Abstract
In this study, pure nickel and Ni-SiC composite coatings were prepared by the conventional electrodeposition technique from nickel sulfamate electrolytic bath containing dispersed SiC particles. The samples obtained after the electrodeposition were subjected to the ultrasonic nanocrystal surface modification (UNSM) technique to improve the surface- and interface-related properties of the coatings. The surface morphology, elemental composition, surface roughness, microstructure, and crystallinity were observed and analyzed by using scanning electron microscope, energy-dispersive x-ray spectroscopy, roughness tester, and x-ray diffraction techniques, respectively. Electrochemical corrosion behavior of the obtained samples was evaluated in 3.5 wt.% NaCl solution by using three electrodes configuration. XRD result revealed the enhanced crystallinity of the UNSM-treated samples. A significant improvement in surface morphology, Vickers microhardness, wear and coefficient of friction, and anti-corrosion property was observed in the UNSM-treated nickel and Ni-SiC coatings compared to the UNSM-untreated samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal matrix composites (MMCs) have drawn considerable attention in recent years due to their excellent mechanical, tribological, electrical, thermal, and electrochemical corrosion properties (Ref 1,2,3,4,5,6). Hence, these composite coatings can be widely applied in the mechanical components to reduce wear and friction between the counter components and the protective components to resist over electrochemical corrosion. Metal matrix composite coatings can be fabricated by several techniques such as liquid-phase processes, solid–liquid processes, deposition techniques, in situ process, and two-phase processes (Ref 7). The deposition technique comprises immersion plating, electroplating, spray deposition, chemical vapor deposition (CVD), physical vapor deposition, spray forming, etc. Out of these several deposition techniques, electroplating technique is a simple, cost-effective, and an easy way to fabricate the composite coatings. The second phase (organic or inorganic particles of nanosize to micron size), to be codeposited, is dispersed in the electrolytic bath during the electrodeposition process.
Being an engineering metal, nickel-based composite coatings by the electrodeposition technique have been extensively studied in recent years (Ref 8,9,10). Reinforcement of a wide range of ceramic particles in the nickel matrix revealed the outstanding final properties of the deposit than the pure nickel coating (Ref 10,11,12). However, the outcome properties of the composite coatings have been found to be closely dependent on the deposition parameters, the second-phase particles and their distribution profile in the nickel matrix, and the amount of the second-phase particles in the composite (Ref 5, 13, 14). On the other hand, silicon carbide (SiC), being chemically inert, cheap and readily available abrasive ceramic material at an industrial point of view, a particular interest is paid for its reinforcement in the nickel plating to produce the Ni-SiC composite coating (Ref 15).
Post-surface modification is one of the important parts of metal finishing industries that is often carried out to well finish the surfaces free from pores, minimize or eliminate the weak points for the chemical attack, and to reduce the wear and friction properties. Ultrasonic nanocrystal surface modification (UNSM) is one of the effective approaches to produce final surface finishing (Ref 16). The principle of the UNSM technique is found elsewhere (Ref 17). In a typical UNSM technique, a WC tip or Si3N4 ball, attached to an ultrasonic horn, strikes the specimen surface up to 2 × 104 shots mm−2 in a very short time (Ref 18). Hence, the surface properties can be altered with respect to its surface roughness, hardness, compressive residual stress, and surface texture. Hence, the UNSM technique is a simple, effective, and economical approach for producing fine surfaces with improved surface properties. Therefore, in this study, we have successfully implemented the ultrasonic nanocrystal surface modification technique to the electrodeposited nickel and Ni-SiC composite coatings, for the first time, to investigate its effectiveness on the morphological, tribological, and electrochemical corrosion properties.
Experimental
Electrodeposition of Ni and Ni-SiC Composite Coatings
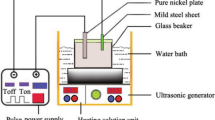
Electrodeposition of pure nickel and Ni-SiC composite coatings was carried out in a 250-mL glass beaker. The sulfamate-type electrolytic bath was utilized to prepare the coatings, of which the concentration and compositions are listed in Table 1. Pure Ni balls were used, inside a titanium basket, as the anode and a polished copper plate of exposed area 1.5 cm × 1.5 cm as the cathode. The cathode was degreased and cleaned in ultrasonic environment for 5 min before the plating. Cetyltrimethylammonium bromide (CTAB) and sodium dodecyl sulfate (SDS) were used as surfactant and an anti-pitting agent, respectively. SiC particles (average size 270 nm) were dispersed in the plating bath under stirring environment. Direct current (current density 80 mA/cm2) was used during the electrodeposition process. The electrodeposition time was set to 120 min.
The electrodeposited samples were cleaned by running distilled water, followed by ultrasonic cleaning for 5 min to remove the loosely adsorbed SiC particles. The samples were then subjected to the ultrasonic nanocrystal surface modification process. Microstructure and phase composition of the samples were evaluated by SEM (Nano-eye, mini-SEM) and XRD (Rigaku DMAX 2200). Vickers microhardness (Buehler Ltd., USA) test was carried out in sample surface by applying 0.98 N load for 10 s at 10 different locations, and the values were averaged.
Ultrasonic Nanocrystal Surface Modification (UNSM)
The principle of UNSM is based on the instrumental conversion of harmonic oscillations of an acoustically tuned body into powerful impulses of ultrasonic high frequency. The energy generated from the oscillations was used to impact the specimen surface from 20,000 to 40,000 shots per square millimeter by a tungsten carbide (WC) ball with a diameter of 2.38 mm attached to an ultrasonic horn. The UNSM process parameters used in this study to treat the electrodeposited specimens are listed in Table 2.
Tribology Test
Wear and coefficient of friction tests were evaluated using a tribometer (CSM instruments; TRN 01-04879) under ball-on-disk method. Steel ball (SAE52100) of a measured average hardness approx. 830 HV and a diameter of 12.7 mm was used as a counterpart, while the prepared sample as disk. A constant load of 10 N was set with the sliding speed of 5 cm/s for 15 min at a radius of 5 mm under dry condition (at 25 °C and 35% humidity). The worn surfaces of the samples were observed by SEM, and the wear debris was analyzed by EDS. Coefficient of friction was recorded simultaneously during the wear test. The specific wear rate of the disk specimens is calculated using the following equation:
where V is the wear volume, N is the normal load applied, and l is the total sliding distance.
Corrosion Test
Electrochemical corrosion test of the samples was performed in a three-electrode cell configuration in 3.5 wt.% neutral NaCl solution at room temperature. A saturated calomel electrode (SCE) was used as the reference electrode and a platinum mesh as counter electrode. The corrosion resistance behavior of the samples was evaluated by using the potentiostat (IVIUMSTAT electrochemical interface, Netherlands). The Tafel curves were recorded at the potential range from −0.6 to +0.6 V versus open-circuit potential (OCP) at 10 mV/s scan rate.
Results and Discussion
Surface Morphologies and Microstructures
Figure 1 shows the surface morphologies of electrodeposited Ni and Ni-SiC composite coatings before and after the UNSM treatment. Surface morphology of pure nickel coating showed some pyramidal structures with random distribution of dissimilar grains exhibiting somewhat rougher surface. It appears that SiC reinforcement may have hindered the successive nickel grain growth and produced further smooth surface in Ni-SiC composite coating (Ref 19). A significant difference in the surface morphologies of the samples is observed before and after the UNSM treatment. Furthermore, despite the visual differences, the measured surface roughness values of both the coatings significantly reduced after the UNSM treatment. It is interesting to note that even after the UNSM treatment, pure nickel coating still possessed the surface with tiny depressions throughout the surface (Fig. 1c). On the other hand, in the Ni-SiC composite coatings after UNSM treatment, no such structures have been observed (Fig. 1d). The reason behind these differences might be the consequence of different microstructures and microhardness values of pure nickel and Ni-SiC composite coatings. During the process of UNSM treatment, an acoustically tuned body is brought to resonance by oscillating ultrasonic transducer which impacts the sample surface at very high frequency that may either deform or cause a severe plastic deformation to the soft surfaces. If the sample is hard enough so that it can bear such impacts, then the effect of UNSM treatment becomes more significant in improving the surface-related properties of the material. Hence, the UNSM-treated Ni-SiC composite coating possessed a fine, smooth, and compact surface compared to the treated pure nickel coating. Figure 2 shows the SEM cross-sectional image of Ni-SiC composite coating with EDS elemental mapping. It shows that the SiC particles are distributed throughout the coating with little agglomeration. The agglomerated particles are submicron to micron in size. Hence, the agglomeration can be ascribed to the involvement in only few SiC particles. Therefore, the result suggests that although the surfactant and stirring condition are used to disperse the particles in the electroplating bath, there still exists some degree of agglomeration of nanoparticles in the deposit.
X-ray diffraction patterns of pure nickel and Ni-SiC composite coatings are shown in Fig. 3. Pure nickel coating shows preferred [100] texture orientation as revealed by the elevation of (200) reflection peak intensity, while the Ni-SiC composite coating revealed the mixed preferred orientation of [211] texture as evidenced by the relatively intense (111) and (311) reflection peaks (Ref 15). The incorporation of SiC nanoparticles into the Ni matrix might have hindered the regular growth of the nickel crystallites; therefore, the intensity of preferred (200) reflection peak appears to be attenuated in Ni-SiC composite coating. After the UNSM treatment, the intensity of (200) reflection peak, again, sharply increased in both the coatings, indicating that the crystallinity of UNSM-treated samples increased significantly. The re-arrangement of nickel atoms might have occurred in a long-range order during the UNSM process. Such atomic re-arrangements might result in the evolution of different textural orientations or the parent textural configuration with an improved crystallinity. The effect of UNSM on crystallinity is more noticeable in Ni-SiC composite coating as represented by the sharp increase in intensity of (200) reflection peak.
Microhardness
Vickers microhardness of pure nickel and Ni-SiC composite coatings, before and after the UNSM treatment, is shown in Fig. 4. It is distinctly noticed that the Ni-SiC composite coating possessed higher microhardness value than the pure nickel coating. Increase in microhardness of Ni-SiC is mainly attributed to the reinforcement of SiC nanoparticles into the nickel matrix. As revealed by EDS analysis, approx. 4 wt.% of SiC nanoparticles were successfully incorporated into the nickel matrix. Incorporation of SiC nanoparticles not only tune the nickel grains, but also act as load bearing agent during indentation and slippage of planes. Hence, the produced metal matrix composite with refined grains and SiC reinforcements both led to improve the microhardness of Ni-SiC composite coatings (Ref 20, 21). On the other hand, after UNSM treatment, further improvements in microhardness of the samples were observed. Yasuoka et al. (Ref 16) also observed the improvement in the hardness and fatigue strength of SUS304 austenite stainless steel using ultrasonic nanocrystal surface modification. However, the increment in Vickers microhardness, after UNSM treatment, was not as significant as that of nanoparticulates reinforcement in the nickel matrix (i.e., between the Ni and Ni-SiC coatings). On the other hand, electrodeposited coating is somehow experienced with the hydrogen embrittlement despite the practice of using different surfactants. During the UNSM treatment, possible elimination of such hydrogen embrittlement is another reason behind the variations in Vickers microhardness.
Tribological Properties
In order to evaluate the tribological behavior of the samples, wear and coefficient of friction tests were carried out in a dry condition. An analysis of wear scars by SEM and the corresponding wear rate of the specimens are presented in Fig. 5 and 6, respectively. The width/or depth of a wear tracks formed in the sample can provide a valuable information regarding the wearability of the sample (Ref 22). Among all the samples, pure nickel coating possessed the largest wear width, indicating that the coating has a poor wear-resistant capability compared to others, while, after the UNSM treatment, the nickel coating demonstrated an improved wear resistant, revealed by the reduced wear width and a decreased wear rate. The magnified view of wear scars and the wear debris is shown in the insets of each image in Fig. 5. It shows that pure nickel, without UNSM-treated, experienced a severe wear compared to the UNSM-treated nickel coating. The Ni-SiC demonstrated improved wear resistant compared to the pure nickel coating (without UNSM treatment). However, compared to the UNSM-treated nickel, no credible improvement in wear observed in Ni-SiC composite. As an evidence, the wear debris produced in the Ni-SiC composite coating without UNSM treatment possessed a plow up of relatively larger wear debris and seemed abrasive–adhesive nature of wear. On the other hand, a significant effect on wear resistance has been observed in the Ni-SiC composite coating after UNSM treatment (see Fig. 6). Also, in Fig. 5(d), it can be observed that an enhanced wear resistance of the UNSM Ni-SiC sample is characterized by the least width of the wear track, relatively smaller size and amount of wear debris, and smaller wear grooves. An analysis of the wear debris by EDS reveals that a higher amount of Fe transferred from the steel counter ball into the UNSM-treated samples as shown in Fig. 7. The presence of higher amount of Fe in the wear debris of UNSM-treated samples is due to the effect of increased hardness of the UNSM-treated samples. Since the wear test was carried out in a dry condition at open atmosphere, the appearance of oxygen in the wear debris might be resulted from the possible oxidized wear products such as Fe2O3, FeO, or NiO (Ref 23). Hence, the improvement in wear characteristics of UNSM Ni-SiC is mainly correlated with the synergistic effect of reinforced SiC nanoparticles, improved Vickers microhardness, and the effect of UNSM treatment (i.e., formation of nanocrystalline deposit with fine grains, and the compactness of the coating surface). Similar trend has been observed in the coefficient of friction, recorded during the wear test, as shown in Fig. 8. Pure nickel coating possessed the highest coefficient of friction, followed by Ni-SiC coating without UNSM treatment. It is also observed that Ni-SiC (without UNSM treatment) and nickel (with UNSM treatment) have shown nearly similar values of coefficient of friction. Although a significant reduction of the coefficient of friction was observed in Ni-SiC coating over pure nickel, effect of UNSM treatment led nickel coating to achieve the values of coefficient of friction similar to that of Ni-SiC composite. Hence, in this regard, the UNSM treatment in nickel has similar effect to that of SiC reinforcement into nickel. Beyond this achievement, a further improvement in coefficient of friction was obtained in the UNSM-treated Ni-SiC composite coating. After the steady state, an average value of coefficient of friction of Ni-SiC composite coating remains constant around 0.4, which is the least value obtained among all the samples in this study.
Electrochemical Corrosion
The electrochemical corrosion behavior of pure nickel and Ni-SiC composite coatings, before and after the UNSM treatment, was observed by monitoring the open-circuit potential after immersion into the 3.5 wt.% sodium chloride (NaCl) solution until reaching a relatively stable value. The potentiodynamic polarization curves of the different samples in neutral 3.5 wt.% NaCl solution are shown in Fig. 9. The pure nickel demonstrated a little active–passive transitions, while the Ni-SiC composite coating has shown the wider passive region and the positive shift in corrosion potential. The corrosion current density, corrosion potential, and the corrosion rates are summarized in Table 3. The data clearly reveal that the Ni-SiC composite coating exhibited enhanced corrosion protection in comparison with the pure nickel coating. The result is attributed to the presence of embedded SiC nanoparticles, refined grains of Ni matrix, and the passivation of the active sites for corrosion initiation (Ref 19). On the other hand, it is interesting to note that the pure nickel coating after UNSM treatment exhibited the improved corrosion resistance than that of Ni-SiC coating without the UNSM treatment. A larger shift of corrosion potential toward the positive value occurred. The outcome is mainly ascribed to the effect of UNSM treatment in nickel coating. As mentioned previously, after the UNSM treatment, an improvement in the crystallinity, smooth and compact surface, and hardness can be observed in the sample. Improvements in these parameters usually favor the enhancement in corrosion protection properties. Furthermore, Ni-SiC composite coating after UNSM treatment revealed a significant improvement in anti-corrosion behavior. It is known that the electrochemical corrosion is a kind of surface phenomenon where the corrosion initiation takes place from the weak point known as active site for corrosion. Corrosion initiation is considered to be one of the most important steps in corrosion behavior of the material. Besides the improvement in surface- and interface-related characteristics of the coatings by UNSM treatment, the embedded inert SiC nanoparticles in Ni-SiC composite coatings further prevented the initiation of corrosion, leading to the shift in corrosion potential (E corr) toward more positive value. Similarly, a deeper corrosion current density (I corr) also reveals the improved corrosion-resistant properties of the UNSM-treated Ni-SiC composite coating.
Conclusion
Pure nickel and the Ni-SiC composite coatings were successfully prepared from the conventional electrodeposition process. Electrodeposited nickel and Ni-SiC composite coatings were subjected to the ultrasonic nanocrystalline surface modification (UNSM) technique. A compact and smooth surface with increased microhardness of the coatings was obtained as a result of UNSM treatment. Similarly, XRD results revealed the improvement in crystallinity of the samples after the UNSM treatment. Tribological properties such as wear and coefficient of friction were significantly improved in UNSM-treated samples in comparison with the parent coatings. Electrochemical corrosion behavior in 3.5 wt.% NaCl solution revealed the substantial improvement in anti-corrosion behavior. Therefore, the surface modification by the application of UNSM technique in the electrodeposited coatings appears beneficial in terms of improving mechanical, tribological, and surface-/interface-related properties.
References
Y. Yang and Y.F. Cheng, Fabrication of Ni-Co-SiC Composite Coatings by Pulse Electrodeposition—Effects of Duty Cycle and Pulse Frequency, Surf. Coat. Technol., 2013, 216, p 282–288
J.-H. Ouyang, X.-S. Liang, J. Wen, Z.-G. Liu, and Z.-L. Yang, Electrodeposition and Tribological Properties of Self-Lubricating Ni-BaCr2O4 Composite Coatings, Wear, 2011, 271, p 2037–2045
G. Gyawali, B. Joshi, K. Tripathi, and S.W. Lee, Preparation of Ni–W–Si3N4 Composite Coatings and Evaluation of Their Scratch Resistance Properties, Ceram. Int., 2016, 42, p 3497–3503
P. Cojocaru, L. Magagnin, E. Gomez, and E. Valles, Nanowires of NiCo/Barium Ferrite Magnetic Composite by Electrodeposition, Mater. Lett., 2011, 65, p 2765–2768
C.T.J. Low, R.G.A. Wills, and F.C. Walsh, Electrodeposition of Composite Coatings Containing Nanoparticles in a Metal Deposit, Surf. Coat. Technol., 2006, 201, p 371–383
D.B. Miracle, Metal Matrix Composites—From Science to Technological Significance, Compos. Sci. Technol., 2005, 65, p 2526–2540
B.C. Kandpal, J. Kumar, and H. Singh, Production Technologies of Metal Matrix Composite: A Review, Int. J. Res. Mech. Eng. Technol., 2014, 4, p 27–32
F.C. Walsh and C. Ponce de Leon, A Review of the Electrodeposition of Metal Matrix Composite Coatings by Inclusion of Particles in a Metal Layer: An Established and Diversifying Technology, Trans. Inst. Metal Finish., 2014, 92, p 83–98
Y. Zhou, F.Q. Xie, X.Q. Wu, W.D. Zhao, and X. Chen, A Novel Plating Apparatus for Electrodeposition of Ni-SiC Composite Coatings Using Circulating-Solution Co-deposition Technique, J. Alloys Compd., 2017, 699, p 366–377
S. Dehgahi, R. Amini, and M. Alizadeh, Microstructure and Corrosion Resistance of Ni-Al2O3-SiC Nanocomposite Coatings Produced by Electrodeposition Technique, J. Alloys Compd., 2017, 692, p 622–628
G. Gyawali, S.H. Cho, and S.W. Lee, Electrodeposition and Characterization of Ni-TiB2 Composite Coatings, Metal Mater. Int., 2013, 19, p 113–118
S. Spanou, E.A. Pavlatou, and N. Spyrellis, Ni/Nano-TiO2 Composite Electrodeposits: Textural and Structural Modifications, Electrochim. Acta, 2009, 54, p 2547–2555
H. Goldasteh and S. Rastegari, The Influence of Pulse Plating Parameters On Structure and Properties of Ni–W–TiO2 Nanocomposite Coatings, Surf. Coat. Technol., 2014, 259, Part C, p 393–400
H.-K. Lee, H.-Y. Lee, and J.-M. Jeon, Codeposition of Micro- and Nano-sized SiC Particles in the Nickel Matrix Composite Coatings Obtained by Electroplating, Surf. Coat. Technol., 2007, 201, p 4711–4717
G. Gyawali, K. Hamal, B. Joshi, A. Rajbhandari, and S. Wohn Lee, Microstructural and Electrochemical Analysis of Ni–SiC Composite Coatings Prepared in Presence of Additives, Mater. Lett., 2014, 126, p 228–231
M. Yasuoka, P. Wang, K. Zhang, Z. Qiu, K. Kusaka, Y.-S. Pyoun, and R.-I. Murakami, Improvement of the Fatigue Strength of SUS304 Austenite Stainless Steel Using Ultrasonic Nanocrystal Surface Modification, Surf. Coat. Technol., 2013, 218, p 93–98
A. Amanov, I.S. Cho, Y.S. Pyoun, C.S. Lee, and I.G. Park, Micro-dimpled Surface by Ultrasonic Nanocrystal Surface Modification and Its Tribological Effects, Wear, 2012, 286–287, p 136–144
A. Amanov, Y.-S. Pyun, J.-H. Kim, and S. Sasaki, The Usability and Preliminary Effectiveness of Ultrasonic Nanocrystalline Surface Modification Technique on Surface Properties of Silicon Carbide, Appl. Surf. Sci., 2014, 311, p 448–460
G. Gyawali, S. Cho, D. Woo, and S. Lee, Pulse Electrodeposition and Characterisation of Ni-SiC Composite Coatings in Presence of Ultrasound, Trans. Inst. Metal Finish., 2012, 90, p 274–281
E.A. Pavlatou, M. Stroumbouli, P. Gyftou, and N. Spyrellis, Hardening Effect Induced by Incorporation of SiC Particles in Nickel Electrodeposits, J. Appl. Electrochem., 2006, 36, p 385–394
M.R. Vaezi, S.K. Sadrnezhaad, and L. Nikzad, Electrodeposition of Ni-SiC nano-composite Coatings and Evaluation of Wear and Corrosion Resistance and Electroplating Characteristics, Colloids Surf. A, 2008, 315, p 176–182
K. Tripathi, G. Gyawali, A. Amanov, and S.W. Lee, Synergy Effect of Ultrasonic Nanocrystalline Surface Modification and Laser Surface Texturing on Friction and Wear Behavior of Graphite Cast Iron, Tribol. Trans., 2017, 60, p 226–237
P. Gyftou, M. Stroumbouli, E.A. Pavlatou, P. Asimidis, and N. Spyrellis, Tribological Study of Ni Matrix Composite Coatings Containing Nano and Micro SiC Particles, Electrochim. Acta, 2005, 50, p 4544–4550
Acknowledgments
This research was supported by the Global Research Laboratory Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (MEST) of Korea (Grant Number: 2010-00339).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gyawali, G., Joshi, B., Tripathi, K. et al. Effect of Ultrasonic Nanocrystal Surface Modification on Properties of Electrodeposited Ni and Ni-SiC Composite Coatings. J. of Materi Eng and Perform 26, 4462–4469 (2017). https://doi.org/10.1007/s11665-017-2891-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-017-2891-4