Abstract
In the present study, the formation of superhydrophobic (SHP) structure on the surface of Mg alloy was investigated by immersion in the CuCl2 and NiSO4 solutions following by soaking in the stearic acid (SA) solution. The results revealed the presence of some stearic acid bonds on the surface of Mg alloy. The contact angle of the surface after the process measured about 151.5°, which could be due to the presence of flake-like morphology and the adsorption of hydrophobic substances of SA. X-ray diffraction pattern showed the presence of NiO as the resistant phase against the diffusion of species. Besides, the values of noise and corrosion resistance regarding SHP Mg were at least three orders of magnitude higher than that of bare Mg alloy due to the formation of SHP structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnesium alloys have shown special remarkable properties including high specific strength, low density, high thermal conductivity, excellent machinability, high damping capacity, good weldability under the controlled ambient, excellent castability, and recyclability. These alloys can be applied in the automotive, aerospace, electronics, medical, and other industries (Ref 1,2,3). However, the inherent poor electrochemical corrosion resistance of Mg alloys due to their low standard electrode potential is a major drawback for their applications (Ref 4, 5). Besides, magnesium possesses high chemical activity and the formed oxide film on the surface of its alloys is porous and non-protective under the natural state (Ref 6). Another problem is that the chemical activity of industrial magnesium alloys is mainly inhomogeneous. For instance, AZ91D magnesium alloy which has been extensively used in the automobile industries is made of α phase (Mg) and β phase (Mg17Al12) (Ref 7).Various surface treatment techniques have been used to reduce the chemical and electrochemical activity of magnesium including physical vapor deposition (Ref 8, 9), electroless plating (Ref 10,11,12), plasma electrolytic oxidation (PEO) (Ref 13,14,15), chemical conversion treatments (Ref 16,17,18), hydroxyapatite coatings (Ref 19,20,21), and the other coatings (Ref 22,23,24).
Surface modification of Mg alloys due to their high activity and inhomogeneity is difficult (Ref 7). Moreover, the formation of the corrosion products is quite complicated and not well understood. For example, when AZ91 Mg alloy is immersed in aqueous solutions, the film on the surface of the α phase consists of three layers including the outer layer of Mg(OH)2, the middle layer of MgO, and the inner layer of Al2O3. The formed surface film on the β phase is different from that on α phase which leads to the inhomogeneity of surface chemistry (Ref 9,10,11). Consequently, it is challenging to control the formation of corrosion products under the laboratory conditions (Ref 25, 26).
In recent years, fabrication of superhydrophobic surfaces has become an area of active fundamental research. In general, similar techniques used for micro- and nanostructure manufacturing, such as lithography, etching, chemical and electrochemical deposition, casting, plasma treatment, and replication have been used for producing superhydrophobic surfaces (Ref 27). There are two main requirements to fabricate a superhydrophobic surface: (1) the surface should be rough and (2) it should have a low surface energy. These two requirements lead to two methods of producing superhydrophobic surfaces. The first method is to make a rough surface from an initially hydrophobic material, and the second method is to modify an initially rough surface by changing the surface chemistry or applying a hydrophobic material (Ref 27,28,29,30). Few researchers have investigated on the SHPS of Mg. The investigated processes categorize on the basis of chemical, electrochemical or physical surface treatment including hydrothermal technique (Ref 31), chemical and electrochemical deposition (Ref 32,33,34,35,36), conversion coating (Ref 37, 38), polymer coating (Ref 39), and etching processes (Ref 40, 41).
The main aim of present study is to creation of a superhydrophobic surface of Mg alloy to protect its surface against corrosion in the aggressive media. Hence, AZ31 alloy was treated in the chemical solution of CuCl2, NiSO4, and stearic acid to form a corrosion-resistant superhydrophobic surface. On the other words, this work is a combination of chemical etching and chemical deposition of the protective compound. In current study, electrochemical behavior of SHP Mg including electrochemical impedance spectroscopy, potentiodynamic polarization, and electrochemical noise spectroscopy was studied. This surface of SHP Mg alloy showed excellent corrosion resistance with superhydrophobicity during 168 h.
Experimental
Materials
AZ31 Mg alloy samples (3% aluminum and 1% zinc) with the dimension of 30 mm × 25 mm × 2 mm were used. Citric acid (99.5%—Samchun), copper chloride (99.8%—Merck), nickel (II) sulfate hexahydrate (extra pure—Samchun), stearic acid (99.7%—Merck), and ethanol (99%) were used without any further purification.
Sample Preparation
Magnesium plates were first abraded with SiC papers up to #2000 and then the plates were ultrasonicated in the methanol for 1 min and subsequently dried by immersion in the 100 ml of 2 wt.% to obtain a high surface activity. At the next stage, the samples were immersed in the 3.4 wt.% copper chloride in the aqueous solution at 90 °C for 5 min followed by immersion in the nickel (II) sulfate hexahydrate solution (0.8 g/100 ml H2O) at 80 °C for 10 min. The obtained samples were immersed in the ethanolic solution of stearic acid (CH3(CH2)16COOH) for 4 h at the room temperature. Finally, the samples were dried at 120 °C for 15 min.
Sample Characterization
The morphology of treated sample (SHP Mg) was studied with a high-resolution scanning electron microscope (CamScan Mira). Contact angles were measured by optical contact angle measuring device (Data physics OCA15 plus). X-ray diffraction (XRD) pattern of the superhydrophobic surface of Mg alloy was obtained by a Philips PW 1730 diffractometer using Cu Kα radiation (40 kV, 40 mA) in the range of 20°-90°. Fourier transform infrared (FTIR) spectroscopic analysis regarding the coatings was performed by Nicolet IR 100 using KBr disk. The scanning ranges were from 4000 to 500 cm−1. The corrosion resistance of the SHP Mg and Mg alloy was tested using an AUTOLAB PGSTAT 30 potentiostat equipped with a three-electrode system with an Ag/AgCl reference electrode consisting of a saturated KCl solution, platinum as the counter electrode, and the sample (SHP Mg and Mg alloy) as the working electrode. Electrochemical impedance spectroscopy (EIS) was conducted in the frequency ranges from 100 mHz to 100 kHz with a perturbation amplitude sinusoidal signal of 10 mV. Corrosion properties of SHP Mg and Mg alloy 3.5 wt.% NaCl and deionized water solutions were investigated by electrochemical impedance tests at various times [1 h, 12 h, 1 day, and 1 week (168 h)]. Potentiodynamic polarization tests were performed in the potential ranges from − 300 to 400 mV versus Ag/AgCl. The scanning rate was set at 2 mV s−1. Corrosion current density (icorr) and corrosion potential (Ecorr) were determined by NOVA software. The corrosion resistance of the working electrode (1 cm2) was investigated in 3.5 wt.% NaCl and deionized water solutions. Three replications were tested to ensure repeatability of data. ZView 3.5c software was used to analyze EIS data.
Electrochemical noise (EN) analysis was conducted in the system containing two identical working electrodes of SHP Mg or Mg and a saturated Ag/AgCl as the reference electrode. These three electrodes were dipped in 3.5 wt.% NaCl solution. The potential and current noise data collected in the time domain were transformed to the frequency domain using the fast Fourier transform (FFT). The electrochemical noise data were recorded for 1024 s at immersion times of 1 h. All electrochemical corrosion measurements were repeated three times to verify the reproducibility of the results.
Results and Discussion
Figure 1(a)-(d) shows FESEM morphology of Mg (a and b) and SHP Mg (c and d) surface with the different magnifications. In the case of Mg with lower magnification (Fig. 1a), the bright and dark phases stand for α-Mg and β-Mg17Al12 phases, respectively (Ref 7). Moreover, the measured contact angle corresponding to Mg alloy was 48°. In the case of SHP Mg, the flake-like morphology was observed after the immersion and modification of Mg surface, which could act as a barrier to the water droplets. As can be observed, the surface morphology after chemical treatment changed completely and composed of the porous and uniform flakes which were evenly distributed on the surface with a preferred direction. These flakes possessed the thickness in the range of 20-50 nm. These flakes could trap the air which led to the prevention of liquid penetration into the coated surface (Ref 42). The measured contact angle was about 151.5°, which depicted superhydrophobicity. The large contact angle between the water and air could prevent the penetration of water or aggressive species toward the surface according to Cassie–Baxter equation (Ref 43). It has been reported (Ref 44) that a robust micro/nanostructure is a key factor for a SHP surface. The hierarchical micro/nanoscaled structure supplied more space to capture the air to achieve superhydrophobicity.
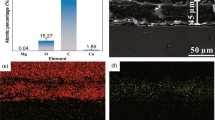
Figure 2(a) and (b) demonstrates x-ray diffraction pattern and energy-dispersive spectroscopy (EDS) related to the SHP Mg. XRD pattern (Fig. 2a) displays the presence of Mg (JCPDS 35-0821), NiO (JCPDS 47-1049), and MgO (JCPDS 01-1235) on the surface of SHP Mg. The XRD patterns showed broad peaks about 37.8° which could be attributed to the (111) plane of NiO phase. That strong (111) plane might be the reason for the preferred direction of flake morphology of SHP Mg. Figure 2(b) illustrates EDS spectra corresponding to the SHP Mg surface. As it can be observed, the coating was constituted of Ni, Mg, S, and O. The presence of Mg could be attributed to the low thickness of chemical deposit and surface roughness. Sulfur and oxygen could arise from the solution during NiO deposition. Although, it shows copper acted as the etchant material and did not deposit on the surface.
Figure 3 shows the process of nickel deposition on the surface of Mg alloy. In the primary stage, the chemical activity of β (Mg17Al12) phase was lower than α (pure Mg) phase (Ref 45). Therefore, α phase dissolved earlier and supplied the Mg2+ cation and electrons. Then, the Ni2+ cations could react with the electrons and hydroxyl ions to form Ni(OH)2 as follows:
However, the formation of NiO might be influenced by the presence of sulfate in the solution as stated in some papers. These particles can react with persulfate (S2O82−) from NiSO4 solution (Ref 46):
This reaction could act as the rate-determining step in the growth of NiO film (Ref 46). Therefore, it can be mentioned that the formation of NiO might be due to dehydration of Ni(OH)2 or reaction with sulfate substances in the solution. The formed phase could deposit on α phase. Consequently, α phase was covered with NiO deposits and dissolution of Mg stopped. In the next stage, the β phase started to dissolve and the production of Mg2+ and Al3+ cations and electrons proceeded. Finally, α phase and β phase were deposited with NiO and the reaction reached the equilibrium. It could be known that the difference of chemical activity between α phase and β phase was the main reason which led to the variation in the amounts of NiO deposition on the two phases and the appearance of the rough surface. In the etching process, the surface was corroded unevenly by chloride ions and the surface possesses higher roughness. In the next stage, Ni ions from NiSO4 solution tended to deposit on the surface and finally a flake-like morphology was created on the uneven surface of Mg alloy.
Figure 4 depicts FTIR spectra of the stearic acid and SHP Mg. The asymmetric and symmetric stretching vibrations of C–H peaks were located at about 2850 and 2920 cm−1, which could be attributed to the presence of the aliphatic groups on the superhydrophobic surface arising from stearic acid. The observed band about 1105 and 1700 cm−1 could be related to the C=O and –COO groups, respectively. C–O and C–C bonds were detected about 1120 and 1635 cm−1, respectively. Also, the band appears about 837 cm−1 indicating C–OR contributing to the superhydrophobic surface. Besides, the peaks at 2910 and 2860 cm−1 corresponded to the asymmetric and symmetric stretching vibrations of –CH2 or –CH3 groups (Ref 47,48,49). These adsorbing bonds corresponding to the SHP Mg could be attributed to the functional groups of SA indicating the presence of SA on the SHP surface. In fact, these observations suggested the formation of carboxylate (CH3(CH2)16COO−) on the Mg substrate.
Figure 5(a) and (b) shows Nyquist spectra corresponding to Mg alloy and SHP Mg alloy after different immersion times (1, 12, 24, and 168 h) in the 3.5 wt.% NaCl. In the case of Mg alloy, the semicircle plot which was related to the capacitive loop in the high–medium-frequency region was associated with the charge transfer resistance and the electric double-layer capacitance. Corrosion resistance (Rct) was measured as the content of Z′ when − Z″ = 0, which was usually calculated at the intermediate frequencies (Ref 24). In the case of SHP Mg, the inductive loop was disappeared which indicated the significant reduction in electrolyte penetration to the interface. The inductive loop can be explained by the different mechanisms including pitting, hydroxide formation, and adsorption (Ref 24). By surface modification of Mg alloy during the mentioned processes, the active surface of this alloy was kept away from chemical species and adsorbing ions. SHP Mg alloy showed two distinct time constants, in which the first was related to the coating resistance at the high-frequency domain arising from chemical deposited film in the NiSO4 containing solution. The second one was attributed to the charge transfer resistance at the low range frequency domain linked to the Mg charge transfer (Ref 24). Second semicircle occurred in the low-frequency domain of the Nyquist plots reflected the electrochemical reaction under the coating. As immersion time was prolonged, the radius of semicircle decreased, indicating the reduction in the charge transfer resistance (Rct) and permeation of aggressive ions to the interface (Ref 24, 45). By passing the time, diameter of the loop related to charge transfer resistance decreases, implying the diffusion of aggressive species through the NiO layer.
Figure 6(a) and (b) shows equivalent circuits corresponding to Mg alloy and SHP Mg alloy, respectively. These models include the solution resistance Rs, a series combination of resistance, R, and inductance, L, in parallel with charge transfer resistance Rct, and the constant phase element (CPE). CPE was used instead of a pure capacitance (C) accounting for non-ideal capacitive response of the interface. The impedance of a CPE is equal to A−1 (i ω)−n, where A is the constant corresponding to the interfacial capacitance, i is the imaginary number, ω is the angular frequency, and n is an exponential factor in the range between − 1 and 1. Depending on the value of n, CPE can illustrate resistance (n = 0, A = R), capacitance (n = 1, A = C), inductance (n = − 1, A = L), and Warburg impedance (n = 0.5, A = W) in the different situations. Pure capacitance behavior is indicated by n = 1, while in practice n often ranges from zero to 1. Heterogeneity of the water diffusion phenomenon could impose deviation from the pure capacitor (Ref 24, 50, 51, and 52). Besides, n is associated with the degree of roughness and the geometry of the coatings (Ref 50). Table 1 shows the obtained parameters from simulation by ZView software. The proposed equivalent circuits fitted very well emphasized by the low value of χ2, which is one of the most important parameters in the curve fitting process (Ref 45, 50). The reported values regarding χ2 were less than 0.01 for all specimens. Rct for Mg alloy after immersion at 1 h, 12 h, 24 h, and 1 week was obtained as 290, 110, 75, and 52 Ω cm2, respectively. This parameter for SHP Mg alloy at the same times reported as 1500, 1100, 950, and 640 kΩ cm2, which could be explained by the effect of superhydrophobicity behavior on the surface of Mg alloy. According to the reported data, corrosion resistance regarding SHP Mg was at least four orders of magnitude higher than that of for bare Mg alloy at each time of immersion. The significant corrosion resistance of SHP Mg alloy can be due to the presence of superhydrophobic surface with the flake-like morphology and the adsorption of low energy substances of stearic acid (Ref 28).
According to Table 1, Rc (coating resistance) value reduced from 3010 to 700 kΩ cm2 for SHP Mg alloy, while Mg alloy showed no obvious time constant related to the coating resistance. In other words, Rc regarding SHP Mg alloy has been appeared due to the barrier properties of NiO compound. Besides, the observed reductions in the Rc values show the degradation of coatings by chloride or hydroxyl ion penetration. By passing the time, coatings showed the higher affinity to the aggressive ions and displayed higher conduction. In other words, the observed decrease in the Rc or increase in CPEc values was attributed to the accumulation of conducting species in the porosities of the coatings (Ref 24). Similarly, CPE is related to the corrosion reaction area, i.e., low CPE implies that relatively low area of the exposed surface is corroded. The fitted values of CPE showed a continuous decrease in the double-layer coating capacitance. The values of n (CPE-P) for all the coatings were closer to unity, indicating a capacitance behavior. However, the deviation observed in the n values was normally arising from the heterogeneity in the coating or water diffusion, which led to the non-uniform current distribution (Ref 53, 54). Although, bare Mg alloy showed inductor element (L) in the low-frequency region. This parameter varied from 145.5 to 62.5 H during the immersion, which could be a sign of a decrease in the induction behavior due to the formation of corrosion products and reduction in active surface exposing the electrolyte.
Table 2 represents two important parameters corresponding to the corrosion properties. |Z|100 mHz and θ10 kHz are suitable parameters to determine the electrochemical properties of the coated surface (Ref 24). If these parameters possess the higher values, corrosion resistance regarding the samples would be greater. Impedance magnitude at 100 mHz (|Z|100 mHz) and phase angle at 10 kHz (θ10 kHz) emphasize the qualitative behavior regarding the corrosion phenomena. If charge transfer resistance and/or double-layer capacitance values are significant, the electrochemical behavior of metal–electrolyte interface will become capacitive. In this case, the current would principally cross through the capacitor and, therefore, the value of phase angle reaches about 90°. In addition, if resistance and/or capacitance values are negligible, the electrochemical response of the metal–electrolyte interface will turn resistive. Consequently, the current would frequently pass through the resistor, and therefore, the value of phase angle would be close to 0° (Ref 24, 55). According to Table 2, the maximum obtained value related to the phase angle at 10 kHz (θ10 kHz) was about 81°. It was reported that the value of θ for intact coatings is close to 90° (Ref 24, 55). At the initial times of immersion, corrosive ions do not have enough times to pass through the coating. By passing the time, phase angle regarding SHP Mg alloy decreased due to the penetration of aggressive species in the protective NiO layer. However, at each time of immersion, θ10 kHz and |Z|100 mHz corresponding to SHP Mg alloy showed the higher magnitude than that of bare Mg alloy due to its better corrosion resistance. As observed |Z|100 mHz reduced from 2.5 to 1.1 MΩ cm2 and 290 to 65 Ω cm2 for SHP Mg and Mg alloy, respectively. Besides, θ10 kHz related SHP Mg decreased from 81 to 70°, while the values of this parameter corresponding to Mg alloy changed from 7 to 2°, which could be due to the effect of superhydrophobic layer on the surface of Mg alloy.
Figure 7 demonstrates potentiodynamic polarization plots corresponding to Mg alloy and SHP Mg in the 3.5 wt.% NaCl solution. Corrosion current density (Icorr) regarding SHP Mg alloy and Mg alloy was estimated about 1.47 nA cm−2 and 82.7 µA cm−2, respectively. As it can be seen, corrosion current density of SHP Mg was lower of about three orders of magnitude than that of Mg sample. The lower corrosion current density of SHP Mg shows the higher corrosion density of this sample in the chloride media. Besides, corrosion potential of SHP Mg was recorded much more positive than Mg sample. It could be attributed to the higher mobility of surface regarding SHP Mg. As stated earlier, NiO phase formed on the surface of Mg by chemical treatment. It could act as a barrier against corrosive species and water molecules and, therefore, the corrosion resistance of SHP Mg shows the higher value in comparison with Mg.
Figure 8 shows power spectral density (PSD) of current related to SHP Mg alloy and Mg alloy after 1 h of immersion in 3.5 wt.% NaCl solutions. In the case of Mg alloy, more severe corrosion can result from the higher PSD (I) values. Therefore, a drop in the noise level could represent a lower electrochemical activity (Ref 56, 57). The better behavior of SHP Mg alloy could be evidenced by using a quantity denoted as noise resistance (Rn). This parameter was calculated from the ratio of the standard deviations of voltage fluctuations divided by the identical amount of current variations. The higher Rn value shows the higher corrosion resistance of mentioned surface (Ref 56, 57). The calculated Rn for SHP Mg alloy and Mg alloy after 1 h of immersion in the NaCl solution obtained 709 and 0.501 kΩ cm2, respectively. Therefore, it could be shown that the corrosion resistance of SHP Mg alloy was greater than Mg alloy as the EIS and polarization tests emphasized this behavior.
Figure 9 shows the relationship between the water contact angle of SHP Mg alloy and the surface before modification by stearic acid with the exposure time. The CA on the surface only slightly decreased to 149.5° from 151.5° after the exposure time of 168 h, while for the untreated sample with stearic acid this value decreased from 49 to 33°. This phenomenon demonstrates that superhydrophobic surfaces have long-term stability, and this will improve the corrosion resistance of magnesium alloy as mentioned before. However, the density of the hydrophobic functional groups on the surface could be decreased by immersing the samples in the NaCl solution, which led to the reduction in contact angle by passing the time. This can indicate that on most parts of the sample surface desorption of the SA molecules or degradation of the flake-like morphology occurred (Ref 33, 58).
Figure 10(a) and (b) illustrates Nyquist spectra and potentiodynamic polarization plots corresponding to Mg alloy and SHP Mg alloy after immersion 1 h in the deionized water. According to Fig. 10(a), Mg alloy showed a semicircle plot which was related to the charge transfer response followed by the induction loop in the low-frequency region. In the case of SHP Mg, the inductive loop was disappeared which indicated the significant reduction in electrolyte penetration to the interface. Rct for Mg alloy after immersion 1 h in the water was obtained as 830 Ω cm2. This parameter for SHP Mg alloy reported as 12 MΩ cm2, which could be explained by the effect of superhydrophobicity behavior on the surface of Mg alloy. The higher reported Rct in the water solution compared 3.5 wt.% NaCl solution could be due to the aggressive behavior of chloride ion exposing the surface. Figure 10(b) shows potentiodynamic polarization plots corresponding to Mg alloy and SHP Mg in the water solution. Corrosion current density (Icorr) regarding SHP Mg alloy and Mg alloy was estimated about 0.27 nA cm−2 and 27 µA cm−2, respectively. As it can be observed, corrosion current density of SHP Mg was much lower than that of Mg alloy, which stated earlier.
Figure 11 illustrates the relationship between the contact angle and the pH value regarding SHP Mg. Contact angles (CA) varied from 148.5° to 152° in the pH range of 2-12, which emphasizes the relative chemical stability of superhydrophobic film under the strong acidic or basic environment. The CA declination from 150° in strong acidic (pH 2) and basic (pH 12) environment could be associated with the loss of air pockets (Ref 59). The lower CA at aggressive media (pH 2 or 12) could be due to the non-homogeneous formation of superhydrophobic film due to the presence of two α phase (Mg) and β phase (Mg17Al12) (Ref 7). These distinct phases could result in the formation of uneven thickness layer on the surface and, therefore, provide the relative susceptibility of the surface toward the penetration of massive aggressive ions.
Conclusion
In this work, superhydrophobic Mg alloy was synthesized by the immersion of Mg plates in the CuCl2/NiSO4 solution following by SA modifier. Modified Mg alloy showed a contact angle of 151.5°, which attributed to the flake-like morphology with nano-sized thickness. This parameter reduced to 149.5° after 168 h of immersion in the NaCl solution. Besides, due to the prevention of water droplet diffusion to the coating, SHP Mg showed a significant corrosion resistance in the chloride media. Icorr regarding SHP Mg recorded more than four orders of magnitude lower than AZ31 Mg alloy. In addition, noise resistance and Rct regarding the SHP Mg alloy possessed the greater values compared to Mg alloys, which was due to the presence of resistant phase of NiO and superhydrophobic tail of stearic species on the surface. Although, SHP Mg showed a contact angle ranged from 148°-152° at the different pHs.
References
A. Atrens, G.L. Song, M. Liu, Z.M. Shi, F.Y. Cao, and M.S. Dargusch, Review of Recent Developments in the Field of Magnesium Corrosion, Adv. Eng. Mater., 2015, 17, p 400–453
Z. Kang and W. Li, Facile and Fast Fabrication of Superhydrophobic Surface on Magnesium Alloy by One-Step Electrodeposition Method, J. Ind. Eng. Chem., 2017, 50, p 50–56
M. La, H. Zhou, N. Li, Y. Xin, R. Sha, S. Bao, and P. Jin, Improved Performance of Mg-Y Alloy Thin Film Switchable Mirrors After Coating with a Superhydrophobic Surface, Appl. Surf. Sci., 2017, 403, p 23–28
G. Song, A. Atrens, G. Song, and A. Atrens, Recent Insights into the Mechanism of Magnesium Corrosion and Research Suggestions, Adv. Eng. Mater., 2007, 9, p 177–183
X.B. Chen, N. Birbilis, and T.B. Abbott, Effect of [Ca2+] and [PO4 3−] Levels on the Formation of Calcium Phosphate Conversion Coatings on Die-Cast Magnesium Alloy AZ91D, Corros. Sci., 2012, 55, p 226–232
R. Udhayan and D.P. Bhatt, On the Corrosion Behavior of Magnesium and Its Alloys Using Electrochemical Techniques, J. Power Sources, 1996, 53, p 103–107
Z. Wang, Q. Li, Z. She, F. Chen, L. Li, X. Zhang, and P. Zhang, Facile and Fast Fabrication of Superhydrophobic Surface on Magnesium Alloy, Appl. Surf. Sci., 2013, 271, p 182–192
J. Senf and E. Broszeit, Wear and Corrosion Protection of Aluminum and Magnesium Alloys Using Chromium and Chromium Nitride PVD Coatings, Adv. Eng. Mater., 1999, 1, p 133–137
G. Wu, X. Zeng, and G. Yuan, Growth and Corrosion of Aluminum PVD-Coating on AZ31 Magnesium Alloy, Mater. Lett., 2008, 62, p 4325–4327
Z.C. Wang, F. Jia, L. Yu, Z.B. Qi, Y. Tang, and G.-L. Song, Direct Electroless Nickel–Boron Plating on AZ91D Magnesium Alloy, Surf. Coat. Technol., 2012, 206, p 3676–3685
D. Seifzadeh and Z. Rajabalizadeh, Environmentally-Friendly Method for Electroless Ni-P Plating on Magnesium Alloy, Surf. Coat. Technol., 2013, 218, p 119–126
E. Georgiza, J. Novakovic, and P. Vassiliou, Characterization and Corrosion Resistance of Duplex Electroless Ni-P Composite Coatings on Magnesium Alloy, Surf. Coat. Technol., 2013, 232, p 432–439
J. Liang, P.B. Srinivasan, C. Blawert, M. Störmer, and W. Dietzel, Comparison of Electrochemical Corrosion Behaviour of MgO and ZrO2 Coatings on AM50 Magnesium Alloy Formed by Plasma Electrolytic Oxidation, Corros. Sci., 2009, 51, p 2483–2492
P.B. Srinivasan, J. Liang, C. Blawert, M. Störmer, and W. Dietzel, Effect of Current Density on the Microstructure And Corrosion Behaviour of Plasma Electrolytic Oxidation Treated AM50 Magnesium Alloy, Appl. Surf. Sci., 2009, 225, p 4212–4218
Z. Yao, Y. Xu, Y. Liu, D. Wang, Z. Jiang, and F. Wang, Structure and Corrosion Resistance of ZrO2 Ceramic Coatings on AZ91D Mg Alloys by Plasma Electrolytic Oxidation, J. Alloys Compd., 2011, 509, p 8469–8474
Y. Chen, B.L. Luan, G.-L. Song, Q. Yang, D.M. Kingston, and F. Benseba, An Investigation of New Barium Phosphate Chemical Conversion Coating on AZ31 Magnesium Alloy, Surf. Coat. Technol., 2012, 210, p 156–165
G. Wang, M. Zhang, and R. Wu, Molybdate and Molybdate/Permanganate Conversion Coatings on Mg-8.5 Li Alloy, Appl. Surf. Sci., 2012, 258, p 2648–2654
X. Wang, L. Zhu, X. He, and F. Sun, Effect of Cerium Additive on Aluminum-Based Chemical Conversion Coating on AZ91D Magnesium Alloy, Appl. Surf. Sci., 2013, 280, p 467–473
T. Masanari and S. Hiromoto, Growth Mechanism of Hydroxyapatite-Coatings Formed on Pure Magnesium and Corrosion Behavior of the Coated Magnesium, Appl. Surf. Sci., 2011, 257, p 8253–8257
R. Rojaee, M. Fathi, and K. Raeissi, Electrophoretic Deposition of Nanostructured Hydroxyapatite Coating on AZ91 Magnesium Alloy Implants with Different Surface Treatments, Appl. Surf. Sci., 2013, 258, p 664–673
S. Sonmez, B. Aksakal, and B. Dikici, Influence of Hydroxyapatite Coating Thickness and Powder Particle Size on Corrosion Performance of MA8M Magnesium Alloy, J. Alloys Compd., 2014, 456, p 125–131
R.-G. Hu, S. Zhang, J.-F. Bu, C.-J. Lin, and G.-L. Song, Recent Progress in Corrosion Protection of Magnesium Alloys by Organic Coatings, Prog. Org. Coat., 2012, 73, p 129–141
L. Xu and A. Yamamoto, In vitro Degradation of Biodegradable Polymer-Coated Magnesium Under Cell Culture Condition, Appl. Surf. Sci., 2012, 258, p 6353–6358
M. Yeganeh and M. Saremi, Corrosion Inhibition of Magnesium Using Biocompatible Alkyd Coatings Incorporated by Mesoporous Silica Nanocontainers, Prog. Org. Coat., 2015, 79, p 25–30
X. Zhang, G. Wu, X. Peng, L. Li, H. Feng, B. Gao, K. Huo, and P.K. Chu, Mitigation of Corrosion on Magnesium Alloy by Predesigned Surface Corrosion, Sci. Rep., 2015, 5, p 1–11
P.R. Cha, H.S. Han, G.F. Yang, Y.C. Kim, K.H. Hong, S.C. Lee, J.Y. Jung, J.P. Ahn, Y.Y. Kim, S.Y. Cho, J.Y. Byun, K.S. Lee, S.J. Yang, and H.K. Seok, Biodegradability Engineering of Biodegradable Mg Alloys: Tailoring the Electrochemical Properties and Microstructure Of Constituent Phases, Sci. Rep., 2013, 3, p 2367–2372
M. Nosonovsky and B. Bhushan, Multiscale Dissipative Mechanisms and Hierarchical Surfaces, Springer, Berlin, 2008
Z. Guo, W. Liu, and B.-L. Su, Superhydrophobic Surfaces: From Natural to Biomimetic to Functional, J. Colloid Interface Sci., 2011, 353, p 335–355
M. Yeganeh and N. Mohammadi, Superhydrophobic Surface of Mg Alloys: A Review, J. Magn. Alloys, 2018, 6(1), p 59–70
Y. Liu, J.D. Liu, S.Y. Li, J.A. Liu, Z.W. Han, and L.Q. Ren, Biomimetic Superhydrophobic Surface of High Adhesion Fabricated with Micronano Binary Structure on Aluminum Alloy, ACS Appl. Mater. Interface, 2013, 5, p 8907–8914
F. Zhang, C. Zhang, L. Song, R. Zeng, S. Li, and H. Cui, Fabrication of the Superhydrophobic Surface on Magnesium Alloy and Its Corrosion Resistance, J. Mater. Sci. Technol., 2015, 31, p 1139–1143
L. Feng, Y. Zhu, W. Fan, Y. Wang, X. Qiang, and Y. Liu, Fabrication and Corrosion Resistance of Superhydrophobic Magnesium Alloy, Appl. Phys. A, 2015, 120, p 561–570
T. Ishizaki and N. Saito, Rapid Formation of a Superhydrophobic Surface on a Magnesium Alloy Coated with a Cerium Oxide Film by a Simple Immersion Process at Room Temperature and Its Chemical Stability, Langmuir, 2010, 26, p 9749–9755
Y. Liu, X. Yin, J. Zhang, S. Yu, Z. Hana, and L. Ren, A Electro-Deposition Process for Fabrication of Biomimetic Super-Hydrophobic Surface and Its Corrosion Resistance on Magnesium Alloy, Electrochim. Acta, 2014, 125, p 395–403
Y. Liu, J.Z. Xue, D. Luo, H.Y. Wang, X. Gong, Z.W. Han, and L.Q. Ren, One-Step Fabrication of Biomimetic Superhydrophobic Surface by Electrodeposition on Magnesium Alloy and Its Corrosion Inhibition, J. Colloid Interface Sci., 2017, 491, p 313–320
Y. Liu, G. Lu, J. Liu, Z. Han, and Z. Liu, Fabrication of Biomimetic Hydrophobic Films with Corrosion Resistance on Magnesium Alloy by Immersion Process, Appl. Surf. Sci., 2013, 264, p 527–532
Z. Qiu, J. Sun, R. Wang, Y. Zhang, and X. Wu, Magnet-Induced Fabrication of a Superhydrophobic Surface on ZK60 Magnesium Alloy, Surf. Coat. Technol., 2016, 286, p 246–250
S.V. Gnedenkov, V.S. Egorkin, S.L. Sinebryukhov, I.E. Vyaliy, A.S. Pashinin, A.M. Emelyanenko, and L.B. Boinovich, Formation and Electrochemical Properties of the Superhydrophobic Nanocomposite Coating on PEO Pretreated Mg-Mn-Ce Magnesium Alloy, Surf. Coat. Technol., 2013, 232, p 240–246
Z. Kang, X. Lai, J. Sang, and Y. Li, Fabrication of Hydrophobic/Super-Hydrophobic Nanofilms on Magnesium Alloys by Polymer Plating, Thin Solid Film, 2011, 520, p 800–806
H. Wang, Y. Weia, M. Liang, L. Hou, Y. Li, and C. Guo, Fabrication of Stable and Corrosion-Resisted Super-Hydrophobic Film on Mg Alloy, Colloid Surf. A Physicochem. Eng. Asp., 2016, 509, p 351–358
W. Xu, J. Song, J. Sun, Y. Lu, and Z. Yu, Rapid Fabrication of Large-Area, Corrosion-Resistant Superhydrophobic Mg Alloy Surfaces, ACS Appl. Mater. Interfaces, 2011, 3, p 4404–4414
M. Liang, Y. Wei, L. Hou, H. Wang, and C. Guo, Fabrication of a Super-Hydrophobic Surface on a Magnesium Alloy by a Simple Method, J. Alloys Compd., 2016, 656, p 311–317
A.B.D. Cassie and S. Baxter, Surface Roughness and Contact Angle, Trans. Faraday Soc., 1944, 40, p 546–551
F. Song, C. Wu, H. Chen, Q. Liu, J. Liu, R. Chen, R. Li, and J. Wang, Water-Repellent and Corrosion-Resistance Properties of Superhydrophobic and Lubricant-Infused Super Slippery Surfaces, RSC Adv., 2017, 7, p 44239–44246
H. Feng, S. Liu, Y. Du, T. Lei, and T. Yuan, Effect of the Second Phases on Corrosion Behavior of the Mg-Al-Zn Alloys, J. Alloys Compd., 2017, 695, p 2330–2338
S.-Y. Han, D.-H. Lee, Y.-J. Chang, S.-O. Ryu, T.-J. Lee, and C.-H. Chang, The Growth Mechanism of Nickel Oxide Thin Films by Room-Temperature Chemical Bath Deposition, J. Electrochem. Soc., 2006, 153, p 382–386
H. Wan and X. Hu, One-Step Solve-Thermal Process for the Construction of Anticorrosion Bionic Superhydrophobic Surfaces on Magnesium Alloy, Mater. Lett., 2016, 174, p 209–212
W.F. Ng, M.H. Wong, and F.T. Cheng, Stearic Acid Coating on Magnesium for Enhancing Corrosion Resistance in Hanks’ Solution, Surf. Coat. Technol., 2010, 204, p 1823–1830
C. Wu, Q. Liu, R. Chen, J. Liu, H. Zhang, R. Li, K. Takahashi, P. Liu, and J. Wang, Fabrication of ZIF-8@SiO2 Micro/Nano Hierarchical Superhydrophobic Surface on AZ31 Magnesium Alloy with Impressive Corrosion Resistance and Abrasion Resistance, ACS Appl. Mater. Interface, 2017, 9, p 11106–11115
M. Saremi and M. Yeganeh, Application of Mesoporous Silica Nanocontainers as Smart Host of Corrosion Inhibitor in Polypyrrole Coatings, Corros. Sci., 2014, 86, p 159–170
A.D. King, N. Birbilis, and J.R. Scully, Accurate Electrochemical Measurement of Magnesium Corrosion Rates; A Combined Impedance, Mass-Loss and Hydrogen Collection Study, Electrochim. Acta., 2014, 121, p 394–406
M. Yeganeh, M. Eskandari, and S.R. Alavi-Zaree, A Comparison Between Corrosion Behaviors of Fine-Grained and Coarse-Grained Structures of High-Mn Steel in NaCl Solution, J. Mater. Eng. Perform., 2017, 26, p 2484–2490
J. Jayaraj, S.A. Raj, A. Srinivasan, S. Ananthakumar, U.T.S. Pillai, N.G.K. Dhaipule, and U.K. Mudali, Composite Magnesium Phosphate Coatings for Improved Corrosion Resistance of Magnesium AZ31 Alloy, Corros. Sci., 2016, 113, p 104–115
A. Keyvani, M. Yeganeh, and H. Rezaeyan, Electrodeposition of Zn-Co-Mo Alloy on the Steel Substrate from Citrate Bath and Its Corrosion Behavior in the Chloride Media, J. Mater. Eng. Perform., 2017, 26, p 1958–1966
A. Keyvani, M. Yeganeh, and H. Rezaeyan, Application of Mesoporous Silica Nanocontainers as an Intelligent Host of Molybdate Corrosion Inhibitor Embedded in the Epoxy Coated Steel, Prog. Nat. Sci. Mater. Int., 2017, 27, p 261–267
R. Naderi and M.M. Attar, Effect of Zinc-Free Phosphate-Based Anticorrosion Pigment on the Cathodic Disbondment of Epoxy-Polyamide Coating, Prog. Org. Coat., 2014, 77, p 830–835
B.P. Markhali, R. Naderi, M. Mahdavian, M. Sayebani, and S.Y. Arman, Electrochemical Impedance Spectroscopy and Electrochemical Noise Measurements as Tools to Evaluate Corrosion Inhibition of Azole Compounds on Stainless Steel in Acidic Media, Corros. Sci., 2013, 75, p 269–279
T. Ishizaki, Y. Masuda, and M. Sakamoto, Corrosion Resistance and Durability of Superhydrophobic Surface Formed on Magnesium Alloy Coated with Nanostructured Cerium Oxide Film and Fluoroalkylsilane Molecules in Corrosive NaCl Aqueous Solution, Langmuir, 2011, 27, p 4780–4788
J. Yuan, J. Wang, K. Zhang, and W. Hu, Fabrication and Properties of a Superhydrophobic Film on an Electroless Plated Magnesium Alloy, RSC Adv., 2017, 7, p 28909–28917
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeganeh, M., Omidi, M. & Eskandari, M. Superhydrophobic Surface of AZ31 Alloy Fabricated by Chemical Treatment in the NiSO4 Solution. J. of Materi Eng and Perform 27, 3951–3960 (2018). https://doi.org/10.1007/s11665-018-3479-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-018-3479-3