Abstract
Improving interfacial bonding between carbon fibers recovered from used carbon composites can reduce costs and address environmental concerns surrounding landfill of carbon composite materials. This study investigated the mechanical and chemical characteristics of carbon fibers exposed to different plasma treatment times in an argon and oxygen atmosphere. After plasma treatment for 1 min, there was no difference in the tensile and morphological properties of commercial carbon fibers and plasma-treated carbon fibers. However, oxygen penetrated into the carbon fiber with the oxygen atoms close to each other, resulting in increase in lactone groups that were not observed in commercial carbon fibers, while the hydroxyl and carbonyl groups were removed in the form of CO and CO2 and thus decreased in number. In addition, the polar surface energy of the carbon fibers markedly increased after the plasma treatment as compared to that of the commercial carbon fibers. After plasma treatment for 3 min, carbon fiber surface damage was observed, and tensile properties were greatly reduced. In addition, lactone, hydroxyl, and carbonyl groups on the carbon fiber surface decreased due to the exposure of C-C and C = C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Carbon fiber is a lightweight material, with high specific strength and elasticity and excellent thermal, electrical, and physical properties, in addition to high corrosion resistance and chemical resistance. Due to the high cost of carbon fibers and environmental problems caused by landfill of carbon composite materials, carbon composites, combined with resin, are used as reinforcing materials only in a few high-tech industries (e.g., aviation, space, and wind power) (Ref 1). Technologies to recover and recycle used carbon composites could reduce the cost of carbon fibers and extend their use to other industries (Ref 2,3). Studies have reported various methods of recovering recycled carbon fibers from waste carbon composites (Ref 4,5). However, the properties of recycled carbon fibers using these methods are poorer than those of commercial carbon fibers. Thus, they have limited reuse value. Carbon composites are composed of carbon fibers and resin. As the carbon fiber surface is smooth and chemically stable, interfacial bonding between the fibers and resin is poor. To improve the interfacial bonding force between carbon fiber and resin, surface treatment of carbon fiber is required (Ref 6,7,8). The latter is particularly important in the case of polypropylene, which is a nonpolar resin.
In surface treatment, functional groups, such as free radicals, or carboxyl, carbonyl, or hydroxyl groups, on the surface of carbon fiber strengthen interfacial bonding (Ref 9,10,11,12). To improve interfacial bonding, generally, the chemical properties of the surface of carbon fiber are altered through oxidation (Ref 13), electrochemical (Ref 14), plasma treatment (Ref 15,16,17), heat treatment, or sizing treatment methods (Ref 18). Of these, plasma treatment imparts reactivity to the surface at the depth of the molecular layer, thereby rapidly improving the interfacial bonding force of the carbon fiber surface (Ref 16, 17, 19). Previous research has reported on target atoms on a specimen’s surface being treated in various atmospheres, including nitrogen, oxygen, argon, and atmosphere, with the porous layer on the carbon fiber surface being removed through plasma treatment to reduce voids and improve adhesion with resins (Ref 16, 17, 19). As a result of plasma treatment in atmosphere and CO2 atmosphere, oxygen functional groups readily formed on the surface of carbon fibers in atmosphere plasma, thereby improving the adhesive force between the fibers and resin (Ref 20). However, oxygen functional groups were not observed in a carbon dioxide (CO2) atmosphere due to the absence of oxygen functional groups (Ref 20). In another study, J. Lin reported that plasma treatment in an inert gas argon atmosphere did not generate a new functional group, had an effect on sizing removal only, and interface adhesion strength was improved, in addition to sizing removal after nitrogen and atmospheric plasma treatment (Ref 21). Other studies reported that plasma treatment of carbon fibers in an atmospheric atmosphere decreased the contact angle and increased the surface roughness (Ref 22, 23). There have been many studies on atmospheric characteristics and conditions during plasma treatment, but research on the mechanisms of change in the chemical structure of the carbon fiber surface remains insufficient.
In this study, a plasma treatment process was introduced to improve the characteristics of recycled CF to the level of commercial carbon fibers, and optimal conditions were proposed to improve the interface properties of carbon fibers and resins using mechanical and chemical analysis based on the length of plasma treatment. The mechanisms of change in the chemical state of the carbon fiber surface and in the oxygen functional group due to the plasma treatment time were also determined.
2 Materials and Methods
2.1 Materials
The carbon fiber used in this study comprised recycled carbon fibers recovered from existing carbon composite, and Table. 1 compares the characteristics of the commercial carbon fibers of Toray with the characteristics of the recycled carbon fibers used in this research. The commercial carbon fibers are referred to as “untreated.”
2.2 Plasma Treatment
Carbon fibers were immersed in acetone at 60 °C for 30 min for desizing, followed by plasma treatment in an argon (99.99% purity) and oxygen (99.99% purity) atmosphere. The power of the plasma generator was 250 W. The injection amount was set to 4 L/minute (argon) and 0.02 L/minute (oxygen), respectively. The plasma density was 0.52 W/cm2, and the distance between the head and the carbon fiber was 2 mm. The carbon fiber moving speed was 50 mm/s. The plasma treatment times varied from 0.5 min to 7 min.
2.3 Characteristic Analysis
A field emission scanning electron microscope (FE-SEM) at an acceleration voltage of 20 kV was used to observe changes in the carbon fiber surface after the plasma treatment. Qualitative and quantitative analyses were performed using an energy-dispersive x-ray spectroscopy (XPS). To evaluate the mechanical properties of the untreated and plasma-treated carbon fibers, a short fiber tensile test was performed under conditions of 25 mm gage length and 5 mm/minute elongation in accordance with the ASTM D 3822 standard. The test was conducted 25 times per condition, and the average of the results was used.
X-ray photoelectron spectroscopy (XPS) with a Nexsa XPS system (Korea Basic Science Support Institute, Jeonju) was used to investigate changes in chemical functional groups on the surface of the carbon fibers in accordance with the plasma treatment time. The specimen was investigated with monochromatic Alαa (1486.6 eV), and a high-resolution spectrum was obtained with a passing energy of 50 eV and a beam size of 400 μm. To determine the effect of the plasma treatment time on surface energy, the contact angle was measured using the Wilhelmy plate method. Carbon fibers were dropped into hydrophilic water and hydrophobic diiodomethane at a constant injection rate of 6 mm/minute. The contact angle measurement was evaluated three times per condition, and the surface energy was calculated based on the contact angle obtained when the carbon fiber was immersed in water and diiodomethane and then removed.
3 Results and Discussion
3.1 Carbon Fiber Surface
Figure 1 shows the SEM observations of the surfaces of the carbon fibers in accordance with the plasma treatment times. The diameters of the carbon fibers changed in accordance with the treatment time, as illustrated in Fig. 2. Compared with the surface of the untreated, after 1 min, the surfaces were smooth, with no significant change. In contrast, after plasma treatment for 3 min, the carbon fiber surface was slightly degraded. After plasma treatment for 5 min, the carbon fiber surface was severely damaged, with the average diameters of the fibers reduced to about 4.8 μm. Plasma treatment for 7 min resulted in a rapid decrease in fiber diameter and fiber loss, leading to loss of function. It was determined that the degree of exposure to excessive energy increased as the time increased when the carbon fiber was treated with plasma. Thus, the numbers and sizes of pores present on the surface were increased by a chemical reaction of the carbon fiber, thereby greatly reducing the diameter.
3.2 Tensile Properties of the Carbon Fibers
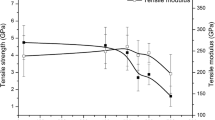
Figure 3 shows the change in tensile characteristics of the carbon fibers according to the plasma treatment time. Overall, the tensile strength and elongation of the carbon fibers decreased in accordance with the plasma treatment time. The tensile strength of the carbon fiber was similar to that of the untreated up to a plasma treatment time of 1 min. However, as the plasma treatment time increased, the tensile strength started to decrease, and the average tensile strength decreased to about 3.9 GPa at a plasma treatment time of 3 min. At a plasma treatment time of 5 min, the average tensile strength decreased sharply to about 52% compared to that of the untreated. After plasma treatment for 7 min, severe carbon fiber damage was observed, with carbon fiber loss, making it difficult to measure the tensile strength. Based on these results, it was confirmed that 1 min of the treatment time showed the same tensile characteristics as the untreated, but the plasma treatment of 3 min or more resulted in degradation of the tensile strength of the carbon fibers, with a large amount of surface damage. After plasma treatment for 1 min, which is the optimal treatment time in a high-energy process, even a short treatment time damaged the carbon fiber surface and weakening of its mechanical properties (Ref 24).
3.3 Chemical Properties of the Carbon Fibers
XPS was used to analyze the chemical state of the carbon fiber surface according to the plasma treatment time. XPS C1s and O1s spectra are shown in Fig. 4. Table 2 shows the elemental composition of the carbon fiber surface with respect to the plasma treatment time. When compared to the desizing carbon fibers, the amount of carbon decreased and the amount of oxygen increased in the plasma-treated samples. As shown in the spectrum of C1s (Fig. 4 a), hydroxyl groups (C-O) and carbonyl groups (C = O) on the surface of the carbon fibers decreased significantly after desizing and continued to decrease in accordance with the plasma treatment time. On the other hand, the lactone groups (O = C-O) were similar to those in the untreated after desizing. However, lactone groups were rapidly generated after plasma treatment for 1 min and gradually decreased after treatment for 3 min or more (Fig. 4 a). Referring to the spectrum of O1s, after desizing, the oxygen present on the surface of the carbon fiber was significantly reduced compared to that of the untreated. After a plasma treatment time of 1 min, the area and width of the oxygen were significantly increased as compared with the desizing. The amount of oxygen decreased after plasma treatment for 3 min and continued to decrease in accordance with the treatment time (Fig. 4 b). In terms of the O/C ratio, it increased to 0.25 following 1 min of plasma treatment after desizing. The O/C ratio decreased after plasma treatment times of 3 min or more.
Figure 5 and Table 3 show the peak separation results of C1s and the change of peak according to the composition of the functional groups generated on the carbon fiber surface by the plasma treatment. After desizing, the numbers of C-O and C = O groups decreased significantly compared to those in the untreated. The number of C-O and C = O groups decreased in accordance with the plasma treatment time. O = C-O bonds formed after 1 min of plasma treatment. However, at plasma treatment times of 3 min or more, O = C-O bond formation decreased.
According to previous research, when heat-treated in a nitrogen atmosphere, the oxygen atoms in C-O and C = O on the surface of the carbon fiber are removed to O2 by the heat as the treatment temperature increases, thus decreasing the oxygen functional groups (Ref 25). In an oxygen atmosphere above 500 °C, the oxygen functional groups rapidly increase due to the generation of O = C-O, which affects the improvement of interfacial bonding strength (Ref 25). When the surface treatment was performed using nitric acid, the C = C bonds were broken and the oxygen functional groups increased (Ref 7). In contrast, plasma treatment in an argon atmosphere does not result in the generation of new oxygen functional groups on the surface of carbon fibers due to the use of an inert gas (Ref 21). Agnieszka Kyzioł et al. reported that, during argon plasma treatment, the C-H bonds on the surface were cut to produce free radicals and there was no change in the oxygen functional groups (Ref 26). When the treated biomaterial is exposed to air, it reacts quickly with oxygen, causing surface oxidation and peroxide formation (Ref 26). Polar chemical functional groups, such as -NH2 (amino groups) and -OH (hydroxyl groups), are generated on the surface of carbon fibers. These polar groups may form hydrogen bonds and covalent bonds with resin, thereby contributing to improved interfacial adhesion (Ref 27,28). Previous research reported that when oxygen atoms on the carbon fiber surface were exposed to plasma energy, O = C-O bonds were generated as the distance between the oxygen atoms decreased (Ref 29). Another study showed that O = C-O bonds may be generated by attaching oxygen to an end of the ring (Ref 30). In this research, as C-O and C = O on the carbon surface after the plasma treatment were greatly reduced compared to desizing, and the binding of O = C-O was generated and increased compared to the untreated, the binding ring between carbons was broken by the plasma energy, CO2 was removed, and the carbon and oxygen bonds were greatly increased. Activation of functional groups may improve the surface energy of the carbon fibers, and they may chemically react with resin. The resulting increase in surface energy may strengthen interfacial bonds between the carbon fibers and resin (Ref 31).
The contact angle was measured by using a hydrophilic and hydrophobic wetting liquid, and the surface energy according to the plasma treatment time was calculated by substituting the value of Eq. 1.
As shown in Fig. 6, after plasma treatment for 1 min, the contact angle was decreased compared to the untreated. However, after plasma treatment for 3 min, the contact angle increased compared to that at 1 min. The contact angle increased significantly at a plasma treatment time of 5 min. From the surface energy results obtained from the contact angle, it can be seen that surface energy increased in response to plasma treatment times up to 1 min. It then decreased, with a significant decrease observed after 5 min. In terms of the ratio of polarity surface energy among surface energies, the treatment time was the highest in 1 min and then gradually decreased, indicating that the increase in surface energy of the plasma-treated carbon fiber was caused by an increase in polarity surface energy (Fig. 7a). The ratio of polarity/surface energy is an index of oxygen functional groups introduced on the surface of carbon fibers. This ratio was highest (36.63%) after a treatment time of 1 min. It started to decrease after 3 min and significantly decrease after 5 min (Fig. 7b). After 1 min of treatment, the polar surface energy is increased due to the introduction of a lactone group; when the treatment time is more than 3 min, lactone, hydroxyl, and carbonyl groups are decreased, thus decreasing polar surface energy. Plasma treatment is reported to cause partial oxidation on the surface of carbon fiber, thereby facilitating adsorption of carbon fiber and polar molecules (Ref 32). As the surface energy of carbon fiber increases, the binding force to a resin is enhanced (Ref 33). In previous studies, as the temperature increased during heat treatment in a nitrogen atmosphere, the oxygen functional groups increased and the free energy of the polar surface decreased. On the other hand, it has also been reported that the contact angle decreases and the free energy of the polar surface increases upon the introduction of active oxygen functional groups during heat treatment in an oxygen atmosphere (Ref 30). In this study, the contact angle decreased after the plasma treatment, and the interfacial bonding force increased because atoms penetrated the exposed carbon fiber surface in the oxygen atmosphere. On the other hand, the plasma treatment damaged exposed carbon fibers, and a relationship between oxygen functional groups combined with carbon on the surface was broken, thereby increasing the contact angle and reducing polar surface energy.
Based on an analysis of its mechanical and chemical characteristics, the mechanisms of surface changes and oxygen functional groups of carbon fiber after different plasma treatment times are shown schematically in Fig. 8. The sizing agent in rCF was removed using acetone treatment to reduce an oxygen functional group, and as the time of plasma treatment increased up to 1 min, the oxygen atoms in C-O and C = O present on the surface of the carbon fiber were removed with CO or CO2. On the other hand, it is thought that the oxygen functional groups are significantly increased by breaking the C–C and C = C bonds and attaching oxygen atoms, or by actively forming O = C-O as the oxygen atoms come close to each other. However, after more than 3 min of treatment time, the oxygen atoms were continuously penetrating the carbon fiber and reacting violently with the carbon atoms inside and on the surface, causing damage to the carbon fiber.
4 Conclusions
This research investigated the effect of plasma treatment in an argon and oxygen atmosphere on mechanical and chemical properties of carbon fiber surfaces at different treatment times. There was no difference in the surface characteristics or tensile properties of the plasma-treated carbon fibers compared to untreated after treatment for 1 min. According to the change in the oxygen functional group, C-O and C = O present in the carbon fiber were removed in the form of CO and CO2, and oxygen in the oxygen atmosphere penetrated into the carbon fiber to significantly generate and increase O = C-O. The surface energy was highest after plasma treatment for 1 min, which is attributable to a significant rise in surface energy due to the increase in the lactone groups introduced into the carbon fiber. An increase in surface energy is expected to improve interfacial bonding with resin in carbon composite materials, thereby having a positive effect on the physical properties of these materials. In this study, damage to the carbon fiber surface was observed when the plasma treatment exceeded 1 min, with the damage increasing in accordance with an increase in the treatment time. After a plasma treatment time of 5 min, carbon fiber tensile strength decreased significantly compared to that of the untreated. It was due to the exposure of C–C and C = C on the surface of the treated carbon fibers and small number of C-O, C = O, and O = C-O, which reduced the surface energy. In future, composites may be manufactured by sizing surface-treated carbon fiber under optimal conditions, and the mechanical characteristics will be compared and evaluated to contribute to the commercialization of automobile parts.
References
H.-K. Kang, J.-Y. Kim, H.-Y. Kim and Y.-O. Choi, Study of Stabilization Process of PAN Precursor and its Characteristics Change by Plasma Treatment, Korean Soc. Compos. Mater., 2021, 34(1), p 23–29. https://doi.org/10.7234/COMPOSRES.2021.34.1.023
D. J. Hayne, F. Stojcevski, D. B. Knorr, N. T. Tran and L. C. Henderson, Surface Modification of Carbon Fibres Using Ring-Opening Metathesis Polymerization, Compos. Part A: Appl. Sci. Manuf., 2021, 145, p 106374. https://doi.org/10.1016/j.compositesa.2021.106374
D. J. Eyckens, J. D. Randall, F. Stojcevski, E. Sarlin, S. Palola, M. Kakkonen, C. Scheffler and L. C. Henderson, Examining Interfacial Interactions in a Range of Polymers using poly(ethylene oxide) Functionalized Carbon Fibers, Compos. Part A: Appl. Sci. Manuf., 2020, 138, p 106053. https://doi.org/10.1016/j.compositesa.2020.106053
H. Sun, G. Guo, S.A. Memon, W. Xu, Q. Zhang, J.-H. Zhu and F. Xing, Recycling of Carbon Fibers from Carbon Fiber Reinforced Polymer Using Electrochemical Method, Compos. A, 2015, 78, p 10–17. https://doi.org/10.1016/j.compositesa.2015.07.015
R. Fukui, T. Odai, H. Zushi, I. Osawa, K. Uzawa and J. Takahashi, Recycle of Carbon Fiber Reinforced Plastics for Automotive Application, Ninth Japan Inter. SAMPE Symposium, 2005, 29, p 44–49.
F.-L. Jin and S.-J. Park, Preparation and Characterization of Carbon Fiber-Reinforced Thermosetting Composites: A Review, Carbon, 2015, 16(2), p 67–77. https://doi.org/10.5714/CL.2015.16.2.067
J. Li and C.L. Cai, The Carbon Fiber Surface Treatment and Addition of PA6 on Tensile Properties of ABS Composites, Curr. Appl. Phys., 2011, 11(1), p 50–54. https://doi.org/10.1016/j.cap.2010.06.017
J. Sun, F. Zhao, Y. Yao, Z. Jin, X. Liu and Y. Huang, High Efficient and Continuous Surface Modification of Carbon Fibers with Improved Tensile Strength and Interfacial Adhesion, App. Surf. Sci., 2017, 412, p 424–435. https://doi.org/10.1016/j.apsusc.2017.03.279
S. Wang, Z.-H. Chen, W.-J. Ma and Q.-S. Ma, Influence of Heat Treatment on Physical-Chemical Properties of PAN-based Carbon Fiber, Ceram. Int., 2006, 32(3), p 291–295. https://doi.org/10.1016/j.ceramint.2005.02.014
L. B. Nohara, G. P. Filho, E. L. Nohara, M. U. Kleinke and Mirabel Cerqueira Rezende, Evaluation of Carbon Fiber Surface Treated by Chemical and Cold Plasma Processes, Mater. Res., 2005, 8(3), p 281–286. https://doi.org/10.1590/S1516-14392005000300010
H.S. Cho, Hydrophilization of PP Fiber through Atmospheric Pressure Plasma Processing, Text. Coloration Finish., 2021, 33(3), p 113–119. https://doi.org/10.5764/TCF.2021.33.3.113
T. Lee, S. Lee and K. Song, Natural Fiber Reinforced Biocomposites and Biodegradation, Fashion Inform. Technol., 2010, 7, p 10–21. ((in Korean))
J. Yu, L. Meng, D. Fan, C. Zhang, F. Yu and Y. Huang, The Oxidation of Carbon Fibers through K2S2O8/AgNO3 System that Preserves Fiber Tensile Strength, Compos. B Eng., 2014, 60, p 261–267. https://doi.org/10.1016/j.compositesb.2013.12.037
Z. Wang, X. Huang, G. Xian and H. Li, Effects of Surface Treatment of Carbon Fiber: Tensile Property, Surface Characteristics, and Bonding to Epoxy, Poly. Compos., 2016, 37(10), p 2921–2932. https://doi.org/10.1002/pc.23489
X. Wei, W. Zhang, L. Chen, X. Xia, Y. Meng, C. Liu, Qifu Lin, Y. Jiang and S. Gao, Evaluation of Graphitization and Tensile Property in Microwave Plasma Treated Carbon Fiber, Diam. Related Mater., 2022, 126, p 109094. https://doi.org/10.1016/j.diamond.2022.109094
Racim Radjef, Karyn L. Jarvis, Bronwyn L. Fox and Sally L. McArthur, Comparing the Properties of Commercially Treated and Air Plasma Treated Carbon Fibers, Surf. Coat. Technol., 2021, 408, p 126751. https://doi.org/10.1016/j.surfcoat.2020.126751
K.R. Sumesh, J. Anton, Petr Spatenka and H. J. Sourkova, Experimental Studies on the Influence of Plasma Treatment of Polyethylene in Carbon Fiber Composites: Mechanical and Morphological Studies, Polymers, 2022, 14(6), p 1095. https://doi.org/10.3390/polym14061095
S. Zhang, W.B. Liu, L.F. Hao, W.C. Jiao, F. Yang and R.G. Wang, Preparation of Carbon Nanotube/Carbon Fiber Hybrid Fiber by Combining Electrophoretic Deposition and Sizing Process for Enhancing Interfacial Strength in Carbon Fiber Composites, Compos. Sci. Technol., 2013, 88, p 120–125. https://doi.org/10.1016/j.compscitech.2013.08.035
Y. Shin, Y. Qiao, N. Canfield, Z. Yu, H.M. Meyer, D.R. Merkel, E.K. Nickerson, N.S. Kanbargi, A. Ortiz, A.K. Naskar and K.L. Simmons, Significant Slowdown of Plasma-Optimized Surface Energy Deactivation by Vacuum Sealing for Efficient Adhesive Bonding, Compos. B Eng., 2022 https://doi.org/10.1016/j.compositesb.2022.110001
H. Lee, H. Wei and J. Takahashi, The Influence of Plasma in Various Atmospheres on the Adhesion Properties of Recycled Carbon Fiber, Macromol. Res., 2015, 23(11), p 1026–1033. https://doi.org/10.1007/s13233-015-3141-y
J. Lin, C. Sun, J. Min, H. Wan and S. Wang, Effect of Atmospheric Pressure Plasma Treatment on Surface Physicochemical Properties of Carbon Fiber Reinforced Polymer and its Interfacial Bonding Strength with Adhesive, Compos. Part B: Eng., 2020, 199, p 108237. https://doi.org/10.1016/j.compositesb.2020.108237
C. Dighton, A. Rezai, S.L. Ogin and J.F. Watts, Atmospheric Plasma Treatment of CFRP Composites to Enhance Structural Bonding Investigated Using Surface Analytical Techniques, Int. J. Adhes. Adhes., 2019, 91, p 142–149. https://doi.org/10.1016/j.ijadhadh.2019.03.010
H. Zhang and W. Li, Plasma-grafting Polymerization on Carbon Fibers and Its Effect on Their Composite Properties, Appl. Surf. Sci., 2015, 356, p 492–498. https://doi.org/10.1016/j.apsusc.2015.08.016
S.H. Han, H.J. Oh and S.S. Kim, Evaluation of Fiber Surface Treatment on the Interfacial Behavior of Carbon Fiber-Reinforced Polypropylene Composites, Compos. B, 2014, 60, p 98–105. https://doi.org/10.1016/j.compositesb.2013.12.069
G. Kim, H. Lee, K. Kim and D.U. Kim, Effects of Heat Treatment Atmosphere and Temperature on the Properties of Carbon Fibers, Polymers, 2022, 14(12), p 2412. https://doi.org/10.3390/polym14122412
A. Kyzioł and K. Kyzioł, Surface Functionalization with Biopolymers via Plasma-Assisted Surface Grafting and Plasma-Induced Graft Polymerization—Materials for Biomedical Applications, Biopolymer Grafting: Applications. Elsevier, 2018, p 115–151. https://doi.org/10.1016/B978-0-12-810462-0.00004-1
C. Sun, J. Min, J. Lin and H. Wan, Effect of Atmospheric Pressure Plasma Treatment on Adhesive Bonding of Carbon Fiber Reinforced Polymer, Polymers, 2019, 11(1), p 139. https://doi.org/10.3390/polym11010139
J. Song, Q. Yuan, X. Liu, D. Wang, F, Fu, and W. Yang, Combination of Nitrogen Plasma Modification and Waterborne Polyurethane Treatment of Carbon Fiber Paper Used for Electric Heating of Wood Floors, BioResources, 2015, 10(3): 5820−5829. https://doi.org/10.15376/biores.10.3.5820-5829.
J. Kim, Y. Yamada and S. Sato, Oxygen Migration and Selective CO and CO2 Formation from Epoxidized Fullerenes, J. Phys. Chem. C, 2014, 118(13), p 7076–7084. https://doi.org/10.1021/jp4120332
E. Bozaci, K.Y. Sever, M. Sarikanat, Y. Seki, A. Demir, E. Ozdogan and I. Tavman, Effects of the Atmospheric Plasma Treatments on Surface and Mechanical Properties of Flax Fiber and Adhesion between Fiber-Matrix for Composite Materials, Compos. B, 2013, 45(1), p 565–572. https://doi.org/10.1016/j.compositesb.2012.09.042
L. Jian, Effect of Sizing Agent on Interfacial Properties of Carbon Fiber-reinforced PMMA Composite, Compos. Adv. Mater., 2021, 30, p 1–6. https://doi.org/10.1177/2633366X20978657
C. Sun, J. Min, J. Lin, H. Wan, S. Yang and S. Wang, The Effect of Laser Ablation Treatment on the Chemistry, Morphology and Bonding Strength of CFRP joints, Int. J. Adhes. Adhes., 2018, 84, p 325–334. https://doi.org/10.1016/j.ijadhadh.2018.04.014
G. Yang, T. Yang, W. Yuan and Y. Du, The Influence of Surface Treatment on the Tensile Properties of Carbon Fiber-Reinforced Epoxy Composites-Bonded Joints, Compos. B, 2019, 160, p 446–456. https://doi.org/10.1016/j.compositesb.2018.12.095
Acknowledgments
This study was supported by the Improvement Strategy of Material & Component Technology Development Program (No. 20012924) funded by the Ministry of Trade, Industry, and Energy in Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, H., Kim, G., Kim, K. et al. Effect of Plasma Treatment Condition on Mechanical and Chemical Properties of Carbon Fibers. J. of Materi Eng and Perform 32, 415–422 (2023). https://doi.org/10.1007/s11665-022-07632-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-022-07632-4