Abstract
This study investigated the possibility of improving strength and density of polystyrene (PS) parts produced by selective laser sintering (SLS) without additives or post-processing. Post-processing methods are used by researchers to achieve optimal mechanical properties. An efficient method was proposed to systematically evaluate the PS powders and optimize the SLS process, in order to overcome the challenges of sintering the PS parts. The tailored SLS powder was produced from PS granule, and its properties including particle size and particle morphology, powder bed density and Hausner ratio (HR) were analyzed in order to evaluate powder quality. The FT-IR test was also utilized to investigate the possible effects of cryogenic milling and SLS processes on the material’s chemical compounds. Then, the effects of input parameters, including powder bed temperature as well as power and speed of the laser on the parts’ mechanical properties, were investigated. The sample’s maximum tensile strength and elongation were, respectively, 17.06 ± 0.9 MPa and 2.75% ± 0.55. The results revealed that it is possible to strengthen PS samples without additives and post-processing. However, due to the powder particle burning and confinement in the molten pool, the increase in energy density beyond the optimum level created spherical pores in the structure that weakened the sample’s strength. It is indicated that the energy density of the laser can be increased to achieve higher strength of specimens and dimensional accuracy can be controlled. TGA analysis of the SLS samples demonstrates when the energy density exceeds 0.4 J/mm2, degradation and decomposition take place in the specimens.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Laser sintering (LS) is an additive manufacturing (AM) method that is used extensively in important industries (Ref 1). In LS, the powdered materials are spread in the production area using a roller or a blade and selectively melted with laser energy. The 3D components are made in layers and the powder acts as the support material which can be reused later. The most important advantage of SLS compared to other AM technologies is its ability to process a wide range of materials, including polymers, metals, ceramics and composites. Due to their variety, performance and application in various industries, polymers are used widely in SLS, whose technological outlook points to extensive adoption in various fields (Ref 2). The need for more polymer processing materials is felt more than ever after considering the advancements in AM with regards to prototyping and final components, as it currently fails to meet the requirements of current commercial products. The capabilities of this technology are clear to see, and in order to compete with traditional methods, the current materials should be qualitatively and quantitatively improved. At the same time, the shortage of powder producers, and the high costs of production and manufacturing systems have increased the cost of services. The growing interest of the SLS parts in different industries has resulted in high demand for new inexpensive materials. This method allows for producing geometrically complex and mechanically accurate components with minimal processing and is therefore considered an important research opportunity, and various sectors of the industry have recognized LS’s potential (Ref 3). New material can expand the LS method’s ability to produce various parts; and in the meantime, using powder raw material slows the applicability of LS as most polymer materials are available as pellet and granules. The physical and inherent properties of any new material should be analyzed before use in LS. The most important properties include powder flowability, melting point, further crystallization and laser absorption (Ref 4). The presence of powder particles of different sizes may affect flow efficiency and powder compression in the production bed, which in turn increases the final component’s porosity and reduces mechanical strength. Tiwari et al. (Ref 5) determined the ideal particle size range to be 50 to 90 µm for polymers, wherein smaller particles effectively fill the gaps between larger particles.
The polymers utilized in SLS belong to thermoplastic class one and can be categorized into amorphous and semi-crystalline polymers. Due to their low melting viscosity in the SLS process, semi-crystalline polymers show a higher processing ability that can be used for obtaining fully compressed SLS parts with great mechanical properties (Ref 6). Also, semi-crystalline polymers have a specific processing window, which makes it possible to more easily optimize the powder bed temperature and produce parts with better properties. Semi-crystalline polymers such as polyethylene (PE) (Ref 7,8,9), polypropylene (PP) (Ref 10, 11), polyamide 6 (Ref 12,13,14), polyamide 11 (Ref 2, 15), polybutylene terephthalate (PBT) (Ref 16,17,18,19), polyether ketone (PEK) (Ref 20,21,22), polyether ether ketone (PEEK) (Ref 23,24,25,26) and thermoplastic elastomers such as thermoplastic polyurethane (TPU) (Ref 27, 28) have been investigated extensively, and some have been released to the market.
According to the literature, SLS parts produced by amorphous polymers, such as polycarbonate (PC) (Ref 29, 30) and polystyrene (PS) (Ref 31,32,33,34,35,36), are commonly weak due to inadequate sintering and high porosity (Ref 7, 29), which describes the requirement of wax infiltration after processing. Polystyrene is a practical thermoplastic polymer and can be widely used as the SLS material to fabricating prototype model due to its low thermal expansion (Ref 37, 38). Therefore, any attempt in order to increase the mechanical properties and eliminating the post-processing stage of the PS fabricated samples could develop the material applications.
In addition, the expense associated with producing parts using expensive AM devices has placed such equipment out of reach for most researchers. The expense of commercial materials is another challenge that has greatly impacted the end price of produced parts (Ref 7). These circumstances have led many researchers to produce in-house powders for their research projects using various methods. The literature illustrates that producing powders appropriate for SLS is difficult. There are some attempts to produce PBT (Ref 17), PC (Ref 39), polycaprolactone (PCL) (Ref 40) and PS (Ref 34) home-made powders. There are several methods to produce SLS powder. There are several methods to produce SLS powder, such as ball milling, grinding, cryogenic milling and spray drying. Cryogenic milling is a conventional method for producing SLS powder and has been providing promising results (Ref 17, 41). Mys et al. (Ref 42) have investigated PS powder production using spray drying method. Due to the solution’s tendency to minimize surface tension, one of the advantages of spray drying is creating spherical particles. Nevertheless, low solubility of PS is a disadvantage which prevents the process and the enlargement of spherical particles. A significant reduction of crystallization was also observed, which led to the assumption of no significant degradation. Hence, this PS production method requires further optimization before use.
According to narrow processing window of PS, fabrication of parts using the SLS method is significantly more difficult than materials such as polyamide. Therefore, the SLS PS specimens required several post-processing operations to enhance their strength. Yang et al. (Ref 33) processed PS polymer and high impact PS using the SLS method. The preheating temperature used for processing was 92 to 100 °C. The relative density of produced samples was 48.2%, the tensile strength and elongation were, respectively, 1.57 MPa and 5.03% for PS. They also determined the energy density for producing elasticity samples according to the best dimensional accuracy. It appears that their low energy density was the reason for their poor mechanical properties, which resulted in surface fusion and weak bond between powder particles. Zheng et al. (Ref 43) studied PS post-processing methods to improve part strength. The tensile strength of the SLS PS sample was increased from 1.6 MPa to 37.78 ± 0.42 MPa after infiltration with two different epoxy resins. Shi et al. (Ref 32) also studied on SLS process of PS. They showed the produced components had good dimensional accuracy, although the components lack the optimal mechanical properties. Therefore, they used epoxy resin to increase the mechanical properties. However, they observed shrinkage of the samples after the post-processing operation, which had a negative effect on the dimensional accuracy of components.
Polystyrene is a suitable material for use in investment casting due to its low thermal expansion. In previous studies, the main challenge in SLS of PS polymer has been poor mechanical properties of components. Although, post-processing operations enhance mechanical properties, it can lead to increase in cost and time of manufacturing and possibly reducing the dimensional accuracy of parts. Therefore, in this study, the production of parts with appropriate accuracy, optimal mechanical properties without post-processing operations and reduction of cost and manufacturing time have been investigated. According to this purpose, an inexpensive method of PS powder production from granule through cryogenic milling was used in the first stage. After obtaining the powder’s properties, single-layer samples were created to determine the processing window. Multilayer samples with different energy densities were produced at the end, and density and tensile strength were measured in order to evaluate the mechanical performance of samples. The SEM imaging, FT-IR and TGA analyses were also performed to find a better explanation for the results.
Materials and Methods
Material
Polystyrene granules were prepared to produce the SLS powder. PS granules (GPPS1540 grade, Tabriz Petrochemical Company, Iran) were used for producing the required PS powder. Table 1 shows the PS granule’s properties.
Cryogenic Milling
Cryogenic milling is a method that can be used for grinding granules. The granules were converted into powder using the milling at 240 RPM and − 40°C (in the presence of liquid nitrogen) for 180 minutes. Cryogenic milling device consists of a steel chamber, a number of ZrO2 spherical balls, a stirrer and a liquid nitrogen inlet. Due to the significant effect of particle size on flowability and the SLS process’s success, the powder was filtered to the suitable sizes using the sieve, where all particles larger than 125 µm and smaller than 25 µm were separated using 120 and 500 mesh size sieves, respectively. The achieved powder was then graded using the 170, 230, 325 and 500 sieves in the 90-125, 63-90, 45-63 and 25-45 µm ranges and finally mixed together with the proper percentages. In addition to powder size, particle size is also very significant; and the D10, D50 and D90 were used for measurement, while SEM analysis and image processing were used for particle geometry analysis.
Powder Bed Density
Powder bed density was measured due to the importance of sintered part density. In order to measure powder bed density, a container with a specific volume was placed on the bed, and the powder was spread on the production table using a roller at 60, 76, 103 and 120 mm/s speeds (Fig. 1). Powder bed density was then obtained by removing the measurement container and calculating the powder weight.
Hausner Ratio (HR)
The HR was used for powder flowability analysis. HR is the tapped to bulk density ratio (Eq. (1)) and is classified as follows (Ref 44):
-
1.
HR < 1.25: easily fluidized
-
2.
1.25 < HR < 1.4: decreasing fluidization behavior
-
3.
HR > 1.4: fluidization problems
In order to measure HR, bulk material density was measured first, and the PS powder was poured into a graduated container and placed on the shaker for 60 s. Then, the tapped powder volume was measured. This test was repeated five times in ambient conditions. Finally, HR was obtained by dividing tapped powder density by bulk density.
Differential Scanning Calorimetry (DSC)
The DSC method (SDOM device, Sanaf Co, Iran) was used for determining the PS glass transition temperature. The samples were heated up to 300°C at a rate of 10°C per minute, kept at equilibrium for 5 minutes, and cooled at a rate of 10°C per minute in the N2 environment.
Fourier Transform Infrared (FT-IR)
FT-IR is used for analyzing the chemical composition of polymers or composites. Researchers use FT-IR to study surface reflection properties, isotope analysis, obtain concentration, identify unknown and organic compounds, pollutants and impurities. This study used the FT-IR test (Tensor27 device, Bruker GmbH, Germany) for analyzing and monitoring possible changes in the PS polymer’s chemical compound after the cryogenic milling and SLS process.
Thermogravimetric Analysis (TGA)
TGA provides information about physical phenomena, such as phase transitions, absorption, adsorption and desorption and also chemical phenomena including chemisorption, solid-gas reactions and thermal decomposition. It is a special useful technique to study polymeric materials, including thermoplastics, thermosets, elastomers, composites, plastic films, fibers, coatings, paints and fuels. In this study, the TGA (PerkinElmer 6000 device, USA) was utilized to investigate possible thermal decomposition of PS polymer chains in the SLS process. Samples between 18 and 56 mg were heated at a rate of 20°C /min from room temperature to 800°C under a flowing nitrogen atmosphere.
Morphology Analysis
The scanning electron microscopy (SEM) (Philips XL 30) analysis was used for the determination of particle size and cross-section morphology of the produced specimens.
Selective Laser Sintering (SLS)
An SLS device (IUT P-100, Isfahan University of Technology) was used for producing samples. The device uses a carbon dioxide laser with a spot diameter of approximately 0.2 mm as the heat source. The powder is spread using a counter-rotating roller and preheated by infrared heaters.
Laser power (p), scanning velocity (v), scan distance (s) and powder bed temperature are the most important parameters in the SLS process that affect part quality. Energy density (ED) is often used for calculating the input energy, which is obtained using Eq 2 (Ref 29).
In the first step, a series of preliminary tests were performed to determine the suitable powder bed temperature. Then, the tensile samples were produced according to ASTM D638 Type V standard to evaluate mechanical properties. The fabrication parameters are shown in Table 2.
Parts Density Measurement
The density of SLS samples was measured before evaluating mechanical properties using the Archimedes method (ASTM D792). Since the PS density approaches that of water, ethanol was used as the immersion liquid for the measurements. Mechanical tests were taken a few days after density measurements to ensure ethanol evaporation from the samples.
Result and Discussion
Powder Characteristics
Particle Size
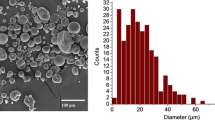
In the pre-sintering steps, powder evaluation usually includes determination of the size distribution, flowability, endo- and exothermal and rheological properties of polymer powders. Proper powder flowability leads to homogeneous distribution in each layer. Measuring the produced powder particle size shows that the irregular grains had a somewhat wide size distribution of 25 to 125 µm. Figure 2a and 2b, respectively, shows the morphology and size distribution of PS powder particles. The powder sizes of D10, D50 and D90 were, respectively, 54, 89 and 120 µm.
Powder Bed Density
Table 3 shows the powder bed density results. According to the results, it can be concluded that the highest and lowest powder bed densities (approximately 35.21 ± 0.38 and 32.88 ± 0.62, respectively) corresponded to the roller speeds of 60 and 120 mm/s. Although the powder bed density decreased by increasing the roller speed, the measured values of the densities revealed that increasing the roller speed from 60 to 103 mm/s does not significantly change the powder bed density. In fact, the drop in powder bed density occurred when the speed increased to 120 mm/s. Therefore, based on this section results, the roller speed during the SLS process was set to 103 mm/s as it decreases fabrication idle times while do not have noticeable negative effect on the powder bed density.
Hausner Ratio
The HR was calculated for powder flowability analysis after adjusting the PS powder size. The HR value calculated for the powder was 1.27 ± 0.04, which indicates adequate flowability for the produced PS powder (as it is close to 1.25).
Glass Transition
Figure 3 shows the curve obtained from the DSC test. The PS glass transition temperature on the curve is approximately 106°C. Accordingly, it is preferable to set the powder base temperature below the glass transition temperature. This study considered 80, 85, 90 and 100°C temperature levels to find the optimum powder bed temperature.
Fourier Transform Infrared (FT-IR)
The FT-IR analysis was performed to determine possible impurities and stability of chemical compounds before and after cryogenic milling and the SLS process. This analysis was performed to ensure there was no effect on the PS structure resulting from cryogenic milling and the SLS process. Figure 4 shows FT-IR analysis results of the granule, powder and printed PS parts. The peaks of this analysis appear according to the type of chemical bond, and Fig. 4 proves that all samples have a similar curve. In order to better explain the diagram, the peaks in the 600-780 range represent the C-H flexural bond outside the benzene rings, the peaks in the 1400-1500 range represent the tensile C-C bond of the benzene ring, the peaks in the 2850-2950 range represent methylene bond (H-C-H) and the peaks in the 3000-3100 range indicate the tensile C-H bond in the aromatic ring. This clearly demonstrates that the PS chemical compound is not disturbed by the cryogenic milling and the SLS process. In other words, using cryogenic milling can save the initial costs of providing commercial powder and maintain the properties of the fabricated SLS samples.
Sample Fabrication
Single-layer samples were produced using different powder bed temperatures varying from 80 to 100°C. The results showed that the bed temperatures above 100°C resulted in powder particles coalescence, while temperatures below 90°C led to severe distortion and twisting that prevents the spread of the powder’s next layers (Fig. 5). Therefore, the suitable temperature of PS processing was set between 90 and 100°C.
After evaluating the optimum powder bed temperature, the multilayered parts were fabricated by the SLS process. The various energy densities from 0.16 to 0.53 J/mm2 were implemented to produce multilayer cubic samples (10×10×3.2 mm). Table 4 illustrates the results of cube height measurements for different energy densities.
As shown by Table 4, increasing energy density increases part height, such that an energy density of 0.53 J/mm2 increased product height by about 0.67mm, while the height of the specimens with an energy density of 0.16 J/mm2 was very close to their nominal value (3.2 mm). Therefore, when the energy density is 0.16 J/mm2, the dimensional accuracy of the parts is desirable and there isn't need for modification. As the energy density increased from 0.16 to 0.53 J/mm2, the height of the samples was increased drastically. Also, a deviation in the X and Y plane from the nominal dimensions was observed for the parts fabricated with higher energy levels.
Therefore, it can be concluded that using low energy densities in the previous researches could be contributed to eliminate the negative effects on the fabricated parts dimensions (Ref 32, 33, 43). In the meantime, part strength is directly correlated to energy density, and it is therefore important to resolve dimensional error to achieve the maximum strength. In this research, a correction method was utilized to overcome the dimensional deviation from the nominal values. The dimensional error in the X and Y direction of the parts was resolved through CAD model correction. On the other hand, the number of the parts layer was modified to maintain the overall parts height constant. After resolving the dimensional error problem, the standard tensile parts were fabricated according to Table 2 (Fig. 6).
As it can be seen in Fig. 6, the increase in energy density has caused to change the color of the specimens from white to semi-transparent brown. Benefiting from the previously mentioned correction method, the parts dimensions were the same for the different samples (the negative effect of energy density was eliminated) and three samples were produced for each case.
Mechanical Properties
Figure. 7 and 8 shows the tensile strength and elongation of SLS samples with various processing parameters. Figure 7 indicates that increasing the energy density increases tensile strength, and the sample with a 0.4 J/mm2 energy density has the highest strength (17.52 MPa ± 0.9). Afterward, increasing the energy density from 0.4 J/mm2 led to reduce the tensile strength from its optimum value.
As shown in Fig. 8, increasing energy density also increases elongation of the parts. The 0.4 J/mm2 sample has the highest elongation at break at approximately 2.75 ± 0.55%.
In order to investigate the causes of this phenomenon, the density and morphology of samples were evaluated.
Density
The relative density of printed parts was measured due to the effect of energy density on mechanical properties. It is clear that the overall trend of the density graph (Fig. 9) resembles the mechanical test’s results (Fig. 7, 8). Increasing the energy density increases the relative density of parts. The maximum density of samples produced by 0.4 J/mm2 energy density was about 81%.
According to Fig. 7, 8 and 9, it can be concluded that the parts fabricated with 0.4 J/mm2 exhibited the maximum value in the tensile, elongation and density tests. Comparing the results with previous studies, it is clear that the mechanical properties of parts improved without using post-processing. The higher implemented energy density alongside the modified particle size of the powder and optimal speed of the roller could be the main reasons that enhanced the mechanical performance of the fabricated samples. It should be mentioned here that due to the correction method explained in 3.2, the dimensions of the parts fabricated with all energy densities were the same as the CAD file model (No dimensional deviation was observed even for the parts fabricated with energy densities above 0.4 J/mm2).
Cross-Section Morphology
SEM analysis was utilized to find an explanation about the mechanical performance of the samples. Figure 10 shows the fracture surface morphology of tensile samples at various energy densities. At an energy density of 0.16 J/mm2 (Fig. 10a), the laser energy was able to melt just the small particles and larger particles remained unmelted in the structure, which creates a weak bond between particles and porosity of over 60%. Increasing the energy density to 0.4 J/mm2 (Fig. 10b) provides the appropriate energy required for fusing larger particles that leaves behind scant unmelted particles, while there are still signs of spherical pores in the structure. Increasing the energy density to 0.45 J/mm2 (Fig. 10c) led to more spherical pores inside samples. The number and sizes of pores in the cross-section of samples were fabricated by 0.53 J/mm2 (Fig. 10d) and were increased in comparison with the others, which could explain the lower density of the parts fabricated with energy densities above 0.4 J/mm2.
Figure 10(b) illustrates that the powder particles are infused properly and the bond between particles is well-formed. Likewise, the number and size of spherical pores are fewer than Fig. 10(c) and (d). The irregular shape of the powder particles suggests that form of the pores resulting from inadequate fusion should be irregular. As the energy density increased from 0.4 J/mm2, the irregular shape porosities were reduced in the structure, but spherically formed pores were generated. The number of spherical pores inside the structure was increased by increasing energy density (As can be seen in Fig. 10c and d). Therefore, the spherical pores that existed in the structure should be a result of other phenomena that required further investigation.
Effect of energy density on polymer chains
According to the observations, signs of material burning were noticed during the fabrication of the samples, especially when the energy densities above 0.4 J/mm2 were utilized (Fig. 11). This phenomenon brings this idea to mind that the spherical pores (observed in SEM imaging) can be attributed to trapped gas inside the molten pool. Therefore, it can be deduced increasing the energy density increased the amount of gas produced, and more spherical pores were formed inside the structure. The results also can explain the density and mechanical performance findings, where both mentioned factors are debilitated when the input energy density exceeds 0.4 J/mm2.
According to the results, it is evident that the more the energy density, the higher the mechanical strength. Meanwhile, in the case of high energy densities, burning of the material is a limitation. Observation of the smoke during the SLS process using high energy density alongside changing the color of the parts (Fig. 6) could be signs of burning and decomposition of polymer chains. In order to profound investigation, TGA and DSC analyses were performed on the samples.
In the SLS process, materials have to undergo a high thermal load such as the preheating, the laser scanning and the cooling process (Ref 45). Furthermore, high-energy lasers scission of polymer chains might happen in the sintered, which causing a molecular weight reduction of the SLS PS and reduces degradation temperature of the SLS PS samples (Ref 46). TGA results of the PS powder and SLS parts are shown in Fig. 12. According to the results, the starting temperature of the degradation for powder and the specimen with an energy density of 0.4 J/mm2 is approximately 400°C. The degradation of samples produced with 0.53 J/mm2 energy density is started from 370°C which 30°C lower than the 0.4 J/mm2 samples. The ending of the degradation process for the powder and specimen with an energy density of 0.4 J/mm2 is 496°C and for the specimen with an energy density of 0.53 J/mm2 is 465°C, which can be caused by complete burning of the polymer composition. Therefore, it can be stated that the degradation graph trend for the powder and 0.4 J/mm2 fabricated samples were the same that could be the sign of no material burning during the process. In fact, the energy densities up to 0.4 J/mm2 did not harm the polymer structure and chains effectively. However, the shifting of the degradation curve of the 0.53 J/mm2 specimens could be attributed to the fact that degradation and decomposition have occurred during the SLS process. It seems that utilizing high energy density has led to brake the C-C bonds of polymer chains, and the observed smoke was contributed to form C-O bonding due to the oxygen present in the chamber.
Suggestion of the authors to overcome the problem is optimizing the particle size of the powder. As it was reported in this study, the distribution of the powder particles was − 125/+ 45 microns (Fig. 2). It is evident that the smaller particles require lower amount of energy density to coalesce in comparison with the larger ones. In this case, when the lower levels of energy densities (lower than 0.4 J/mm2) just fused the small particles, the larger ones remained unmelted in the structure. As the energy density increased above 0.4 J/mm2, the appropriate energy to melt the larger powder particles was applied, however, it caused to burning of the smaller powder particles. Thus, powder particle size distribution should be as uniform as possible to reduce negative effects of high energy density. In other words, homogenizing powder particle size will allow for selecting the energy density so as to supply the energy required for particle fusions, prevent smoking (bobble generation the molten pool) and achieve parts with the maximum density and enhanced mechanical performance without post-processing.
Importance of Production Cost
In a part of the present study, the required PS powder was prepared in the laboratory. This was due to the fact that the expense of tailored material for AM processes has increased the production costs, such that the powder has a direct impact of 33% to 75% on the final price of SLS parts (Ref 2). Therefore, in-laboratory production of tailored powders for the SLS process can greatly reduce the process costs. The results of this study in comparison with commercial materials (Ref 47,48,49) show that despite using non-commercial material (laboratory powder production), the obtained results represent a significant advantage in strength (Table 5). The first row in Table 5 illustrates the mechanical characteristic of the samples in this study.
The significance of mechanical results is becoming more brilliant by considering powder production costs in comparison with commercial powders. In this study, a low-cost granule ($3 per kilogram) was used to produce PS powder. Considering the costs of cryogenic milling, sieving and other complementary activities, the final cost of the powder was lower than $20 per kilogram, which is much cheaper than commercial powders. For example, one kilogram of PS-200 PS powder produced by Advanced Laser Material Company (ALM) costs about $95. Accordingly, producing parts through SLS does not necessarily require expensive commercial powders, and laboratory production of the suitable powder will save budgetary expenses while also leading to an expectation of good results in terms of strength.
Conclusions
The present study intended to produce PS parts with improved mechanical strength without using post-processing compared to the results reported by previous researchers. Another distinguishing aspect of this study was using home-made powder to reduce production costs as much as possible while maintaining the parts’ final properties. Powder analysis results showed that the powder produced from granule through cryogenic milling had suitable features for the SLS process. The main reason for the poor strength of polystyrene components in previous investigations was the low energy density of the laser. This investigation indicated that the energy density of the laser can be increased and dimensional accuracy can be controlled. Furthermore, it is shown that powder particle size is significant in strength and dimensional accuracy.
Further analysis of the powder bed temperature and energy density showed that specimens with an energy density of 0.4 J/mm2 have the highest strength (17.52 ± 0.9 MPa) and elongation at break (2.75 ± 0.55). Likewise, the highest density of approximately 81% corresponded to the parts produced with an energy density of 0.4 J/mm2. Increasing energy density above 0.4 J/mm2 reduced tensile strength, elongation at break and part density. The presence of spherical pores in the structure of samples at energy densities above 0.4 J/mm2, as a result of material burning, made a negative effect on the performance. When the energy density exceeds 0.4 J/mm2, the material burned and smoke was seen. TGA and DSC analysis results showed that the samples were degraded and the polymer chains were broken. The sample’s color change has also supported this assumption.
Further narrowing the range of powder size particle can noticeably reduce pores and improve the mechanical properties of parts at higher densities. It can be concluded that using optimized powder particle size, alongside implementing higher energy density and dimensional controlling of the samples by correction of CAD file, could result in PS SLS samples without post-processing. Expanding the possibility of producing parts from low-cost materials in AM methods can assist the process’s wider adoption.
References
A. Mazzoli, Selective Laser Sintering in Biomedical Engineering, Med. Biol. Eng. Comput., 2013, 51(3), p 245–256.
R.D. Goodridge, C.J. Tuck and R.J.M. Hague, Laser Sintering of Polyamide and Other, Prog. Mater Sci., 2012, 57(2), p 229–267.
M. Vasquez, J. Cross, N. Hopkinson and B. Haworth, Developing New Laser Sintering Materials for Snowboarding Applications, Proc. Eng., 2012, 34, p 325–330.
S. Dadbakhsh, L. Verbelen, T. Vandeputte, D. Strobbe, P. Van Puyvelde and J.P. Kruth, Effect of Powder Size and Shape on the SLS Processability and Mechanical Properties of a TPU Elastomer, Phys. Proc., 2016, 83, p 971–980.
S.K. Tiwari, S. Pande, S. Agrawal and S.M. Bobade, Selection of Selective Laser Sintering Materials for Different Applications, Rapid Prototyp. J., 2015, 21(6), p 630–648.
C. Yan, Y. Shi and L. Hao, Investigation into the Differences in the Selective Laser Sintering Between Amorphous and Semi-crystalline Polymers, Int. Polym. Process., 2011, 26(4), p 416–423.
R.D. Goodridge, R.J.M. Hague and C.J. Tuck, An Empirical Study into Laser Sintering of Ultra-High Molecular Weight Polyethylene (UHMWPE), J. Mater. Process. Technol., 2010, 210(1), p 72–80.
J. Bai, B. Zhang, J. Song, G. Bi, P. Wang and J. Wei, The Effect of Processing Conditions on the Mechanical Properties of Polyethylene Produced by Selective Laser Sintering, Polym. Test., 2016, 52, p 89–93.
Y. Khalil, A. Kowalski and N. Hopkinson, Influence of Energy Density on Flexural Properties of Laser-Sintered UHMWPE, Addit. Manuf., 2016, 10, p 67–75.
W. Zhu et al., Study on the Selective Laser Sintering of a Low-Isotacticity Polypropylene Powder, Rapid Prototyp. J., 2016, 22(4), p 621–629.
W. Zhu, C. Yan, Y. Shi, S. Wen, J. Liu and Y. Shi, Investigation into Mechanical and Microstructural Properties of Polypropylene Manufactured by Selective Laser Sintering in Comparison with Injection Molding Counterparts, Mater. Des., 2015, 82, p 37–45.
S.K. Dong, J.A. Young, W.B. Sung, C.-H. Kim, and H.-I. Kim, Development of polyamide-6 powders and its effect on the fabrication by selective laser sintering, in ICALEO 2006—25th International Congress on Applications of Laser and Electro-Optics, Congress Proceedings, vol. 2006, no. 1, p. 1807 (2006)
W. Zhou, X. Wang, J. Hu and X. Zhu, Melting Process and Mechanics on Laser Sintering of Single Layer Polyamide 6 Powder, Int. J. Adv. Manuf. Technol., 2013, 69(1–4), p 901–908.
L. Verbelen, S. Dadbakhsh, M. Van Den Eynde, J.P. Kruth, B. Goderis and P. Van Puyvelde, Characterization of Polyamide Powders for Determination of Laser Sintering Processability, Eur. Polym. J., 2016, 75, p 163–174.
A. Salazar, A. Rico, J. Rodríguez, J. Segurado Escudero, R. Seltzer and F.M. De La Escalera Cutillas, Monotonic Loading and Fatigue Response of a Bio-based Polyamide PA11 and a Petrol-Based Polyamide PA12 Manufactured by Selective Laser Sintering, Eur. Polym. J., 2014, 59, p 36–45.
S. Arai, S. Tsunoda, A. Yamaguchi and T. Ougizawa, Effects of Short-Glass-Fiber Content on Material and part properties of Poly(Butylene Terephthalate) Processed by Selective Laser Sintering, Addit. Manuf., 2018, 21, p 683–693.
S. Arai, S. Tsunoda, R. Kawamura, K. Kuboyama and T. Ougizawa, Comparison of Crystallization Characteristics and Mechanical Properties of poly(butylene terephthalate) Processed by Laser Sintering and Injection Molding, Mater. Des., 2017, 113, p 214–222.
J. Schmidt et al., A novel Process for Production of Spherical PBT Powders and Their Processing Behavior During Laser Beam Melting, AIP Conf. Proc., 2016, 1713(1), p 140008.
J. Schmidt et al., Optimized Polybutylene Terephthalate Powders for Selective Laser Beam Melting, Chem. Eng. Sci., 2016, 156, p 1–10.
Y. Wang, E. James and O.R. Ghita, Glass bead filled Polyetherketone (PEK) composite by High Temperature Laser Sintering (HT-LS), Mater. Des., 2015, 83, p 545–551.
O. Ghita et al., High Temperature Laser Sintering (HT-LS): An investigation into mechanical properties and shrinkage characteristics of Poly (Ether Ketone) (PEK) structures, Mater. Des., 2014, 61, p 124–132.
O.R. Ghita, E. James, R. Trimble and K.E. Evans, Physico-chemical behaviour of Poly (Ether Ketone) (PEK) in High Temperature Laser Sintering (HT-LS), J. Mater. Process. Technol., 2014, 214(4), p 969–978.
S. Berretta, Y. Wang, R. Davies and O.R. Ghita, Polymer Viscosity, Particle Coalescence and Mechanical Performance in High-Temperature Laser Sintering, J. Mater. Sci., 2016, 51(10), p 4778–4794.
S. Berretta, O. Ghita and K.E. Evans, Morphology of Polymeric Powders in Laser Sintering (LS): From Polyamide to New PEEK Powders, Eur. Polym. J., 2014, 59, p 218–229.
Y. Wang, D. Rouholamin, R. Davies and O.R. Ghita, Powder Characteristics, Microstructure and Properties of Graphite Platelet Reinforced Poly Ether Ether Ketone Composites in High Temperature Laser Sintering (HT-LS), Mater. Des., 2015, 88, p 1310–1320.
S. Berretta, K.E. Evans and O. Ghita, Processability of PEEK, a New Polymer for High Temperature Laser Sintering (HT-LS), Eur. Polym. J., 2015, 68, p 243–266.
L. Verbelen et al., Analysis of the Material Properties Involved in Laser Sintering of Thermoplastic Polyurethane, Addit. Manuf., 2017, 15, p 12–19.
S. Yuan, F. Shen, J. Bai, C.K. Chua, J. Wei and K. Zhou, 3D Soft Auxetic Lattice Structures Fabricated by Selective Laser Sintering: TPU Powder Evaluation and Process Optimization, Mater. Des., 2017, 120, p 317–327.
H.C.H. Ho, I. Gibson and W.L. Cheung, Effects of Energy Density on Morphology and Properties of Selective Laser Sintered Polycarbonate, J. Mater. Process. Technol., 1999, 89–90, p 204–210.
Y. Shi, J. Chen, Y. Wang, Z. Li and S. Huang, Study of the Selective Laser Sintering of Polycarbonate and Postprocess for parts Reinforcement, Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl., 2007, 221(1), p 37–42.
K. Dotchev and S. Soe, Rapid Manufacturing of Patterns for Investment Casting: Improvement of Quality and Success Rate, Rapid Prototyp. J., 2006, 12(3), p 156–164.
Y. Shi, Y. Wang, J. Chen and S. Huang, Experimental Investigation into the Selective Laser Sintering of High-Impact Polystyrene, J. Appl. Polym. Sci., 2008, 108(1), p 535–540.
J. Yang, Y. Shi, Q. Shen and C. Yan, Selective Laser Sintering of HIPS and Investment Casting Technology, J. Mater. Process. Technol., 2009, 209(4), p 1901–1908.
N. Mys, A. Verberckmoes, and L. Cardon, Processing of syndiotactic polystyrene to microspheres for part manufacturing through selective laser sintering, mdpi.com, 2016.
D. Strobbe, S. Dadbakhsh, L. Verbelen, P. Van Puyvelde and J.P. Kruth, Selective laser Sintering of Polystyrene: A Single-Layer Approach, Plast. Rubber Compos., 2018, 47(1), p 2–8.
Z. Zeng, X. Deng, J. Cui, H. Jiang, S. Yan, and B. Peng, Improvement on Selective Laser Sintering and Post-Processing of Polystyrene (2019)
K.D. Dotchev, S.S. Dimov, D.T. Pham and A.I. Ivanov, Accuracy Issues in Rapid Manufacturing CastFormTM Patterns, Proc. Inst. Mech. Eng. Part B J. Eng. Manuf., 2007, 221(1), p 53–67.
T.S. Hock, S. Trevor, P. Christodoulou and P.K.D.V. Yarlagadda, Experimental Studies on the Accuracy of Wax Patterns Used in Investment Casting, Proc. Inst. Mech. Eng. Part B J. Eng. Manuf., 2003, 217(2), p 285–289.
S. Kloos, M.A. Dechet, W. Peukert and J. Schmidt, Production of Spherical Semi-Crystalline Polycarbonate Microparticles for Additive Manufacturing by Liquid-Liquid Phase Separation, Elsevier, Amsterdam, 2018.
S. Eosoly, D. Brabazon, S. Lohfeld and L. Looney, Selective Laser Sintering of Hydroxyapatite/Poly-ε-Caprolactone Scaffolds, Elsevier, Amsterdam, 2010.
S. Dadbakhsh, L. Verbelen, T. Vandeputte, D. Strobbe, P. Van Puyvelde and J.-P. Kruth, Effect of Powder Size and Shape on the SLS Processability and Mechanical Properties of a TPU Elastomer, Phys. Procedia, 2016, 83, p 971–980.
N. Mys, A. Verberckmoes and L. Cardon, Spray Drying as a Processing Technique for Syndiotactic Polystyrene to Powder Form for Part Manufacturing Through Selective Laser Sintering, Springer, Berlin, 2017.
Z. Zeng et al., “Improvement on Selective Laser Sintering and Post-Processing of Polystyrene. mdpi.com.
G.V. Barbosa-Cánovas, Food Powders: Physical Properties, Processing, and Functionality, Kluwer Academic, New York, 2005.
T.J. Gornet, K.R. Davis, T.L. Starr, and K.M. Mulloy, Characterization of selective laser sintering materials to determine process stability, in Solid Freeform Fabrication Symposium, pp. 546–553, 2002.
B. Singh and N. Sharma, Mechanistic Implications of Plastic Degradation, Polym. Degrad. Stab., 2008, 93(3), p 561–584.
3d Systems, Available: https://www.3dsystems.com/materials/plastic.
ALM, Available: https://alm-llc.com/portfolio-items/ps-200/.
EOS, Available: https://www.eos.info/en/additive-manufacturing/3d-printing-plastic/sls-polymer-materials/ps-polystyrene-primecast.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khazaee, S., Kiani, A., Badrossamay, M. et al. Selective Laser Sintering of Polystyrene: Preserving Mechanical Properties without Post-processing. J. of Materi Eng and Perform 30, 3068–3078 (2021). https://doi.org/10.1007/s11665-021-05606-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-021-05606-6