Abstract
Superior mechanical properties of copper are needed in industries to meet high application requirement. In this study, an electroformed copper with superior mechanical properties is achieved by using a simple ultra-low-concentration copper sulfate bath containing neither chloride nor organic additives. Copper deposits obtained in the copper sulfate concentrations ranging from 30 to 60 g/L exhibit high tensile strength and elongation simultaneously. A maximum tensile strength of 256 MPa is achieved, with an elongation ratio of 31%, at a copper sulfate concentration of 30 g/L, while a maximum elongation ratio of 43% is achieved, with a tensile strength of 216 MPa, at a concentration of 50 g/L. It has been found that the copper sulfate concentration affected the hydrogen content, grain arrangement and orientation index of copper deposits which determine their mechanical properties. When the copper sulfate concentration is less than 30 g/L, the excessive hydrogen content of the deposits thus produced leads to poor compactness and inferior mechanical properties. At concentrations over 50 g/L, a disordered arrangement of grains and a significant increase in the peak of (111) lead to an increase in the tensile strength but a decrease in the elongation ratio. This approach provides an effective and economical method for the copper deposits achieving superior mechanical properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper has high electrical and thermal conductivities and high ductility, and these properties have led to its widespread use in a number of industries, including electronics, MEMS and instrument manufacture. Electroforming is a metal forming process using electrodeposition, and it has attracted much attention in recent years for its high replication precision. Copper is one of the main metals that can be used in electroforming. So copper electroforming has been employed to prepare lots of precision parts, such as battery electrodes, sensors, contacts, the gear of micro motor and so on. With continuous increasing requirements regarding application of copper, superior mechanical properties are needed for improving reliability and prolonging the service life of copper parts. So how to improve the tensile strength and elongation of copper deposits simultaneously becomes an attractive task and a research hotspot in industries (Ref 1,2,3,4,5).
In the conventional preparation method of electroformed copper in an acid sulfate bath, whose copper sulfate concentration was 200 g/L and sulfuric acid concentration was 60 g/L, copper deposits with a tensile strength of 150 MPa and a elongation ratio of 18% were obtained, and it was difficult to meet high application requirement (Ref 6). Then, some studies used external assistance to improve the mechanical properties of electrodeposited copper layers (Ref 1, 6). With the use of ultrasonic assistance, although the tensile strength was greatly increased to 310 MPa, the elongation ratio was decreased to 15% (Ref 6). Then, some studies also used pulse power or periodic reversal power to optimize the mechanical properties. The copper deposits with a tensile strength of 252 MPa and an elongation ratio of 20% were obtained using a periodic reversal process (Ref 7). Apparently, a high tensile strength and a high elongation ratio could not be achieved simultaneously by using above methods.
Until now, the use of additives may be the only way to obtain copper deposits with high tensile strength and elongation simultaneously (Ref 8,9,10). In one investigation, an organic additive was used in an acid sulfate bath to optimize the crystal structure of the deposit, and the copper deposits with a tensile strength of 214 MPa and an elongation ratio of 52% were obtained (Ref 8). Chloride ion is another commonly used additive. In one study, copper was deposited using a periodic reversal process in an acid sulfate bath, with the addition of chloride ions. The copper deposits with a tensile strength of about 281 MPa and an elongation ratio of about 39.5% were obtained (Ref 11). Although the copper deposits with superior mechanical properties can be obtained by using additives, it is difficult to keep the concentration of additive stable during longer electroforming processes, and the concentration is difficult to be detected for maintenance. The concentration can change owing to evaporation of the solution and codeposition of the additives. The mechanical properties of copper deposits can vary greatly as a result of just small fluctuations in additive concentration, and even poor mechanical properties are obtained (Ref 9, 11). So the mechanical properties of copper deposits obtained in an acid sulfate bath with additives are not stable.
In the study described in this paper, to allow convenient maintenance of the electroforming solution and obtain copper deposits with stable mechanical properties, a simple acid sulfate bath without additives was used for copper electroforming. Studies have shown that the use of additives can refine grain and optimize crystal structure by increasing the throwing power and cathodic polarization (Ref 8,9,10). So a high acid concentration of 170 g/L was used to increasing the throwing power (Ref 11, 12). An ultra-low concentration (20-80 g/L) of copper sulfate was used to increase the cathodic polarization by decreasing exchange current density (Ref 13, 14). A direct current was used. The mechanical characteristics and crystal structure of copper deposits were investigated. These results indicate that an ultra-low-concentration sulfate bath without additives can be used to prepare copper deposits with high tensile strength and elongation, effectively and economically.
Experimental Procedure
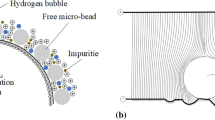
Copper electroforming was carried out on a cylindrical stainless steel mandrel of diameter 48 mm and length 80 mm acting as the cathode, as shown in Fig. 1, which was rotated at the center of the bath to stir the electrolyte in its vicinity and thereby accelerate solution renewal near the cathode for improving the structural uniformity of the copper deposit. Before electroforming, the mandrel was mechanically polished and ultrasonically cleaned in alcohol. Phosphor copper was used as the anode. The experimental conditions were listed in Table 1. The electrolyte solution was prepared using distilled water. The experiment was carried out with six copper sulfate concentrations: 20, 30, 40, 50, 60, and 80 g/L.
The thickness of copper deposits was about 0.3 mm. After electroforming, the mandrel was flushed and dried. A CSS-2202 tensile strength tester was used to measure strength. The tensile rate was kept at 0.2 mm/min. The fracture surface morphology was examined using a Hitachi S-3400N scanning electron microscope. A LECOTCH600 instrument was used to determine the hydrogen content of the deposited copper layer. A JEM-2000EX transmission electron microscope (TEM) was used to characterize the grain size and distribution.
Results and Discussion
Figure 2 shows the test results for the tensile strength and elongation ratio of copper deposits prepared with different copper sulfate concentrations. As the copper sulfate concentration was increased from 20 to 30 g/L, the tensile strength increases by 39% and the elongation ratio by 121%. When the concentration exceeded 30 g/L, the tensile strength of the copper deposits decreased until a concentration of 60 g/L was reached, after which it increases. The elongation ratio continued to increase until a concentration of 50 g/L was reached, after which it decreased. As shown in Fig. 2, the maximum tensile strength of the copper deposit was 256 MPa and was achieved at a copper sulfate concentration of 30 g/L. At this point, the elongation ratio was 31%. The maximum elongation ratio was 43% and was achieved at a copper sulfate concentration of 50 g/L, at which the tensile strength was 216 MPa. The stress-strain curves of the copper deposits prepared at 30 and 50 g/L are shown in Fig. 3. The inset image in Fig. 3 shows the tensile specimen of copper deposits. As clearly shown in Fig. 2, in comparison with copper deposits obtained in traditional electrolytes (Ref 6, 10), the copper deposits obtained in the copper sulfate concentrations ranging from 30 to 60 g L−1 exhibit high tensile strength and elongation simultaneously.
Figure 4 shows the hydrogen content of the copper deposits prepared with different copper sulfate concentrations. As can be seen, when the copper sulfate concentration was increased from 20 to 30 g/L, the hydrogen content of the copper deposit decreased from 32 to 19 ppm, and the tensile strength and elongation ratio were both improved greatly as shown in Fig. 2. The anomalous mechanical properties of copper deposits at 20 g/L may be attributed to hydrogen bubbles embedded in the copper deposits which could cause the formation of fine pores in the copper deposits. As a result, the compactness of the deposits was poor, which led to a great decrease in tensile strength and elongation ratio. When the concentration exceeded 30 g/L, the hydrogen content varied in the range 13–19 ppm, and there was no significant correlation between the variation trends of tensile strength and elongation ratio and the variation trends of hydrogen content in copper deposits. This implies that the effect of hydrogen content on the mechanical properties is weakened when the concentration exceeds 30 g/L.
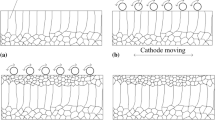
Figure 5 shows TEM images of the grains of copper deposits produced at different copper sulfate concentrations. As can be seen, the grain size increased with an increase in the copper sulfate concentration. It may be attributed to the decrease in cathodic polarization. The exchange current density decreased with a decrease in metal ion concentration. Moreover, a decrease in exchange current density led to an increase in the cathodic polarization, then the grain size increases (Ref 13, 14). As the copper sulfate concentration was increased from 30 to 50 g/L, the trend of tensile strength and elongation ratio was accord with the trend of grain size. When the copper sulfate concentration was 30 g/L, the grain size was finest. At this point, the tensile strength was highest and the elongation ratio was lowest. This is in accordance with the fine-grain strengthening theory (Ref 15). With an increase in grain size, the tensile strength decreased and the elongation ratio increased. Above 50 g/L, the grains became significantly coarser, inhomogeneous in size and of irregular shape. This may be attributed to the decrease in throwing power caused by a decrease in cathodic polarization (Ref 12). So, with an increase in the copper sulfate concentration, the grains became greater and show disorder in arrangement. The disorder arrangement of grains meant the nonuniform growth of grain which might lead to the easy formation of crystal defects. The dislocation lines appear in the grain as shown in the arrow in Fig. 5 when the copper sulfate concentration was above 50 g/L. The dislocation density is defined as the total length of the dislocation lines in the unit volume crystal. So the disorder arrangement of grains may increase the dislocation density of grains. Studies have shown that the increased dislocation density of grains increased the strength and decrease the elongation (Ref 16, 17). So the tensile strength of the copper deposits increased and the elongation ratio decreased. From Fig. 2 and 5, it could be seen that the crystal structure changes are consistent with the trends of variation of the tensile strength and elongation ratio of the copper deposits.
Figure 6 shows the XRD pattern of the copper deposits, also performs to verify the cause of the changing tensile strength and elongation ratio. When the copper sulfate concentration was 20 g/L, there were only peaks of (220) and (331). The intensity of the peak of (220) was the largest of all peaks. As shown in Fig. 6, when the copper sulfate concentration was less than 50 g/L, with an increase in the copper sulfate concentration, the peak of (111), (200) and (311) appeared and slightly increased, and the peak of (220) decreased. Above 50 g/L, the peak of (220) continued to decrease, and the peak of (111) significantly increased in particular. Figure 7 shows the texture coefficient of peaks of the copper deposits produced at different copper sulfate concentrations. The texture coefficient was used to characterize the degree of preferred orientation (Ref 18). As shown, with an increase in the copper sulfate concentration, although the (220) represented a preferential orientation, the texture coefficient of (220) decreased. This means that the degree of preferred orientation of (220) decreased. The texture coefficient of (111) increased with an increase in the copper sulfate concentration. In particular, there was a significant increase in the texture coefficient of (111) above 50 g/L. The preferred orientation was related to the growth rate of each crystal face, and the growth of (111) face was promoted. So the concentration change of copper sulfate may affect the growth of crystallites and their relative speed of growth, which leads to the change of surface diffraction density and orientation index (Ref 19). Study has shown that the peak of (111) of copper deposits with high tensile strength and low elongation ratio represents a preferential orientation (Ref 20). So an increase in peak of (111) may lead to an increase in the tensile strength and a decrease in the elongation ratio.
Figure 8 shows the fracture surface morphology of copper deposits after strength testing. The fracture surface morphology could reflect the mechanical properties of copper deposits. From Fig. 2 and 8, it can be seen that the morphological changes in the fracture surface are consistent with the trends of variation of the tensile strength and elongation ratio of the copper deposits. As shown in Fig. 8, the mode of tensile fracture was dimple fracture. The mechanical properties of the deposits determined the number, dimension and distribution of dimples. From examination of the dimples in all the samples, it was found that the greatest number of dimples in the microstructure occurred at a copper sulfate concentration of 30 g/L. Moreover, at this concentration, the dimples were shallow and small. This implies a high resistance to deformation and a high tensile strength. The most homogeneous distribution of dimples in the microstructure was obtained at a copper sulfate concentration of 50 g/L. Furthermore, the dimples achieved their maximum depth and width at this concentration, as well as exhibiting a high degree of deformation and thus the highest elongation ratio.
Conclusions
An electroformed copper with superior mechanical properties is achieved by using a simple ultra-low-concentration copper sulfate bath containing neither chloride nor organic additives. Copper deposits obtained in the copper sulfate concentrations ranging from 30 to 60 g/L exhibit high tensile strength and elongation simultaneously. It has been found that copper sulfate concentration affected the hydrogen content, grain arrangement and orientation index of copper deposits which determined their mechanical properties. When the copper sulfate concentration is less than 30 g/L, the excessive hydrogen content of the deposits thus produced leads to a poor compactness and inferior mechanical properties. At concentrations over 50 g/L, the disordered arrangement of grains and the significant increase in the peak of (111) lead to an increase in tensile strength but a decrease in elongation ratio. It is indicated that an ultra-low-concentration sulfate bath without additives can be used to prepare copper deposits with high tensile strength and elongation, effectively and economically.
References
Z.W. Zhu, D. Zhu, and N.S. Qu, Synthesis of Smooth Copper Deposits by Simultaneous Electroforming and Polishing Process, Mater. Lett., 2008, 62, p 1283–1286
H. Li, X. Li, G. Bai et al., Copper Electroforming for UV LIGA Technology, IEEE International Vacuum Electronics Conference 2014, p 245–246.
J.H. Ren, Z.W. Zhu, C.J. Shen et al., Abrasive-Assisted Copper Electroforming Process with Offset Anode in Sulfate bAth, Acta Armament., 2015, 36, p 1736–1742
S. Semboshi, Y. Sakamoto, H. Inoue et al., Electroforming of Oxide-Nanoparticle-Reinforced Copper–Matrix Composite, J. Mater. Res., 2015, 30(4), p 1–7
P.C. Huang, K.H. Hou, H.H. Sheu et al., Wear Properties of Ni-Mo Coatings Produced by Pulse Electroforming, Surf. Coat. Technol., 2014, 258, p 639–645
Q. Liao, W. Li, H. Liu et al., Fabrication of Nanostructured Electroforming Copper Layer by Means of an Ultrasonic-Assisted Mechanical Treatment, Chin. J. Aeronaut., 2010, 23, p 599–603
P.R. Sankar, B.Q. Khattak, A.K. Jain et al., Electroforming of Copper by the Periodic Reversal Process, Surf. Eng., 2013, 21(21), p 204–208
S.H. He, The Mechanical Properties of Electroformed Copper, China Electroplating Youth Academic Exchange, 1999
T.G. Woo, I.S. Park, and K.W. Seol, The Effect of Additives and Current Density on Mechanical Properties of Cathode Metal for Secondary Battery, Electron. Mater. Lett., 2013, 9, p 535–539
Q. Liao, L.Q. Zhu, H.C. Liu et al., Effects of Ultrasonic on Microstructure and Mechanical Properties of Electroforming Copper Layer, Mater. Sci. Technol., 2010, 18, p 206–210
K. Tajiri and T. Nakamura, Periodic Reverse Copper Electroforming From a low Cu-Content Acid Copper Sulfate Bath, Plat. Surf. Finish., 2001, 88, p 60–63
L.M. Feng and Y. Wang, Electroplating Technology, Chemical Industry Press, Beijing, 2010
Y.D. Gamburg and G. Zangari, Theory and Practice of Metal Electrodeposition, Springer, New York, 2011, p 56–67
D. Li, Electrochemical Principles, University Press, Beijing, 1999, p 211–214
K. Lu, X.D. Liu, and Z.Q. Hu, Hall–Petch Relation of Nanocrystalline Materials, Chin. J. Mater. Res., 1994, 8, p 385–391
K. Nakai, T. Sakamoto, R. Asakura et al., Nucleation of Bainite at Small Angle Dislocation Network in Austenite and Its Effects on Mechanical Properties in Steels, Isij Int., 2011, 51(2), p 274–279
S.V. Kovsh, V.A. Kotko, I.G. Polotskii et al., Effect of Cyclic Deformation on the Dislocation Structure and Mechanical Properties of Molybdenum, Chromium, Tungsten, Strength. Mater., 1973, 5(11), p 1306–1311
T.C. Liu, Z.C. Lu, D.R. Li et al., Investigation on the Microstructure and Properties of Electrodeposited Iron-Nickel Alloy Film with Nano-structure, J. Funct. Mater., 2007, 38(1), p 138–141
X. Ye, M.D. Bonte, J.P. Celis et al., Role of Overpotential on Texture, Morphology and Ductility of Electrodeposited Copper Foils for Printed Circuit Board Applications, J. Electron. Mater., 1992, 139(139), p 1592–1600
H. Sakai, T. Yokota, T. Asai et al., High Tensile Strength Electrodeposited Copper Foil and Process of Electrodepositing Thereof, US, US 5958209 A[P], 1999.
Acknowledgments
The authors acknowledge financial support from the National Natural Science Foundation of China (Grant No. 51475239) and Jiangsu Key Laboratory of Precision and Micro-Manufacturing Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, C., Zhu, Z., Zhu, D. et al. Copper Deposits with High Tensile Strength and Elongation Electroformed in an Ultra-Low-Concentration Sulfate Bath without Additives. J. of Materi Eng and Perform 26, 987–992 (2017). https://doi.org/10.1007/s11665-016-2494-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-016-2494-5