2O3.
-
A remarkable effect of the N2 gas flow rate was observed when using the fine Al2O3 particles compared with larger particles. Increasing the N2 gas flow rate enhanced the nitriding conversion in flight and after deposition by increasing the nitriding species surrounding the particles.
-
The h-AlN content was improved with the N2 plasma gas flow rate due to decrease of the particle residence time in the plasma jet. This residence time affects the particle melting, reactivity, and evaporation tendency during spraying.
-
The thickness improved with the N2 gas flow due to increased particle velocity, and thick (200 μm) coatings could be fabricated at N2 gas flow of 75.5 L/min.
-
H2 plasma gas is required for RPS of the fine Al2O3/AlN mixture, but a low flow rate is recommended. Further increase of the H2 gas flow increased the particle temperature, which assisted in-flight vaporization of the fine particles and therefore decreased the AlN content as well as the deposition efficiency.
-
The transformation rate of the α-Al2O3 to γ-Al2O3 phase was improved with the H2 gas (due to increase of the temperature) but was suppressed with the N2 gas (due to decrease of the residence time in the plasma).
-
The plasma gases strongly influence the microstructure and properties of the fabricated coatings.
References
H.O. Pierson, Handbook of Refractory Carbides and Nitrides, Noyes, NJ, 1996, p 237-239
A.W. Wemer, Carbide, Nitride and Boride Materials Synthesis and Processing, Chapman & Hall, London, 1997, p 6-68
L.R. Krishna, D. Sen, Y.S. Rao, G.V.N. Rao, and G. Sundararajan, Thermal Spray Coating of Aluminum Nitride Utilizing the Detonation Spray Technique, J. Mater. Res., 2002, 17(10), p 2514-2523
M. Yamada, T. Yasui, M. Fukumoto, and K. Takahashi, Nitridation of Aluminum Particles and Formation Process of Aluminum Nitride Coatings by Reactive RF Plasma Spraying, Thin Solid Films, 2007, 515(9), p 4166-4171
M. Shahien, M. Yamada, T. Yasui, and M. Fukumoto, Cubic Aluminum Nitride Coating through Atmospheric Reactive Plasma Nitriding, J. Therm. Spray Technol., 2010, 19(3), p 635-641
M. Shahien, M. Yamada, T. Yasui, and M. Fukumoto, Fabrication of AlN Coatings by Reactive Atmospheric Plasma Spray Nitriding of Al Powders, Mater. Trans., 2010, 51(5), p 957-961
M. Shahien, M. Yamada, T. Yasui, and M. Fukumoto, Reactive Atmospheric Plasma Spraying of AlN Coatings: Influence of Aluminum Feedstock Particle Size, J. Therm. Spray Technol., 2011, 30, p 580-589
M. Shahien, M. Yamada, T. Yasui, and M. Fukumoto, In Situ Fabrication of AlN Coating by Reactive Plasma Spraying of Al/AlN Powder, Coatings, 2011, 1, p 88-107
Y. Qiu and L. Gao, Nitridation Reaction of Aluminium Powder in Flowing Ammonia, J. Eur. Ceram. Soc., 2003, 23, p 2015-2022
M. Shahien, M. Yamada, T. Yasui, and M. Fukumoto, Synthesis of Cubic Aluminum Nitride Coating from Al2O3 Powder in Reactive Plasma Spray Process, Mater. Trans., 2013, 54(2), p 207-214
M. Shahien, M. Yamada, T. Yasui, and M. Fukumoto, N2 and H2 Plasma Gases’ Effects in Reactive Plasma Spraying of Al2O3 Powder, Surf. Coat. Technol., 2013, 216, p 308-317
L. Zhao, K. Seemann, A. Fischer, and E. Lugscheider, Study on Atmospheric Plasma Spraying of Al2O3 Using On-Line Particle Monitoring, Surf. Coat. Technol., 2003, 168, p 186-190
K. Remesh, H.W. Ng, and S.C.M. Yu, Influence of Process Parameters on the Deposition Footprint in Plasma-Spray Coating, J. Therm. Spray Technol., 2003, 12, p 377-392
L.H. Cao, K.A. Khor, L. Fu, and F. Boey, Plasma Spray Processing of Al2O3/AlN Composite Powders, J. Mater. Process. Technol., 1999, 89-90, p 392-398
Y. Li and K.A. Khor, A Study of Processing Parameters in Thermal-Sprayed Alumina and Zircon Mixtures, J. Therm. Spray Technol., 2002, 11, p p186-p194
A. Kulkarni, A. Vaidy, A. Goland, S. Sampth, and H. Herman, Processing Effects on Porosity-Property Correlations in Plasma Sprayed Yttria-Stabilized Zirconia Coatings, Mater. Sci. Eng. A, 2003, 359, p 100-111
Acknowledgments
This research was support by grants-in-aid for JSPS fellows (232575) of the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahien, M., Yamada, M., Yasui, T. et al. Reactive Plasma Spraying of Fine Al2O3/AlN Feedstock Powder. J Therm Spray Tech 22, 1283–1293 (2013). https://doi.org/10.1007/s11666-013-9992-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-013-9992-x