Abstract
Grain growth behaviors of LZ50 have been systematically investigated for various temperatures and holding times. Quantitative evaluations of the grain growth kinetics over a wide range of temperature (950-1200 °C) and holding time (10-180 min) have been performed. With the holding time kept constant, the average austenite grain size has an exponential relationship with the heating temperature, while with the heating temperature kept constant, the relationship between the austenite average grain size and holding time is a parabolic curve approximately. The holding time dependence of average austenite grain size obeys the Beck’s equation. As the heating temperature increases, the time exponent for grain growth n increases from 0.21 to 0.39. On the basis of previous models and experimental results, taking the initial grain size into account, the mathematical model for austenite grain growth of LZ50 during isothermal heating and non-isothermal heating is proposed. The effects of initial austenite grain size on hot deformation behavior of LZ50 are analyzed through true stress-strain curves under different deformation conditions. Initial grain size has a slight effect on peak stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Railway axles are one of the most important components in railway systems (Ref 1). Its properties mainly depend on its material and manufacturing process. Forging is widely used to produce railway axles, with the mechanical properties of the forged parts depending on their microstructure and grain size. Heating is the first step of forging. Material behaviors during the heating process should be carefully understood for design of controlled forming schedules. One of the key aspects in modeling microstructural change during heating is the austenite grain growth. Numerous studies on modeling of austenite grain size (AGS) evolution have been reported (Ref 2-6). Effect of heating temperature and holding time on the austenite grain growth of the 300 M steel was assessed and the grain growth model was described (Ref 7). Taking the alloying elements into account, an empirical equation for predicting the AGS of global low alloy steels was proposed (Ref 8). Al additions can produce aluminum nitride (AlN) precipitates in austenite, which will contribute to the grain refiner through grain boundary pinning. Based on these studies, the major factors affecting the AGS are the heating temperature, heating rate, holding time, and alloying elements (Ref 9, 10).
LZ50 is widely used for manufacturing railway axles and transmission parts. Heating process modeling is the beginning and an important component for the forging process modeling. Isothermal or non-isothermal heating is the basic procedure for LZ50 to get to the plastic deformation temperature. Generally, the finer grain size is, the higher strengthen and hardness will be. At the same time, plasticity and toughness improve. As the grain size is small, it is difficult for crack to propagate which is very important for railway axle. For railway axle, the grain size grade of blank for forging is no less than 5 grade, and the grain size grade after forging is no less than 6 grade. Precise prediction of the grain size is of importance for the control of the grain size evolution. However, the growth behavior of austenite grain in the heating process of the LZ50 steel has not been reported in the literature. In this paper, the effect of the heating temperature and holding time on the morphology and grain size of austenite in the LZ50 steel is investigated. When the austenite grain evolution is modeled using a classical relationship of isothermal grain growth, the initial grain size is sometimes neglected (Ref 7, 11, 12), on the basis that it is smaller than that after coarsening. This assumption is seldom justified, and in any event, the initial grain size will be a function of the heating temperature since the austenite is generated by nucleation and growth during heating of a mixture of ferrite and cementite (Ref 13). Taking the initial grain size into account, based on the experimental results, the growth model of the austenite grain under the isothermal and non-isothermal heating conditions for the LZ50 steel is presented. Effect of initial austenite grain size on the flow stress is studied by the hot compression tests.
Experimental Procedure
The chemical compositions of the as-received LZ50 steel are shown in Table 1. Additional alloying elements like vanadium are added to improve mechanical properties through grain size control. The AC1 and AC3 temperatures for this alloy are 725 and 760 °C, respectively. In experimental procedure, the geometry of the as-received material is a square blank (230 mm × 230 mm × 2000 mm). The dimensions of experimental specimens machined from the as-received LZ50 steel are Φ8 mm × 12 mm. The hot compression tests were carried on Gleeble-3500 thermomechanical simulator. The heating treatment was carried out in HMF1400-30 electric furnace. The specimens were isothermally heat-treated in a pre-heated furnace at different temperatures (i.e., 950, 1000, 1050, 1100, 1150, and 1200 °C) and kept for various times (i.e., 10, 30, 60,120, and 180 min). After the heating treatment, the specimens were quenched into water immediately. The quenched specimens were cut from the middle, and upon grinding and polishing were etched with a solution of saturated picric acid + alcohol + detergent to reveal the prior austenite grain boundaries. Subsequently, the austenite grains were observed by a Zeiss optical microscope and grain size was measured and evaluated using the average linear intercept method. When measuring the grain size by the intercept method, software MIAPS was used. Firstly, a suitable magnification was chosen. Five views were taken on the sample. Under the view, by use of MIAPS, 5 × 5 grid measuring lines were chosen. Average grain size of the view was measured. At last, measuring results of every view were averaged to get the total average grain size. Image-Pro Plus software was used to observe the austenite grains.
Results and Discussion
Effect of Heating Temperature on Austenite Grain Growth
The morphology of austenite grains of the as-received LZ50 steel is shown in Fig. 1, which indicates that the initial AGS is about 12.5 µm. Figure 2 shows the austenite grain structures of the samples after holding for 2 h at different temperatures. Inhomogeneity of grains in Fig. 2 implies that the austenite grain growth is via big grains swallowing small ones. Subsequently, the grain grows gradually with the increase of the heating temperature and abnormal grain growth appears at around 1150-1200 °C. Table 2 summarizes the average austenite grain size after holding for 2 h at different temperatures. When the heating temperature is lower than 1100 °C, the grain size is small, and the grains grow slowly; when the temperature exceeds 1100 °C, coarse and asymmetric grain appears. The austenite grain is small at the lower temperatures because there are a great number of second-phase particles in the steel below 1100 °C (Ref 14, 15). Additions of Al can produce AlN precipitates in austenite. It has been well documented that Al dissolved into austenite can effectively inhibit the austenite recrystallization through the solute drag effect or Zener pinning effect resulting from strain-induced precipitation of AlN. Vanadium additions contribute significant strengthening through the formation of vanadium carbo-nitrides (V(C,N)). Austenite grain size is small at lower temperature because there are a great number of second-phase particles (AlN, V(C,N)) in the steel below 1100 °C. These second-phase particles pin austenite grain boundary and effectively prevent austenite grain growing in heating process (Ref 16). When soaking temperature is above 1100 °C, most of the precipitates (AlN, V(C,N)) are dissolved. Abnormal grain growth is also taking place for these temperatures due to reduction of pinning function of these second-phase particles.
The migration resistance of the second-phase particles for austenite grain boundary, G m, can be expressed as (Ref 17):
where f is the volume percentage of second-phase particles; r is the radius of second-phase particles; and σ′ is the grain boundary energy. With the increase of f or σ′, the migration resistance of the second-phase particle increases. As the heating temperature increases, the volume percentage of second-phase particles decreases and the radius of the particles decreases as well, which leads to overall reduction in the effectiveness of the second phase in pinning the grain boundary. When the heating temperature is above 1100 °C, the second-phase particles are dissolved. As a result, the pinning effect of these second-phase particles significantly decreases and the grains grow rapidly.
Austenite grains grow through the migration of grain boundaries and the migration of grain boundaries is strongly temperature-dependent. As shown in Fig. 2, the boundaries of the fine austenite grains are bent at first with high-grain boundary energy. With the increase of the heating temperature, the grain boundary energy decreases and the grain boundaries become flat, with the angle between the boundaries being close to 120°. Figure 3 shows the size distribution of the austenite grains in the steel after heating for 2 h at 1000 and 1150 °C. As can be seen from Fig. 3, the grain size distribution of the austenite is close to the lognormal distribution, which is consistent with the results reported by Han et al. (Ref 18), Kurtz et al. (Ref 19), and Banerjee (Ref 20).
The austenite grain growth rate, v, can be expressed as (Ref 21):
where k is a constant, Q m the activation energy (J/mol) for grain growth, R the gas constant (8.314 J/mol/K), T the austenitization (heating) temperature in K, σ′ the grain boundary energy, and d the average austenite grain size. Austenite grain growth rate is proportional to grain boundary energy and has an exponential relationship with the (T −1). With the increase of the heating temperature, the austenite grain growth rate will increase exponentially. The finer the grain size is, the greater the grain boundary energy is, and the larger the austenite grain growth rate will be.
Effect of the Holding Time on the Morphology and Grain Size of Austenite
Figure 4 shows the morphology of the austenite grains in LZ50 after heating at 1050 °C for different holding times. As can be seen from Fig. 4, as the holding time increases, the carbides dissolve into the austenite gradually, the grain growth occurs, the number of grain boundaries decreases, and they become flat with an angle of ~120° in between. According to Fig. 5, the average austenite grain size increases exponentially as the heating temperature increases, as predicted by Eq 2. For each heating temperature, the relationship between the austenite grain size and the holding time is close to a parabolic curve, Fig. 6. It can be seen from Fig. 5 and 6 that the effect of the heating temperature on the austenite grain growth is more profound than that of the holding time. At each heating temperature, as the holding time increases, the austenite grain growth rate decreases and eventually the grain reaches a certain critical size.
According to the physical metallurgy, Beck’s equation can be used for depicting the relationship between the austenite grain size and holding time under isothermal heating, as shown below (Ref 22, 23):
where d is the final average grain diameter, n the time exponent for grain growth, and k the rate constant. Both n and k which depend on the material and holding temperature can be determined experimentally. Taking logarithm of both sides, it can be seen that the ln d is proportional to ln t. From the plot of ln d-versus-ln t, the time exponent for grain growth n for each temperature can be determined from the slope of the line. Figure 7 shows the relationship between ln d and ln t for different heating temperatures. It can be seen that they are indeed proportional to each other. The corresponding time exponent n values are summarized in Table 3. According to the earlier studies (Ref 24, 25), n varies with the material and the heating temperature. It is generally less than 0.5 for most materials and is around 0.3 generally (Ref 7). Furthermore, it increases as the temperature increases. According to Table 3, for LZ50, n increases from 0.21 to 0.39 as the heating temperature increases from 950 to 1200 °C. For similar steel which carbon content is 0.55%, n is from 0.2 to 0.3 (Ref 26). According to Ref 27, for C-Mn steel type, we can calculate n(0.24). For medium steel which carbon content is 0.26%, n is from 0.36 to 0.38 (Ref 28).
Austenite Grain Growth Model
It is mentioned above that the austenite grain growth depends on the heating temperature and holding time. At the same time, initial grain size should be taken into account. The grain growth model which is often used is of the Sellars-Whiteman (Ref 23) type:
where d o and d are the initial and final grain sizes, respectively, T the austenitizing (i.e., heating) temperature, t the time at T (i.e., the holding time), m the grain size exponent, Q the activation energy for grain growth, R the gas constant, and A is a material-dependent constant.
The relationship between the austenite grain growth and austenitizing condition has also been explained by a thermally activated atomic jump process, which is typically expressed by the following Anelli equation (Ref 29):
where B a material-dependent constant, n is the time exponent for grain growth, and the others are the same as defined in Eq 4.
In Eq 4, the initial grain size is considered, but the holding time exponent is ignored. In Eq 5, the time exponent is taking into account, but the initial grain size is neglected. Taking the heating temperature, holding time and initial grain size into account, a new model to predict the austenite grain growth, which combines Eq 4 and 5, is proposed as follows:
In order to determine the constants in Eq 6, taking logarithm of both sides, we can get
In Eq 7, m, A, Q, and n can not be determined by linear regression. To quantitatively express the growth model of austenite grain in the LZ50 steel, which can be derived considering the effect of heating temperature, initial grain size, and holding time, the unknown variables are analyzed by fitting the experimental data. By setting m at different levels, such as 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, and 5.0, experimental data are fitted to obtain the unknown variables.
Setting the holding time constant and taking the derivative with respect to 1/T for Eq 7, we can get
where l is equal to ∂[ln (d m − d m0 )]/∂(1/T)| t . The relationship between ln (d m − d m0 ) and 1/T can be determined by the least-square regression. The average slope of the line will be l. Setting the heating temperature constant and taking the derivative with respect to ln t for Eq 7, we can get
where n is the average slope of the ln (d m − d m0 )-versus-ln t line. After n and l are determined, A can be calculated by Eq 7. If the lines in the above two linear relationship figures are approximately parallel, the activation energy for grain growth is unique, which means that the grain growth mechanism is the same.
In order to improve the calculation accuracy, the square sum of error is taken as objective function. The error for l and n are computed for different m values. The square sum of error y(m) as a function of m is shown in Fig. 8. The relationship between y(m) and m can be obtained through polynomial fitting as follows:
Through mathematical analysis, the grain size exponent m for the minimum y(m) is determined to be 2.82. Putting this m value into Eq 7, n, A and Q can be calculated by Eq 8 and 9. The following values are obtained: n = 0.6821, l = −40,472, Q = 336484 J/mol, and A = 2.19 × 1017. According to Ref 27, 28, we can calculate that Q is 378.197 and 397.679 kJ/mol, respectively, which is similar to our results. Putting these values into Eq 6, the isothermal grain growth model for LZ50 is obtained:
Because the grain growth mechanism is the same, the activation energy for grain growth is unique. The relationship between ln (d m − d m0 ) and 1/T must be linear. As shown Fig. 9, with m equals to 2.82, ln (d 2.82 − d 2.820 ) has a linear relationship with 1/T. The linearly dependent coefficient varies from 95.64 to 99.69%. Figure 10 shows the relationship between ln (d 2.82 − d 2.820 ) and ln t by linear regression. The linearly dependent coefficient is from 98.93 to 99.90%. These results indicate that the grain growth model as given in Eq 11 is accurate and can be utilized for predicting the austenite grain size of the LZ50 steel after the heating treatment at different heating temperatures for various holding times.
It should be noted that the isothermal grain growth model given in Eq 11 has been deduced using the data of the LZ50 steel with an initial grain size of 12.5 μm. In practice, for a forging blank, its initial grain size can vary from a dozen to several dozens of µm. Equation 11 is universally applicable to LZ50, as long as the actual initial grain size is used in the equation.
In practice, the heating of the blank is often a non-isothermal process. Grain growth model can be established for the non-isothermal process using the isothermal data. For a non-isothermal heating process, the function of heating is given as follows:
where t is the heating time (min), T is the heating temperature (K). Δt is time interval which equals to t i+1 − t i . When Δt is small enough, the heating process during Δt can be considered as isothermal. For T < 1123 K, grain size can be assumed as having no change. For T ≥ 1123 K, according to Eq 11, based on the grain size d i and the temperature T i at t i , the holding time \(\bar{t}_{i}\) during the isothermal heating from d 0 to d i can be obtained:
The grain growth model under the non-isothermal process, i.e., the grain size d i+1 at t i+1, is as follows:
Effect of Initial Austenitic Grain Size on the Flow Stress
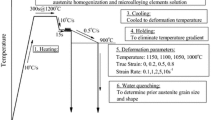
In order to investigate the effect of initial austenitic grain size on flow stress of LZ50, different tests were performed under different heated temperatures, four different deformation temperatures. The specimens were heated to 1100, 1150, 1200, and 1250 °C, held for 10 min to obtain different initial austenitic grain sizes, cooled to the specified deformation temperatures at a rate of 10 °C/s, and then subjected to a compressive deformation at specified strain rates. The four deformation temperatures were 950, 1000, 1050, and 1100 °C. The strain rates used was 0.5 s−1 and the deformation value was 60%. Initial austenitic grain size was obtained through water quenching after the materials were heated to 1100, 1150, 1200, and 1250 °C, respectively, and held for 10 min. The resulting initial austenitic grain sizes of the specimens measured using transversal method are 40, 65, 92, and 120 μm, respectively.
Figure 11 shows the true stress-strain curves at different deformation temperatures (heated temperature is 1200 °C). As shown in Fig. 11, when the deformation temperature is 950 °C, the peak stress is 110 MPa, and when the deformation temperature increases to 1100 °C, the peak stress decreases to 55 MPa. Peak stress decreases with the increase of the deformation temperature. Figure 12 shows the effect of initial austenitic grain size on peak stress. It can be seen from it that under the deformation conditions of T = 950 °C, the peak stresses corresponding to the initial austenitic grain size of 40 and 120 μm differ by 12%. Under the deformation condition of T = 1000 °C, the peak stresses corresponding to the initial austenitic grain size of 40 and 120 μm differ by 12.5%. Under another two deformation conditions of T = 1050 °C and T = 1100 °C, similarly, the peak stresses corresponding to 40 and 120 μm differ by 8 and 16%, respectively. Effect of grain size on the peak stresses is less than the deformation temperatures.
Conclusions
The grain growth kinetics in the axle steel LZ50 have been studied for different heating temperatures (950-1200 °C) and various holding times (10-180 min). The main results are briefly summarized as follows:
-
(1)
The effect of the heating temperature on the austenite grain growth is more profound than that of the holding time. The holding time exponent ranges from 0.21 to 0.39 and increases as the heating temperature increases. At the temperatures above 1100 °C, grain growth showed a sharp increase because most of precipitates (AlN, V(C,N)) is dissolved.
-
(2)
The growth model of the austenite grain in LZ50 for isothermal heating is described as follows:
$$d^{2.82} = d_{0}^{2.82} + 2.19 \times 10^{17} t^{0.6821} \exp \left( {\frac{ - 336484}{RT}} \right)$$For non-isothermal heating of LZ50, the growth model of the austenite grain is
$$\bar{t}_{i}^{0.682} = \frac{1}{{2.19 \times 10^{17} }}(d_{i}^{2.82} - d_{0}^{2.82} ) \cdot \exp \left( {\frac{336484}{{RT_{i} }}} \right)$$$$d_{i + 1}^{2.82} = d_{0}^{2.82} + 2.19 \times 10^{17} (\bar{t}_{i} + \Delta t)^{0.682} \cdot \exp \left( { - \frac{336484}{{RT_{i} }}} \right)$$ -
(3)
In the range of experimental deformation parameters of LZ50, for the certain initial austenitic grain size, the higher the deformation temperature is, the smaller the peak stress of flow stress is. For the certain deformation parameters, effect of the initial austenitic grain size on peak stress is low.
References
T. Makino, T. Kato, and K. Hirakawa, Review of the Fatigue Damage Tolerance of High-Speed Railway Axles in Japan, Eng. Fract. Mech., 2011, 78, p 810–825
Q.Y. Sha and Z.Q. Sun, Grain Growth Behavior of Coarse-Grained Austenite in a Nb-V-Ti Microalloyed Steel, Mater. Sci. Eng. A, 2009, 523, p 77–84
J. Fernández, S. Illescas, and J.M. Guilemany, Effect of Microalloying Elements on the Austenitic Grain Growth, Mater. Lett., 2007, 61, p 2389–2392
Q.B. Yu and Y. Sun, Abnormal Growth of Austenite Grain of Low-Carbon Steel, Mater. Sci. Eng. A, 2006, 420, p 34–38
H. Pous-Romero, I. Lonardelli, D. Cogswell, and H.K.D.H. Bdadeshia, Austenite Grain Growth in a Nuclear Pressure Vessel Steel, Mater. Sci. Eng. A, 2013, 576, p 72–79
C.X. Yue, L.W. Zhang, S.L. Liao, and H.J. Gao, Kinetic Analysis of the Austenite Grain Growth in GCr15 Steel, J. Mater. Eng. Perform., 2010, 19(1), p 112–115
S.S. Zhang, M.Q. Li, Y.G. Liu, and T.Q. Liu, The Growth Behavior of Austenite Grain in the Heating Process of 300 M steel, Mater. Sci. Eng. A, 2011, 528, p 4967–4972
S.J. Lee and Y.K. Lee, Prediction of Austenite Grain Growth During Austenitization of Low Alloy Steels, Mater. Des., 2008, 29, p 1840–1844
A. Chbihi, D. Barbier, L. Germain, A. Hazotte, and M. Goune, Interactions Between Ferrite Recrystallization and Austenite Formation in High-Strength Steels, J. Mater. Sci., 2014, 49, p 3608–3621
S. Maropoulos, S. Karagiannis, and N. Ridley, Factors Affecting Prior Austenite Grain Size in Low Alloy Steel, J. Mater. Sci., 2007, 42(4), p 1309–1320
S. Uhm, J. Moon, C. Lee, J. Yoon, and B. Lee, Prediction Model for the Austenite Grain Size in the Coarse Grained Heat Affected Zone of Fe-C-Mn Steels: Considering the Effect of Initial Grain Size on Isothermal Growth Behavior, ISIJ Int., 2004, 44(7), p 1230–1237
Y. Zhao, J. Shi, W. Cao, M. Wang, and G. Xie, Kinetics of Austenite Grain Growth in Medium-Carbon Niobium-Bearing Steel, Appl. Phys. Eng., 2011, 12, p 171–176
Y.W. Xu, D. Tang, Y. Song, and X.G. Pan, Prediction Model for the Austenite Grain Growth in a Hot Rolled Dual Phase Steel, Mater. Des., 2012, 36, p 275–278
A.J. Papworth and D.B. Williams, Segregation to Prior Austenite Grain Boundaries in Low-Alloy Steels, Scr. Mater., 2000, 42, p 1107–1112
N. Wang, Y.H. Wen, and L.Q. Chen, Pinning Force From Multiple Second-Phase Particles in Grain Growth, Comput. Mater. Sci., 2014, 93, p 81–85
L.M. Rothleutner, R. Cryderman, and C.J. Van Tyne, Influence of Temperature and Holding Time on the Interaction of V, Al, and N in Microalloyed Forging Steels, Metall. Mater. Trans. A, 2014, 45(10), p 4594–4609
Z.C. Liu, Transformation Principles of Material Microstructure, Metallurgical Industry Press, Beijing, 2006, p 100 [in Chinese]
L.Z. Han, R.K. Chen, J.F. Gu et al., Behavior of Austenite Grain Growth in X12CrMoWVNbN10-1-1 Ferrite Heat-Resistant Steel, Acta. Metall. Sin. Engl, 2009, 45, p 1446–1450
S.K. Kurtz and F.M.A. Carpay, Microstructure and Normal Grain Growth in Metals and Ceramics. Part I. Theory, J. Appl. Phys., 1980, 51(11), p 5725–5744
K. Banerjee, M. Matthias, M. Perez, and X. Wang, Nonisothermal Austenite Grain Growth Kinetics in a Microalloyed x80 Linepipe Steel, Metall. Mater. Trans. A, 2010, 41(12), p 3161–3172
D.D. Su, Austenitic Grain Growth and Grain Boundary Remove, Steel Wire Prod., 2004, 30(5), p 51–54 [in Chinese]
P.A. Beck, J.C. Kremer, L.J. Demer, M.L. Ho, and L. Zorth, Grain Growth in High-Purity Aluminum and in an Aluminum-Magnesium Alloy, Trans. AIME, 1948, 175, p 372–394
C.M. Sellars and J.A. Whiteman, Recrystallization and Grain Growth in Hot Rolling, Met. Sci., 1979, 13(3–4), p 187–194
S. Illescas, J. Fernndez, and J.M. Guilemany, Kinetic Analysis of the Austenitic Grain Growth in HSLA Steel with a Low Carbon Content, Mater. Lett., 2008, 62(20), p 3478–3480
S. Jiao, J. Penning, F. Leysen, Y. Houbaert, and E. Aernoudt, Modelling of Isothermal Austenite Grain Growth in a Si-Mn TRIP Steel: Experimental and Calculation, Steel Res., 2000, 71(9), p 345–350
Z.Y. Gao and F.Y. Sun, Study of Austenite Grain Growth Kinetics in Medium Carbon Steel, J. Iron Steel Res., 1989, 4, p 59–63 [in Chinese]
H. Pous-Romero, I. Lonardelli, D. Cogswell, and H.K.D.H. Bhadeshia, Austenite Grain Growth in a Nuclear Pressure Vessel Steel, Mater. Sci. Eng. A, 2013, 567, p 72–79
Y.L. Zhao, J. Shi, W.Q. Cao, M.Q. Wang, and G. Xie, Kinetics of Austenite Grain Growth in Medium-Carbon Niobium-Bearing Steel, J. Zhejiang Univ. Sci. A, 2011, 12, p 171–176
E. Anelli, Application of Mathematical Modeling to Hot Rolling and Controlled Cooling of Wire Rods and Bars, ISIJ Int., 1992, 32(3), p 440–449
Acknowledgments
This project is supported by the National Natural Science Foundation of China (Grant No. 51305289), Shanxi Province International Cooperation Projects (Grant No. 2013081030), and PhD Research Startup Foundation of Taiyuan University of Science and Technology (No. 20122052).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, S., Li, Y. & Zheng, Y. Kinetics of Austenite Grain Growth During Heating and Its Influence on Hot Deformation of LZ50 Steel. J. of Materi Eng and Perform 25, 2661–2669 (2016). https://doi.org/10.1007/s11665-016-2162-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-016-2162-9