Abstract
The study aimed at optimizing the suspension plasma spraying of TiO2 coatings obtained using different suspensions of fine rutile particles in water solution onto aluminum substrates. The experiments of spraying were designed using a 23 full factorial plan. The plan enabled to find the effects of three principal parameters, i.e. electric power input to plasma, spray distance, and suspension feed rate onto microstructure of coatings, content of anatase phase and size of anatase crystals in the coatings. The microstructure of deposits was observed with scanning electron microscope (SEM) and optical microscope and their composition was characterized using energy dispersive spectrometry (EDS). The observations were made on the coatings surface and their cross-sections. The latter made it possible to determine the coatings thicknesses to be in the range from 8 to 33 μm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
TiO2 is a frequently used oxide, which can be used in, e.g. chemical, electronic and optical industries. The oxide may be applied as a thin film deposited by PVD or a thick coating obtained by thermal spraying (Ref 1, 2). The application of thermally sprayed coatings, which is about to emerge is a deposition of photocatalytic coatings (Ref 3-6). The photocatalytic activity seems to be promoted by the appropriate phase composition of coatings, which should possibly include anatase. The tested coatings have been prepared by vacuum or atmospheric plasma spraying of coarse powders and by atmospheric plasma spraying of fine precursors in suspension (Ref 3-6). Suspension plasma spraying has been applied recently to develop the nanostructural coatings (Ref 7). One of the first tested applications was a development of thermal barrier coatings (Ref 8, 9). The physics and chemistry of suspension spray processes were analyzed by Fauchais et al. (Ref 10). At the processing, the liquid feedstock containing fine particles is fed into plasma jet using a peristaltic pump or similar device (see Fig. 1). The suspension is fed to an atomizer and gets injected into plasma jet. The solid content in the atomized droplets starts to agglomerate, melt (some of them will vaporize) and eventually solidify in-flight before impacting on the substrate. The formation of anatase in suspension sprayed coatings was explained by the phenomena occurring at small droplet solidification (Ref 11). The explanation is based onto the calculation made by Li and Ishigaka (Ref 12), which show that at the temperature of solidus lower than 2075 K (melting point in equilibrium condition is equal to T m = 2143 K), the nucleation of anatase occurs and above this temperature—that of rutile. Taking this model the nucleation of anatase would be favored mainly by the temperature at which nucleation from the melt begins and the rate of cooling down of solidified droplet. The latter, for fine particles used at suspension spraying, should depend on the operational processing parameters such as spray distance, electric power input to the torch, linear velocity of torch at spraying among others. The present study focuses on optimizing the spray conditions in view of controlling the anatase content in suspension plasma sprayed deposits.
Experimental
Suspensions Preparation and Injection
In the experiments, suspensions were formulated with the use of rutile TiO2 (Tioxide R-TC90 of Huntsman Tioxide) in the following proportions: 360 g of distilled water + 40 g of rutile + 0.12 g of dispersant. The dispersant was an aqueous solution of sodium polyacrylate (Hydropalat N, Congis). The size distribution of TiO2 powder used in the suspension formulation is shown elsewhere (Ref 11). The mean diameter (volume-surface mean defined by Masters (Ref 13)) equals to d VS = 0.33 μm. The geometry of atomizer/injector is shown in Fig. 2. The parameters related to the suspension delivery are summarized in Table 1.
Plasma Spraying
Aluminum plates of size 15 × 15 × 3 mm were used as coatings substrates. They were plasma sprayed using Praxair SG-100 torch with anode 03083-175 and cathode 03083-129 mounted on 5-axis ABB IRB-6 industrial robot. Throughout all the experiments the following parameters were kept constant:
-
composition of plasma working gas: Ar + H2;
-
flow rates of plasma working gas: q Ar = 45 slpm and \( q_{{{\text{H}}_{2} }} \) = 5 slpm;
-
linear torch velocity: v = 500 mm/s;
-
trajectory of one torch pass is presented in Fig. 3
-
total number of passes was 50 (5 cycles of 10 passes including 1 min of cooling down).
The variable spray parameters, called experimental domain, are collected in Table 2. The full matrix of 23 experiments was realized for samples sprayed using water suspension and two experiments were made in the center. All experiments done are summarized in Table 3.
Powders and Coatings Characterization
Morphology of powders, splats and coatings surfaces and cross-sections was investigated using JEOL-JSM 6480L microscope, equipped with a detector of secondary electrons and an energy dispersive spectrometer (EDS). The cross-sections were prepared metallographically. Cameca CAMEBAX electron probe micro-analyzer was used for qualitative chemical analysis of sprayed coatings. The cross-sections of the sprayed samples were visualized using an optical microscope Olympus CK 40 M with a camera Camedia 3040. The thicknesses of the coatings were determined using software DP-SOFT 5.0. XRD analysis of powders and coatings was carried out using Bruker diffractometer of type D8 using Cu Kα radiation. The phases were identified with use of Diffrac plus EVA software which uses database of International Centre of Diffraction Data JCPDS-ICDD.
Crystallographic Analyses
Initially, five mixtures of anatase and rutile powders were prepared. Rutile was the fine powder used in the suspensions and anatase was a 99.8% pure product of Aldrich. Their composition is shown in Table 4. Subsequently, the X-ray diagrams of the mixtures were realized and the content of anatase was determined from the following equation (Ref 14):
in which I A is intensity of [101] peak of anatase and I R is intensity of [110] peak of rutile. This expression was corrected by taking into account different densities of two phases of TiO2 in the following way:
in which ρA is the density of anatase (3.9 g/cm3) and ρR is the density of rutile (4.2 g/cm3). The calculation was made by taking into account the heights and the areas of peaks and their results are shown in Table 5. The comparison of the methods enabled to retain the analysis of peaks heights with the corrections shown in Eq 2. The crystal sizes were calculated from broadening of Bragg peaks using Laue-Scherer method. The influence of microstrains was taken into account in the way described elsewhere (Ref 15).
Statistical Treatment of Data
Optimization of operational spray parameters was made using three responses, namely:
-
fraction of anatase phase in the coating, Y 1;
-
coating thickness, Y 2;
-
size of anatase crystals, Y 3.
The effects of the process variables onto responses were calculated following to the model valid in the experimental spaceFootnote 1:
where i, j, k are equal to 1, 2, 3 and m can be equal 1, 2, or 3. The coefficient b 0 is the mean of responses of all experiments; b i coefficient represents the effect of the variable X i , and b ij and b 123 are the coefficients which represent the effects of interactions of variables X i *X j and X 1*X 2*X 3, respectively. Experiments were carried out by considering a 23 full factorial design with two levels and three factors, i.e. a total of 8 experiments (Table 3). The advantage of this type of experimental design is that the structure is perfect for estimating the model (Eq 3). The analyzes were made using Nemrod software (Ref 16). They aimed at determination of significant (active) effects influencing quantitative properties of sprayed deposits. The method of statistical analyzes was a creation of the regression equation describing the property and an analysis of the coefficients of this equation. Initially, all effects and their first and second order interaction (as shown in Eq 3) were considered. Then the most active effects and their interactions were selected by application of the Student criterion. The selected coefficients should be greater than the incertitude calculated using reproducibility variance and Student criterion, taken at 95% confidence level and for a degree of freedom that depends, in turn, on number of experiments, number of their repetition, and on the number of regression equation coefficients as shown by Lazić (Ref 17). Later on, the analysis was repeated by taking only these effects in the regression equation into account (and narrowing the incertitude in coefficients determination). Finally, the probability that the effect is active was calculated with the use Bayes approach and the final statistical model of the analyzed property was established.
Results
Scanning Electron Microscope
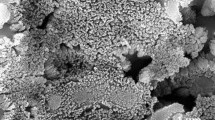
The surfaces of the sprayed samples are presented in Fig. 4. The coatings include submicrometer and micrometer fine particles. Some of them are irregular and some are spherical (see arrows in Fig. 4). Careful analysis enables a distinction of greater agglomerates having size of a few tens of micrometers (see circle in Fig. 4). Such agglomerates are visible rather on the surface of coatings sprayed at greater distance of 12 cm and/or greater suspension flow rate of 30 mL/min. The energy dispersive spectroscopy analysis carried out for the samples T2, T3, T4, and T6 enabled qualitatively only Ti and O to be discovered. The quantitative analysis made for the samples T2 and T4 enabled to find the atomic proportion of these elements of 66.7 at.% O and 33.3 at.% Ti, that corresponds to stoichiometric TiO2. The secondary electron image of sprayed samples indicates an important porosity of the samples (Fig. 5a). The visible porosity could have resulted from the real porosity and from the pull-out effect at the metallographical preparation of the cross-sections.
Electron Micro-probe Mapping, Optical Microscope
The elementary mapping of Al and Ti made using EMPA technique shows sharp interface and important roughness of sprayed samples (Fig. 5b, c). Optical microscope investigation of a cross section of a coating confirms porosity and roughness of sprayed coatings (Fig. 6). The thicknesses of the coatings were calculated by averaging the thicknesses along a line. The results of the measurements are collected in Table 6.
Statistical Analyses
Fraction of Anatase in Deposits
The initial analysis shows that only coefficient b 2 is greater that the 95% significance level (Fig. 7a). The refined analysis with the regression equation limited to three principal variables and one interaction, i.e. X 1*X 3, shows that the interaction effect b 13 is slightly greater than the signification level (Fig. 7b). However, the probability that the b 13 interaction effect is smaller than 50% (see Fig. 7c) and the final regression equation has the following forms:
Statistical analysis of the active effects influencing anatase fraction in sprayed deposits: regression equation coefficients for a model including all effects and their interaction of second and third order (a); refined regression equation coefficients for most active effects (b); determination of probability that the effects in the refined regression equation are active (c)
Coating Thickness
Initial analysis indicates that no coefficient of the regression equation is greater than 95% significance level (Fig. 8a). The analysis limited to three principal effects and X 2*X 3 interaction enabled to find out that the effect b 3 of electric power input to plasma is significant what was confirmed by the probability calculation (Fig. 8b, c)
Statistical analysis of the active effects influencing coating thickness: regression equation coefficients for a model including all effects and their interaction of second and third order (a); refined regression equation coefficients for most active effects (b); determination of probability that the effects in the refined regression equation are active (c)
Anatase Crystal Size
Similarly, initial analysis does not reveal any significant effect and refined analysis indicates that b 1, b 3, and b 12 can be significant (Fig. 9a, b). The probability that these effects are active is lower than 50% (Fig. 9c). Consequently, it is probable that no significant effect of chosen variables influences anatase crystal size.
Statistical analysis of the active effects influencing anatase crystal size in sprayed deposits: regression equation coefficients for a model including all effects and their interaction of second and third order (a); refined regression equation coefficients for most active effects (b); determination of probability that the effects in the refined regression equation are active (c)
Discussion
The droplets of atomized liquid are injected into plasma jet. Knowing that the size of suspension delivery outlet is 700 μm (see Fig. 2) and that the liquid is subsequently atomized, the sizes of droplets must have diameter ranging from a few tens to a few hundreds of micrometers. The droplets are accelerated in the plasma jet and disintegrated into smaller droplets. Simultaneously, water boils and gets evaporated. The solid particles inside any droplet get closer and may start to agglomerate by sintering in flight. The number of solid particles in the droplets is estimated in Table 7. The process can be compared to that of spray-drying described by Masters (Ref 13). However, the processes have the following, significant differences:
-
particularly hot heating medium (plasma jet);
-
boiling of suspension liquid;
-
very short heating time (milliseconds instead of seconds);
-
missing of a binder in the liquid.
The rough estimation of the number of small particles in suspension droplets of different sizes is shown in Table 7. A part of precursor gets molten in the initial part of their trajectory. Fauchais et al. (Ref 10) estimated that the precursors having size of 0.1 μm gets molten after 2.5 mm of its trajectory in the plasma jet. In fact, the plasma gets colder because of evaporation of water and the trajectory may be longer. Subsequently, the fine particles may get agglomerated by sintering with other particles and/or evaporated. The agglomeration progresses until an impact with the substrate. This can be confirmed by aspect of surface of the coatings sprayed using greater spray distance, which has slightly more agglomerates visible (Fig. 4). As the atmosphere around solid precursors contains a lot of vapours of water the solidification of precursors in-flight is probable. Anatase has lower interfacial energy between liquid and solid TiO2 and this phase is likely to nucleates firstly (Ref 12). Moreover, the cooling of a precursor in flight is very rapid and anatase is likely to be formed in its entire volume because of their small size. An increase of anatase phase fraction with the spray distance can be attributed to the increasing number of solidified precursors arriving on the substrate. The precursors being molten at impact with substrate nucleates as anatase but this phase can transform back to rutile on cooling of the coating. This transformation is more probably for larger agglomerates due to the larger quantity of heat of fusion liberated and lower cooling rate. As the size of anatase and rutile crystals, being about 100 nm (see Table 6) is comparable to the size of particles, it is reasonable to suppose that the particles are entirely rutile or entirely anatase. The anatase-particles are generated in flight and rutile-particles are unmelted ones and those, which transform back from anatase-particles when the coating cools down.
Thickness of the coatings depends mainly on power input to plasma. An increase in electric power input results in a decrease of thickness. It can be explained by an increase of velocity of plasma stream with electric power input to plasma. The stream generated with increasing electric power input to the torch is more rapid and more turbulent. Such a stream is likely to blow away these particles, which are not closely adhering to the substrate or to previously deposited coating.
Conclusions
A statistical study was carried out to find out the influence of operational spray parameters of TiO2 on coatings thickness and on some microstructural features such as fraction of anatase in the coatings and the crystal size. The spray distance has most probably the strongest influence on the anatase fraction in coatings. The phenomenon was explained by an increase of number of fine particles solidified in flight before an impact with substrate. The electric power input to the torch was probably influencing thickness of suspension plasma sprayed coatings. The influence of other parameters on tested coatings characteristics was not significant. Further studies are carried out aiming at testing of other solutions formulation and its injection into plasma jet.
Notes
Defined as −1 ≤ X i ≤ +1, i = 1 to 3, variables are normalized with regard to spray parameters in such a way that X i = −1 corresponds to the lower limit of variable, X i = 0 corresponds to the center of experimental domain and X i = 1 corresponds to higher limit of the variable.
References
L. Pawlowski, Dépôts Physiques (PPUR, Lausanne, Switzerland, 2003)
L. Pawlowski, The Science and Engineering of Thermal Spray Coatings, 2nd ed. (John Wiley & Sons, Chichester, England, 2008)
F.-L. Toma, G. Bertrand, S.-O. Chwa, C. Coddet, D. Klein, P. Nardin, and A. Ohmori, Studies of the Photocatalytic Efficiency of Titanium Dioxide Powders and Coatings Obtained by Plasma Spraying, Conference Proceedings on CD-ROM, ITSC 2004, Osaka, Japan, May 10-12, 2004, DVS Verlag, Düsseldorf, Germany
F.-X. Ye, A. Ohmori, and C.-J. Xi’an, The Photoresponse and Donor Concentration of Plasma Sprayed TiO2 and TiO2-ZnO Electrodes, Conference Proceedings on CD-ROM, ITSC 2004, Osaka, Japan, May 10-12, 2004, DVS Verlag, Düsseldorf, Germany
F.-L. Toma, G. Bertrand, D. Klein, L.-M. Berger, and S. Thiele, Photocatalytic Properties of Coatings Sprayed from TiOx and Tin−2Cr2O2n−1 Powders by APS and VPS, Conference Proceedings on CD-ROM, ITSC 2004, Osaka, Japan, May 10-12, 2004, DVS Verlag, Düsseldorf, Germany
F.-L. Toma, G. Bertrand, D. Klein, C. Coddet, and C. Meunier, Photocatalytic Decomposition of Nitrogen Oxides Over TiO2 Coatings Elaborated by Liquid Feedstock Plasma Spraying, Conference Proceedings on CD-ROM, ITSC 2005, Basel, Switzerland, May 2-4, 2005, DVS Verlag, Düsseldorf, Germany
J. Karthikeyan, C.C. Berndt, S. Reddy, J.-Y. Wang, A.H. King, H. Herman, J. Am. Ceram. Soc. 81(1), 121-8 (1998)
T. Bhatia, A. Ozturk, L. Xie, E.H. Jordan, M. Gell, X. Ma, N.P. Padture, J. Mater. Res. 17(9), 2363-72 (2002)
N.P. Padture, K.W. Schichting, T. Bhatia, A. Ozturk, B. Cegeten, E.H. Jordan, M. Gell, S. Jiang, T.D. Xiao, P.R. Strutt, E. Garcia, P. Miranzo, M.I. Osendi, Acta Mater. 49, 2251-7 (2001)
P. Fauchais, J.F. Coudert, V. Rat, J. Fazilleau, and C. Delbos, 1 st International Meeting on Thermal Spraying, Lille, ed. L. Pawlowski, 4-5 December 2003, p 8-13
R. Tomaszek, L. Pawlowski, L. Gengembre, J. Laureyns, Z. Znamirowski, J. Zdanowski, Surf. Coat. Technol. 201, 45-56 (2006)
Y. Li, T. Ishigaki, J. Cryst. Growth 242, 511-516 (2002)
K. Masters, Spray Drying Handbook, 4th ed. (George Godwin, London, England, 1985), p. 67
N. Berger-Keller, G. Bertrand, C. Filiatre, C. Meunier, C. Coddet, Surf. Coat. Technol. 168, 281-290 (2003)
V. Deram, C. Minichiello, R.N. Vannier, A. Le Maguer, L. Pawlowski, D. Murano, Surf. Coat. Technol. 166, 153-9 (2003)
Nemrod, software, version Education 2000 (Marseille, France), LPRAI
Ž.R. Lazić, Design of Experiments in Chemical Engineering, 2004 (Weinheim, Germany), Wiley-VCH, p 262-385
Acknowledgments
Ms. Ophelie Meunier, Maria Aguila, and Mr. Christophe Penverne of ENSCL helped in X-ray diffraction studies of samples. Professors Didier Chicot and Jacky Lesage made SEM installation available.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaworski, R., Pawlowski, L., Roudet, F. et al. Influence of Suspension Plasma Spraying Process Parameters on TiO2 Coatings Microstructure. J Therm Spray Tech 17, 73–81 (2008). https://doi.org/10.1007/s11666-007-9147-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-007-9147-z