Abstract
Nano-hydroxyapatite (HA)/poly(ε-caprolactone) (PCL) coatings with thickness of about 45-55 μm were prepared on the surface of a Mg-Ca alloy by a combination of electrochemical deposition and dip-coating methods. The results indicate that incorporation of nano-HA as an inner layer affects the porosity of the PCL-coated layer as top layer and enhances the corrosion resistance of Mg-Ca alloys. The nano-HA/PCL-coated samples showed higher R p (823.1 kΩ cm2) and lower i corr (5.85E−2 µA/cm2) compared with uncoated. Significant enhancement in the compressive strength was observed in the both nano-HA/PCL and PCL compared with uncoated samples after 10 days immersion in SBF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomaterials have been applied for various biomedical applications such as fixation and stabilization of fractured bones and bone substitutes. Traditional non-degradable metals such as titanium alloys, stainless steel, and cobalt chromium alloys were widely applied owing to their good corrosion resistance and mechanical properties as well as their suitable biological performance (Ref 1-3). Compared with biodegradable metallic implants, non-degradable metals require a second surgical procedure for implant removal (Ref 2, 4). In addition with the development of tissue-engineering techniques, biodegradable materials now attract great attention as a result of its replacement with the host tissue. In addition, they have been used in the delivery of bioactive ions to enhance hard tissue healing (Ref 1). Currently, magnesium (Mg) and its alloys are used as biodegradable materials for implant applications because Mg dissolution does not cause any adverse side effects as well as it is not toxic when exposed to human body fluids (Ref 5). Furthermore, Mg alloys present good mechanical properties, biodegradability, and biocompatibility (Ref 6, 7). However, rapid and uncontrolled corrosion of Mg in the physiological environment results in the production of hydrogen gas. Although the evolved gas is rapidly exchanged with surrounding tissues, the initial evolution of H2 may produce cavities adjacent to the Mg-based implant which are clinically undesirable (Ref 3, 8). Apart from alloying, surface modification methods such as sol-gel, micro arc-oxidation, conversion coatings, physical vapor deposition, polymer coating, hot spraying, electrodeposition, and laser cladding have been investigated as options to slow down the corrosion rate of Mg alloys (Ref 4, 9-11). The electrodeposition method has been selected because of its simplicity, ability to fabricate thick films, ease of particle-size control, compact coating, low temperature processing, cost-effectiveness, ability to coat substrates with complex shapes, and high purity of deposits (Ref 12, 13). The polymer coating can enhance the in vitro corrosion behavior and protect the implant from fast degradation (Ref 13). Poly(ε-caprolactone) (PCL) as a semi-crystalline linear hydrophobic polymer is widely used because of its good mechanical strength, flexibility, biocompatibility, and biodegradability (Ref 14). However, the application of PCL coating for bone regeneration has been limited because of low stiffness and relatively low bioactivity (Ref 14). To address this problem, fabrication of bioactive ceramics as an inner layer can enhance the osteoconductivity of PCL without adversely affecting its mechanical properties (Ref 15, 16). Different types of bioceramics have been used in bone-healing applications; among these hydroxyapatite (HA, Ca10(PO4)6(OH)2) presented good bioactivity and osteoconductivity as a coating material (Ref 13). In addition, HA is the main mineral phase in natural bone which can enhance biological therapies (Ref 13). Several researches (Ref 5, 17, 18) have been conducted with the aim of improving corrosion resistance and bioactivity of Mg alloys by bioceramic-polymer coating. Wang et al. (Ref 17) reported that a DCPD-PCL composite coating on Mg-Zn substrate can slow down the in vitro degradation rate of the Mg alloy. Wong et al. (Ref 19) indicated that the PCL coating on AZ91D magnesium alloys reduces the corrosion rate of the implant. Furthermore, Chen et al. (Ref 20) showed that PCL and polylactic acid (PLA) coatings on high-purity magnesium (HPM) can improve the corrosion resistance of HPM in m-simulated body fluid (SBF). It was also reported (Ref 18) that composite polymeric coating (polyether imide-hydroxyapatite) on biodegradable AZ31 magnesium alloy can enhance corrosion resistance and biocompatibility. However, there are very few reports on the surface modification of Mg alloys via double-layered bioceramic-polymeric coating. Therefore, the objective of this work is preparing double-layered nano-HA/PCL composite coating on Mg-Ca alloy via electrodeposition followed by dip-coating method in order to slow down the Mg alloy degradation rate, thus improving the healthiness of the severely hurt tissues in implant applications.
Experimental Details
Sample Preparation
Magnesium alloys were prepared by melting 99.9% pure magnesium ingots and Mg-32wt.%Ca. The melts, with a constant concentration of Ca (1.2 wt%), were then cast in a 300 °C preheated stainless steel mold to produce an ingot. In preparation for further experiments, several Mg-1.2wt.%Ca specimens, with composition of 0.047% Si, 0.003% Cu, 1.221% Ca, 0.021% Al, 0.012% Fe, 0.004% Ni, and 98.692% Mg with dimensions of 15 mm × 10 mm × 10 mm, were cut from the ingot. Then, they were mechanically wet ground with 320-2000 SiC grit papers until all visible scratches were removed.
Deposition of HA/PCL Coatings
To coat HA using the electrodeposition method, a conventional cell was fitted with a graphite rod as the anode and the Mg-1.2wt.%Ca sheet (15 × 10 × 10 mm) as the cathode. The electrolyte was prepared by dissolution of Ca(NO3)2 (0.042 mol/L), NH4H2PO4 (0.025 mol/L), NaNO3 (0.1 mol/L), and H2O2 (10 mol/L). The addition of NaNO3 leads to an increase in the ionic strength. The pH value of the solution was adjusted to 5.0 by adding HNO3 and (CH2OH)3CNH2 at room temperature. The HA-coated samples were dipped into the 2.5 wt.% PCL (pellets; M w = 80,000 g/mol, Sigma-Aldrich, UK) dichloroform solution (DCM; CH2Cl2, Sigma-Aldrich, UK) for 30 s and withdrawn at fixed speed to form a uniform coating and then dried at room temperature. Prior to dipping of HA, PCL pellets were dissolved in dichloromethane by stirring for 6 h at room temperature. Then they were taken out slowly and placed in air for 24 h.
Microstructural Characterization
An x-ray diffractometer (Siemens-D5000) was used to evaluate the phase transformation, using Cu Kα radiation (λ = 1.5405 Å) generated at 35 kV and 25 mA. The crystallite size was determined using the Scherrer equation (Ref 21):
where t c is the average crystallite size (nm), λ is the x-ray wavelength, β is the diffraction peak width at mid-height, and θ is the Bragg diffraction angle. Fourier-transform infrared (FTIR) spectroscopy was used to determine the surface functional groups of the coated sample. The FTIR spectra were recorded in the spectral range 4000-400 cm−1. Microstructural observation was performed using a scanning electron microscope (JEOL JSM-6380LA), equipped with an EDS. The average pore size and uniformity of the coated layers were analyzed using ImageJ software version 1.47.
Mechanical Properties
For the compressive test, cylindrical specimens with a diameter of 10 mm and a height of 20 mm were immersed in SBF for 10 days and then cleaned in a boiling solution of chromium trioxide (CrO3) to remove the surface corrosion product. The specimens were then dried in warm air. Compressive tests were performed using an Instron-5569 universal testing machine at a displacement rate of 0.5 mm/min, at ambient temperature. For each testing condition, two specimens were examined. The bonding strength of the PCL-coated and nano-HA/PCL composite-coated specimens was measured according to ASTM F1044 standard using a universal testing machine (Instron 5569). Rectangular specimens with the dimensions of 30 mm × 10 mm were prepared. The cross-head displacement rate was 1 mm/min with a 10 kN load cell. Three composite-coated specimens were tested, and the average value was reported.
Electrochemical Impedance Measurements
Rectangular specimens, with a surface area of 1 cm2, were mounted in epoxy resin for electrochemical tests. The test was conducted at 37 °C in an open-air glass cell containing 350 ml Kokubo solution with a pH of 7.44, using a PARSTAT 2263 potentiostat/galvanostat (Princeton Applied Research). A three-electrode cell was used for potentiodynamic polarization tests. The reference electrode was a saturated calomel electrode (SCE), the counter electrode was a graphite rod, and the specimen was the working electrode. The samples were immersed in the SBF for 1 h prior to the electrochemical test to establish the open-circuit potential. All experiments were carried out at a constant scan rate of 0.5 mV/s, initiated at −250 mV relative to the open-circuit potential. The electrochemical impedance spectra (EIS) were measured over a frequency range of 1 Hz to 100 kHz, and the data were analyzed using the ZsimpWin software. The EIS test was also performed using a VersaSTAT 3 machine. Each electrochemical test was duplicated to examine the reproducibility of the results. The corrosion current density (i corr), corrosion potential (E corr), cathodic Tafel slopes (βc), anodic Tafel slopes (βa), polarization resistance (R p), and corrosion rate (P i) were determined. The values of i corr, E corr, βc, βa, and a corresponding P i for each alloy were extracted from the polarization curves. The polarization resistance (R p) was calculated according to the following equation (Ref 22, 23):
The corrosion rate (P i) of the samples, obtained from the corrosion current density, was calculated according to (Ref 24, 25):
Immersion Test
The immersion test procedure was carried out based on the ASTM: G1-03. The specimens were then immersed in a beaker containing 200 ml of Kokubo SBF (Ref 26). The SBF was not replenished during the testing period. The average pH of the SBF was measured (n = 3) after an immersion time of 24 h. The immersion tests were repeated at least once to check the reproducibility of the results. Corroded surfaces were analyzed using scanning electron microscopy (SEM) and EDS following immersion testing. The beakers were sealed with a pH value of 7.4 and incubated at a constant temperature of 37 °C for 3, 7, and 14 days duration. Afterwards, the specimens were rinsed with acetone and deionized water, and corrosion products were removed before weight measurement. The corrosion rate was calculated as follows:
where C R is the corrosion rate, W is the mass loss, A is the surface area exposed to the corrosive media, t is the exposure time, and d is the density. The hydrogen evolution rate of the specimens was also measured during the 168 h immersion in Kokubo solution. Specimens were immersed in a beaker containing 250 ml of SBF, where a funnel was located over the sample to collect evolved hydrogen in a burette (50 ml) placed above the funnel. The SBF was renewed every 24 h after recording the hydrogen evolution rate (ml/cm2/day). At least two specimens were used to verify the reproducibility of the measurements.
Results and Discussion
Characterization of the Coating
Figure 1 shows SEM images of the uncoated, PCL, and nano-HA/PCL composite-coated specimens. Mg-1.2 wt%Ca alloy consisted of α-Mg and Mg2Ca secondary phases (Fig. 1a). The secondary phases are formed in the form of eutectic phases (α-Mg + Mg2Ca) along the grain boundary (Fig. 1e) which has significant effect on the corrosion behavior of Mg alloy because of the formation of micro-galvanic cells between the matrix and secondary phase. The corresponding EDS analysis in the denoted area is composed of Mg and Ca, which is related to the evolution of Mg2Ca phase (Fig. 1d). However, the surface of single-layered polymer coating (PCL) and bi-layered ceramic-polymer (nano-HA/PCL) coating comprised interconnected pore networks. Both single-layered and double-layered coating indicated pore structure with relatively uniform distribution (Fig. 1b and c). This structure permits considerable amounts of media to pass through the polymer coating and decrease the corrosion resistance (Ref 26, 27). But in the double-layered ceramic-polymer coating, most pores in the PCL layer are sealed by nano-HA as an inner layer, serving as a barrier against corrosive electrolytes; nano-HA/PCL coatings can improve the corrosion resistance of the coated sample (Fig. 1c). The pore formation was mainly due to the process of phase separation, in which solvent evaporation in the polymer solution acted as a driving force for phase separation because the polymer solution became thermodynamically unstable during solvent evaporation (Ref 19, 28). This resulted in the formation of either a polymer-rich or polymer-poor phase, where the polymer-rich phase would be solidified, whereas the polymer-poor phase led to pore formation (Ref 19, 29). This kind of distribution provides bonding between the organic coating and metal substrate (Ref 30). EDS analysis results also detected C, O, and Mg in this sample, indicating formation of PCL (Fig. 1e); in particular, the presence of a high amount of C (64.45 at.%), the origin of which could be related to the PCL coating. However, the nano-HA/PCL coating showed a larger pore size (7.4 ± 0.27 μm) than the PCL coating (3.3 ± 0.42 μm) because of the incorporation of nano-HA as an inner layer, which affects the porosity of the PCL-coated layer in the top layer and enlarges the pore diameters, as they might be embedded inside these pores. EDS analysis clearly identified only the elements of Mg, Ca, P, C, and O, indicating that the nano-HA/PCL composite coating on the Mg alloy is free of any impurities. The atomic ratio of Ca/P in the coatings was about 1.31. The strong carbon signal was attributed to the PCL deposited layer (Fig. 1f).
Figure 2 shows the mapping of the elements on the surface of uncoated Mg-Ca, PCL, and nano-HA/PCL-coated samples. The elemental mapping of the uncoated sample showed high concentration of Ca at the grain boundaries, indicating formation of Mg2Ca (Fig. 2a). The mapping of the element result of the PCL-coated sample in Fig. 2(b) shows that the coating was predominantly composed of C, Mg, O, and a trace amount of Ca. The distributions of C and O are relatively uniform in the coating. The mapping of nano-HA/PCL also shows similar elemental distribution with additional Ca and P, which deposited on the substrate (Fig. 2c). Figure 3 shows EDS elemental maps of the cross-sectional PCL and nano-HA/PCL composite-coated specimens, indicating formation of thick and compact coating layers which homogeneously covered the surface of the Mg alloy.
As the cross-section morphology (Fig. 3a) shows, a PCL coating with a thickness of around 40-50 μm formed on the surface of the magnesium alloy. The cross-section morphology of nano-HA/PCL coating (Fig. 3b) shows that the coating with a thickness of about 45-55 μm is composed of two distinct layers. These layers are closely bonded with substrate. The inner layer is nano-HA film with a thin thickness (6-8 μm), while the outer layer is PCL with a thick thickness. Gu et al. (Ref 31) showed that the thickness of the coating layer has a significant effect on the corrosion behavior of the Mg substrate where the thicker coating layer prohibited further corrosion in Mg during tests in chloride solutions (Ref 15).
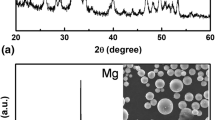
The XRD patterns of the uncoated, PCL-, and nano-HA/PCL-coated samples are shown in Fig. 4. The XRD spectrum of Mg-Ca alloys further confirmed the formation of the Mg2Ca compounds.
The PCL background spectrum mainly consists of two intense peaks at 2θ = 21.6° and 2θ = 23.8° (Fig. 4b) that account for diffraction on the (110) and (200) planes, respectively (PCL has a crystalline structure with polyethylene-like orthorhombic cell disposition, with lattice parameters a = 0.748 nm, b = 0.498 nm, and c = 1.727 nm) (Ref 32). Besides the diffraction peaks belonging to HA at 2θ of 32.7°, 34.4°, and 35.8°, the diffraction pattern of nano-HA/PCL composite showed two additional strong diffraction peaks at Bragg angles 2θ = 21.6° and 23.8°, which were attributed to the (110) and (200) crystallographic planes of semi-crystalline PCL (Ref 32, 33). The average crystallite size of the HA-coating specimens was determined by Scherrer’s method. In this method, the (0 0 2) peaks are the most obvious peaks for which the average particle size of the HA is about 96 nm. Nanocrystalline bioceramics have received considerable attention, owing to their unique and superior properties that are often different from their bulk materials’ counterpart. These materials can possess superior biological properties (Ref 34). Figure 5 shows the TEM micrographs of nano-HA/PCL-coated specimens. The TEM images show that after HA/PCL coating, needle-like particles of nano-HA formed on the pore structure of PCL. It can also be seen that the needle-like particles are about 60-100 nm in diameter and 400-800 nm in length (Fig. 5a). This is in agreement with the crystallite size of the HA-coating specimens calculated using XRD data. After coating with a PCL layer as top layer, dark spherical particles can be observed, which we assumed to be the PCL. The diffraction of HA nanosized crystallites shows a mixture of both ring and spot patterns indicating that the HA structure is semi-crystalline. The lattice spacing of (0 0 2) HA and (1 2 0) HA was estimated to be 0.31 and 0.44 nm, respectively, as shown in Fig. 5b. These values are consistent with the calculated values (0.344 nm for (0 0 2) HA and 0.471 nm for (1 2 0) HA) using the lattice parameters of a = 9.421 Å, b = 9.421 Å, c = 6.882 Å, α = 90°, β = 90°, and γ = 120° (Ref 35-37). Nanostructured coating with the higher standard electrochemical potential compared to the substrate can decrease the Mg alloy corrosion rate and preserve the formed protective oxide films on the Mg alloy during corrosion (Ref 9, 12).
The FTIR spectra of PCL- and nano-HA/PCL-coated specimens are shown in Fig. 6. The PCL spectrum shows characteristic peaks of C-O stretching vibrations at 1253-1114 cm−1 and (CH2)4 bending modes at 2965-2843 cm−1 and 1487-1289 cm−1 (Fig. 6a). A C=O bond stretching vibration that yields peaks at 1615 cm−1 was indicative of an aliphatic ester of PCL on the surface (Ref 38, 39). The FTIR spectrum of the nano-HA/PCL composite sample contained C=O, C-O, and C-H bands corresponding to PCL, while the P-O and O-H bands were attributed to nano-HA (Ref 38, 39). The peak at 960 cm−1 was assigned to the symmetric stretching (v 1) of the P-O bond of the phosphate group. The peak at 632 cm−1 was attributed to the librational mode (v L) of the hydroxyl group, which further confirmed the crystalline nature of nano-HA (Fig. 6b).
Mechanical Properties
The compressive strength of uncoated Mg-Ca, PCL-, and nano-HA/PCL-coated samples before and after immersion in Kokubo solution for 10 days are shown in Fig. 7. As the polymer and ceramic/polymer coating did not affect the bulk mechanical properties, thus, the compressive curves of uncoated and coated samples were similar before immersion. The compressive strengths of PCL- and nano-HA/PCL-coated samples decreased from 272 MPa before immersion to 228 and 200 MPa, respectively, after 10 days of immersion (Table 1). These values are comparable to the compressive strength of human bones (100-230 MPa) in cortical bone and 2-12 MPa in cancellous bone (Ref 26). But, the compressive curves of uncoated Mg-Ca dropped to 159 MPa after 10 days immersion in Kokubo solution because of the presence of pits and cracks caused by high corrosion rates. This indicated that ceramic/polymer coating can delay the loss of the mechanical properties of the substrate (Ref 40). Wong et al. (Ref 19) showed that the polymer-coated samples aided in retaining the mechanical strength of the implant in contrast to uncoated samples during the immersion test. Ductility of the uncoated Mg-Ca alloy before immersion is around 14.3%, whereas the ductility of uncoated, PCL-, and nano-HA/PCL-coated samples decreased to 10.8, 12.2, and 13.6%, respectively, after 10 days immersion in Kokubo solution. The existence of pores and cracks as a result of the corrosion may limit ductility of samples caused by dislocation movement as well as twinning owing to the different natures of the internal bonds and the large mismatch of the coefficient of thermal expansion and elastic modulus between pores and the Mg substrate.
The effectiveness of restriction to ductility of samples depends on the number of such defects (Ref 40). However, in the double-layered coating, a nano-HA coating can avoid the direct contact of the PCL and the Mg matrix with the aggressive solution. Thus, the inner layer (nano-HA) causes to avoid their negative interaction and enhanced their ductility values compared with uncoated Mg-Ca sample. It is reported (Ref 41) that the poor bonding between coating and substrate will cause coating delamination and will adversely affect the performance and reliability of devices. Thus, the bonding strength between the coating and the substrate is one of the important factors for implant materials. Xu et al. (Ref 42) shows that PCL films show poor bonding strength which may be attributed to the molecular structure of the polymer. PCL has a low ratio of O in its weight, which resulted in less electrostatic interaction on the Mg substrate surface, as can be seen in Fig. 8. It has been reported (Ref 43) that the bulging of PCL films can be found after 7 days immersion in an immersion experiment. Therefore, it is necessary to improve the bonding strength between the polymer coating and the Mg substrate. To address this issue, Abdal-hay (Ref 44) showed that the magnesium surface has an electropositive charge and is very active. The existence of oxygen in the polymer chain leads to strong electrochemical attraction between Mg substrate and PCL molecules. Bonding strength depends on oxygen as an active element. The bonding strength of the single-layered PCL coating attained was about 2.2 MPa. However, bonding strength between the nano-HA and PCL layer was about 6.71 MPa. The good interfacial bonding strength of nano-HA with the polymer matrix can be explained by the hydrogen bonding formed between the -OH groups of nano-HA and the =O sites of the PCL polymer (Ref 30). Furthermore, nano-needle-like morphology of HA crystals resulted in decreases of porosity of PCL and increased in the bonding strength of the nano-HA/PCL composite coating compared to the PCL-coated specimen.
Electrochemical Measurement
The electrochemical polarization curves of the uncoated Mg-Ca, PCL-, and nano-HA/PCL-coated samples are shown in Fig. 9. The corrosion potential (E corr) showed that the polymer-coated magnesium alloys shifted the corrosion potential to a more positive potential. The E corr, with reference to the Mg-Ca-uncoated magnesium alloy, increased by −1298.1 mVSCE in PCL samples and −1269.3 mVSCE in nano-HA/PCL composite-coated samples, which indicates that the formed PCL over the HA could resist the penetration of corrosive ions. Electrochemical parameters of uncoated Mg-Ca alloy, and of PCL- and nano-HA/PCL-coated Mg alloys in Kokubo solution are listed in Table 2. The more negative corrosion potential of the uncoated Mg-Ca, compared to the coated alloy, is owing to the formation of a micro-galvanic cell between the Mg2Ca phase and the α-Mg phase which resulted in an increase in the corrosion rate of the uncoated sample. The values of the corrosion current density (i corr) of the both PCL (1.79 E−1 µA/cm2) and nano-HA/PCL (5.85 E−2 µA/cm2) samples were significantly lower than that of the uncoated Mg-Ca sample (267.5 µA/cm2). Both PCL- and nHA/PCL-coated specimens exhibit a significantly lower corrosion current density than HA-coated specimens (6.01 µA/cm2) (Ref 45). However, nano-HA/PCL presented lower i corr compared to the PCL-coated sample which is attributed to the coating porosity, uniformity, and thickness of coating. The presence of the PCL film as a top layer and nano-HA as an inner layer caused significant reduction of the corrosion rate. The samples coated with single-layered PCL suffer from a high level of attack by water molecules. Water diffusion through tiny micro- or nano-defects on the coating layer deteriorated the coatings and destroyed the adhesion of the organic layer on the substrate (Ref 30). PCL as top layer also over the nano-HA surface could hinder the hydrogen evolution reaction, and in turn, the current densities decreased significantly compared to the uncoated sample (Ref 46). The fluctuations are observed in the anodic region of the PCL- and nano-HA/PCL-coated samples. Pores provide the sites to set up galvanic cells in solutions. Hydrogen gas produced by the cathodic reaction forces the coating to rupture and leave the substrate (Ref 47). Here, the fluctuation in the anodic polarization curves was simply the response of this behavior. A corresponding increase in R p from 1.22 kΩ cm2 (for Mg-Ca) to 331.4, and 823.1 kΩ cm2 for PCL-coated Mg and nano-HA/PCL composite-coated, respectively. The large increase in R p values observed for the nano-HA/PCL composite-coated Mg are due to the complete coverage of nano-HA-coated Mg by a rather thicker double-layered coating. In addition, in double-layered coatings, nano-HA coating can avoid the direct contact of the PCL and the Mg matrix so as to avoid their negative interaction. The dependence of the corrosion resistance on the thickness of the PCL coating suggests that the corrosion rate of double-layered-coated Mg can be adjusted by controlling its thickness (Ref 17). According to Eq 2, the corrosion rate of nano-HA/PCL- (0.001 mm/year), and PCL (0.004 mm/year)-coated samples presented significantly lower compared to the HA-coated (0.14 mm/year) (Ref 45) and uncoated (6.11 mm/year) sample. Wong et al. (Ref 19) showed that the addition of a polymer coating on the implant can reduce the corrosion rate of the implant. This obviously indicates the enhancement of protective properties of the specimens with double-layered nano-HA/PCL and single-layered PCL coating at the surface than that of the samples without coatings.
Nyquist plots of the Mg-Ca-uncoated, PCL-, and nano-HA/PCL-coated samples acquired by electrochemical impedance spectra (EIS) are shown in Fig. 10(a). In general, the high corrosion potential and charge-transfer resistance and low corrosion current density indicate that the test specimen has good corrosion resistance (Ref 48). As can be seen, coated and uncoated specimens showed a typical single-capacitive semicircle, which represents the electrochemical process with only one time constant (Ref 49). The capacitive loop in the high frequency region is related to the charge-transfer reaction in the electric double layer formed at the interface between the sample surface and corrosion medium (Ref 50). The reason that only one capacitive loop was phenomenally observed in the Nyquist diagram is probably because the electrochemical reaction area at the metal/coating interface was still small in this immersion period. This led to the difficulty to separate the time relaxation of physical impedance of the coating from that of electrochemical reaction impedance at the metal/coating interface (Ref 50). It can be clearly seen that the incorporation of nanoparticles of HA increased the charge-transfer resistance (Rt) of the PCL-coated sample (2127.15 kΩ cm2) indicating reduced coating porosity and improved barrier performance for corrosion protection of the steel substrate. However, the diameter of the semicircle in the Nyquist plots decreased to 1732.14 kΩ cm2 after single-layered PCL coating, indicating degradation of the coating. This can be attributed to the ionic transport in the coating including, e.g., chloride ion penetration through pinholes (Ref 51). However, the lowest charge-transfer resistance is observed for the uncoated sample (0.782 kΩ cm2). The rate of the electrochemical processes at the substrate/electrolyte interface is controlled by the charge-transfer resistance which is the key factor in determining the corrosion resistance (Ref 51, 52). This suggests that the double-layered coating can significantly hinder the charge-transfer process at the substrate/electrolyte interface. The Bode plots of the EIS spectra obtained for both coated samples are shown in Fig. 10(b). The impedance modulus at low frequency (|Z|) is used to characterize the corrosion protection property of the coatings (Ref 51). It is clearly denoted from the impedance value in the low frequency range that the nano-HA/PCL-coated presented the highest, |Z|, while, uncoated Mg-Ca alloy presented the lowest |Z| at the low-frequency limit which was attributed to the barrier characteristic of the coating. The combination of polarization and impedance tests shows that the double-layered nano-HA/PCL composite coating can efficiently delay penetration of electrolyte and chloride ions through the coating, and thus enhance the barrier property.
Immersion Test
Figure 11 shows the surface morphology of the uncoated, PCL-, and nano-HA/PCL-coated samples immersed for 240 h in SBF. Uncoated Mg-Ca alloy presented crack structure accompanied by a suffering from large round corrosion pits (Fig. 11a). The crack formation is due to dehydration of the corrosion products and associated differential shrinkage. Cracks make the solution contact the matrix easier, accelerating the corrosion of the matrix. EDS analysis in Fig. 11(b) shows the presence of Mg and O and trace amount of C indicating that corrosion products are composed of magnesium hydroxide Mg(OH)2, and the mole ratio of O to Mg was about 2.12. The pore structure of the single PCL layer was remarkably unchanged while some part is burst (Fig. 11c). This can be due to evolution of H2 which caused the increase the amount of the gas pressure under the polymer layer. The H2 permeability of the polymer is insufficient to release the gas at the rate that it is being produced, which leads to excess pressure that results in the bursting of the polymer film (Ref 30). The EDS spectra of the PCL-coated sample indicated that the film composed of Mg, C, O, and small amounts of Ca and P (Fig. 11d). The nano-HA/PCL coating presented larger pore structure because of the nano-HA particles were embedded inside the PCL and enlarged the pore diameters. In the composite coating, formation of large white particles can be observed on the specimen surface (Fig. 11e). The EDS analysis of the corrosion products of nano-HA/PCL coating revealed the presence of Mg, Ca, O, C, and P with Ca/P atomic ratio of around 1.20 which was higher than the PCL coating (1.16) indicating that more carbonate and phosphate ions can be absorbed by the nano-HA/PCL coating (Fig. 11f). The immersion tests also showed that double-layered nano-HA/PCL- and PCL-coated samples maintained their integrity better than the uncoated sample in SBF and reduced the corrosion rate more effectively compared to the uncoated sample.
Figure 12 shows a schematic illustration of the degradation mechanism of PCL- and nano-HA/PCL-coated Mg-Ca alloy. When coated specimens are immersed in the SBF, the SBF penetrated from the pore structure of the PCL coatings to the Mg-Ca substrate. As soon as the corrosive media reached the substrate, galvanic reactions between α-Mg and the Mg2Ca phases occurred (Fig. 12a). As a result of this galvanic cell, the anodic reaction occurred according to the following reactions (Ref 53, 54):
The presence of Mg+ is consistent with the low activation energy and electron transfer kinetics of the electrochemical process. Mg+ is metastable and oxidized readily by H+ with H2 being released from the surface of the specimens according to the following reaction (Ref 54):
Reduction of H+ by Mg leads to hydrogen evolution on the cathodic surface of the Mg alloy. On the cathodic surface (Ref 54),
The metal Mg was transformed into insoluble Mg(OH)2 film due to ready reaction with OH− (Eq 8), and therefore, magnesium hydroxide (brucite) starts forming at the interface coating with the alloy. This stage is also accompanied by hydrogen evolution. The generation of Mg(OH)2 subsequently leads to an increase in the pH of the solution. The generated hydrogen tends to release and push away the PCL films during its release, which weakens the adhesion between the Mg substrate and PCL coatings. The accumulation of gas underneath the coating also leads to formation of cracks and bursting of the polymer film and failures in the PCL coating (Fig. 12b). However, the presence of aggressive chloride ions in SBF can destroy the protective layer by transforming the Mg(OH)2 into more soluble MgCl2 (Ref 20) (Eq 9). Thus, the surface film would be easily dissolved, and the newly exposed active surface will be prone to pit nucleation.
When a large amount of Mg(OH)2 accumulated underneath the PCL coatings, the polymers near the Mg(OH)2 layer gradually became unstable and began to degrade to acids. These acids dissolve the Mg(OH)2 layer and at the same time release to the surrounding solution to neutralize OH− and decrease the pH of the solution (Ref 20).
Dissolution of the Mg(OH)2 layer and consumption of OH− by the released corrosion product of the PCL coating in the SBF solution resulted in acceleration of penetration of the solution in the Mg substrate and decrease in the corrosion resistance of the Mg alloy. Ca-P is formed on the PCL coating layer with spherical morphology because of the dissolution of high amounts of Mg2+ and reaction of phosphate ions (HPO4 2− or PO4 3−) and Ca2+ in the solution with OH− ions to form Ca-P. At this stage, the enlargement of the defects slows down and formation of Ca-P improves; however, some fragments of coating experienced delamination and corrosion products under the coating are exposed to the SBF (Fig. 12c). However, in double-layered nano-HA/PCL, due to the presence of nano-HA as an inner layer, formation of Mg(OH)2 resulted in reaction with other constituents in the SBF, and the local alkalization leads to formation of several precipitates. Upon increasing the immersion time, further diffusion of solution will occur via the pore structure and defects of which caused bursting or delamination of the polymer film.
Figure 13 shows the average mass loss of uncoated, PCL-, and nano-HA/PCL-coated Mg-Ca alloy in Kokubo solution for durations of 3, 7, and 14 days at 37 °C. As shown in the figure, the PCL-coated samples showed a dramatically low weight-loss rate at the initial stages of exposure. It is clear that the weight-loss rate was further decreased because of the deposition of nano-HA and PCL on the Mg substrate which inhibits further corrosion. The weight loss for the initial stages of PCL- and nano-HA/PCL-coated samples were about 0.19 and 0.11 mg/cm2/day, respectively, for the first 7 days of immersion. However, upon increasing the exposure time to 14 days, the weight-loss rate of the coated samples increased, indicating that the low adhesion of coating layers on magnesium substrates does not provide satisfactory protection for a longer immersion time. As can be also observed, single-layered PCL-coated samples presented higher weight-loss rates which can be due to blasting of the PCL layer in a longer immersion time. Uncoated Mg-Ca alloy showed the highest weight-loss rate (3.12 mg/cm2/day). The corrosion rate of uncoated alloys significantly increased with the increasing immersion time in the first 3 days (2.6 mg/cm2/day). This can be due to the fact that in the first few days of the exposure time, a larger surface area was exposed to the solution, resulting in more exothermic reactions thus increasing the corrosion rate. The other reason can be attributed to the presence of high concentration of chloride in Kokubo solution (Ref 51, 55, 56). Increasing the exposure time to 7 days increased the weight-loss rate for the uncoated alloy with a reduced slope compared to the 3 days exposure (3.7 mg/cm2/day). The weight-loss rate of uncoated alloys indicated a drop with prolonged exposure to 14 days. This can be ascribed to the reduced surface area of the alloy for corrosion processes to take place after prolonged exposure times. However, the corrosion rate of both coated samples significantly increased because of the delamination of the protective layers after prolonged exposure to the SBF.
Conclusions
In this work, we have successfully synthesized nano hydroxyapatite-polymer layers coated on Mg-Ca via a combination of electrodeposition and dip-coating methods. The double-layered composite coating consists of a PCL pores layer and a nano-HA dense layer. The PCL layer composed of interconnected pore networks with 40-50 μm thickness had relatively uniform distribution. In contrast, the nano-HA as an inner layer has a more compact structure with nano-needle-like microstructure and a crystallite size of around 96 nm and had 4-6 μm thickness only. During a 14-day in vitro test, weight losses of the PCL- and nano-HA/PCL-coated samples were significantly lower (0.53 and 0.42 mg/cm2/day, respectively) compared to the uncoated sample (2.83 mg/cm2/day). However, both PCL- and nano-HA/PCL-coated samples presented higher compressive strength than that of the uncoated sample. The nano-HA/PCL composite coating indicated better bonding strength (6.7 MPa) compared to PCL coating (2.2 MPa), owing to an incorporation of nano-HA with the PCL-coated layer on the Mg alloy. Therefore, the double-layered nano-HA/PCL composite coating is effective in protecting the Mg alloy against corrosion for a long time.
References
M. Yazdimamaghani, M. Razavi, D. Vashaee, and L. Tayebi, Surface Modification of Biodegradable Porous Mg Bone Scaffold Using Polycaprolactone/Bioactive Glass Composite, Mater. Sci. Eng., C, 2015, 49, p 436–444
H.R. Bakhsheshi-Rad, M.R. Abdul-Kadir, M.H. Idris, and S. Farahany, Relationship Between the Corrosion Behavior and the Thermal Characteristics and Microstructure of Mg-0.5Ca-xZn Alloys, Corros. Sci., 2012, 64, p 184–197
J. Zhou, Q. Li, H. Zhang, and F. Chen, Corrosion Behavior of AZ91D Magnesium Alloy in Three Different Physiological Environments, J. Mater. Eng. Perform., 2014, 23, p 181–186
N.G. Wang, R.C. Wang, C.Q. Peng, and Y. Feng, Corrosion Behavior of Magnesium Alloy AP65 in 3.5 % Sodium Chloride Solution, J. Mater. Eng. Perform., 2012, 21, p 1300–1308
P. Shi, B. Niu, E. Shanshan, Y. Chen, and Q. Li, Preparation and Characterization of PLA Coating and PLA/MAO Composite Coatings on AZ31 Magnesium Alloy for Improvement of Corrosion Resistance, Surf. Coat. Technol., 2015, 262, p 26–32
Z.J. Jia, M. Li, Q. Liu, X.C. Xu, Y. Cheng, Y.F. Zheng, T.F. Xi, and S.C. Wei, Micro-arc Oxidization of a Novel Mg-1Ca Alloy in Three Alkaline KF Electrolytes: Corrosion Resistance and Cytotoxicity, Appl. Surf. Sci., 2014, 292, p 1030–1039
E. Ghali, W. Dietzel, and K.U. Kainer, General and Localized Corrosion of Magnesium Alloys: A Critical Review, J. Mater. Eng. Perform., 2013, 22, p 2875–2891
H.R. Bakhsheshi-Rad, E. Hamzah, S. Farahany, and M. Staiger, The Mechanical Properties and Corrosion Behavior of Quaternary Mg-6Zn-0.8Mn-xCa Alloys, J. Mater. Eng. Perform., 2015, 24, p 598–608
M.C. Lopes de Oliveira, V.S. Marques Pereira, O.V. Correa, and R.A. Antunes, Corrosion Performance of Anodized AZ91D Magnesium Alloy: Effect of the Anodizing Potential on the Film Structure and Corrosion Behavior, J. Mater. Eng. Perform., 2014, 23, p 593–603
H.R. Bakhsheshi-Rad, E. Hamzah, M. Daroonparvar, M.A.M. Yajid, and M. Medraj, Fabrication and Corrosion Behavior of Si/HA Nano-composite Coatings on Biodegradable Mg-Zn-Mn-Ca Alloy, Surf. Coat. Technol., 2014, 258, p 1090–1099
Y. Huang, Y. Yan, and X. Pang, Electrolytic Deposition of Fluorine-Doped Hydroxyapatite/ZrO2 Films on Titanium for Biomedical Applications, Ceram. Int., 2013, 39, p 245–253
M. Razavi, M. Fathi, O. Savabi, B. Hashemi Beni, D. Vashaee, and L. Tayebi, Nanostructured Merwinite Bioceramic Coating on Mg Alloy Deposited by Electrophoretic Deposition, Ceram. Int., 2014, 40, p 9473–9484
M. Razavi, M. Fathi, O. Savabi, S. Mohammad Razavi, B. Hashemi Beni, D. Vashaee, and L. Tayebi, Controlling the Degradation Rate of Bioactive Magnesium Implants by Electrophoretic Deposition of Akermanite Coating, Ceram. Int., 2014, 40, p 3865–3872
E.J. Lee, S.H. Teng, T.S. Jang, P. Wang, S.W. Yook, H.E. Kim, and Y.H. Koh, Nanostructured Poly(ε-caprolactone)-Silica Xerogel Fibrous Membrane for Guided Bone Regeneration, Acta Mater., 2010, 6, p 3557–3565
Y. Huanga, Q. Dinga, X. Panga, S. Hana, and Y. Yana, Corrosion Behavior and Biocompatibility of Strontium and Fluorine Co-Doped Electrodeposited Hydroxyapatite Coatings, Appl. Surf. Sci., 2013, 282, p 456–462
H.R. Bakhsheshi-Rad, E. Hamzah, M. Daroonparvar, R. Ebrahimi-Kahrizsangi, and M. Medraj, In-Vitro Corrosion Inhibition Mechanism of Fluorine-Doped Hydroxyapatite and Brushite Coated Mg-Ca Alloys for Biomedical Applications, Ceram. Int., 2014, 40, p 7971–7982
H. Wang, C. Zhao, Y. Chen, J. Li, and X. Zhang, Electrochemical Property and In Vitro Degradation of DCPD-PCL Composite Coating on the Biodegradable Mg-Zn Alloy, Mater. Lett., 2012, 68, p 435–438
A. Zomorodia, M.P. Garcia, T.M. Silva, J.C.S. Fernandes, M.H. Fernandes, and M.F. Montemor, Corrosion Resistance of a Composite Polymeric Coating Applied on Biodegradable AZ31 Magnesium Alloy, Acta Mater., 2013, 9, p 8660–8670
H.M. Wong, K.W.K. Yeung, K.O. Lam, V. Tam, P.K. Chu, K.D.K. Luk, and K.M.C. Cheung, A Biodegradable Polymer-Based Coating to Control the Performance of Magnesium Alloy Orthopaedic Implants, Biomaterials, 2010, 31, p 2084–2096
Y. Chen, Y. Song, S. Zhang, J. Li, C. Zhao, and X. Zhang, Interaction Between a High Purity Magnesium Surface and PCL and PLA Coatings During Dynamic Degradation, Biomed. Mater., 2011, 6, p 1–8
B.D. Cullity, Elements of X-ray Diffraction, 2nd ed., Addison-Wesley, Massachusetts, 1978
Y. Song, E.H. Hana, D. Shan, C.D. Yim, and B.S. You, The Effect of Zn Concentration on the Corrosion Behavior of Mg-xZn Alloys, Corros. Sci., 2012, 65, p 322–330
G.R. Argade, K. Kandasamy, S.K. Panigrahi, and R.S. Mishra, Corrosion Behavior of a Friction Stir Processed Rare-Earth Added Magnesium Alloy, Corros. Sci., 2012, 58, p 321–326
Z. Shi and M. Liu, Atrens A., Measurement of the Corrosion Rate of Magnesium Alloys Using Tafel Extrapolation, Corros. Sci., 2010, 52, p 579–588
H.R. Bakhsheshi-Rad, M.H. Idris, M.R. Abdul-Kadir, A. Ourdjini, M. Medraj, M. Daroonparvar, and E. Hamzah, Mechanical and Bio-corrosion Properties of Quaternary Mg-Ca-Mn-Zn Alloys Compared with Binary Mg-Ca Alloys, Mater. Des., 2014, 53, p 283–292
T. Kokubo, H.M. Kim, and M. Kawashita, Novel Bioactive Materials with Different Mechanical Properties, Biomaterials, 2003, 24, p 2161–2175
K. Huang, S. Cai, G. Xu, X. Ye, Y. Dou, and M. Ren, Preparation and Characterization of Mesoporous 45S5 Bioactive Glass-Ceramic Coatings on Magnesium Alloy for Corrosion Protection, Appl. Surf. Sci., 2013, 264, p 816–822
Y. Wu and R.L. Clark, Controllable Porous Polymer Particles Generated by Electrospraying, J. Colloid Interface Sci., 2007, 310, p 529–535
P. Dayal, J. Liu, S. Kumar, and T. Kyu, Experimental and Theoretical Investigations of Porous Structure Formation in Electrospun fibers, Macromolecules, 2007, 40, p 7689–7694
A. Abdal-hay, T. Amna, and J.K. Lim, Biocorrosion and Osteoconductivity of PCL/nHAp Composite Porous Film-Based Coating of Magnesium Alloy, Solid State Sci., 2013, 18, p 131–140
X.N. Gu, W. Zheng, Y. Cheng, and Y.F. Zheng, A Study on Alkaline Heat Treated Mg-Ca Alloy for the Control of the Biocorrosion Rate, Acta Biomater., 2009, 5, p 2790–2799
M. Lebourg, J. Suay Antón, and J.L. Gomez Ribelles, Characterization of Calcium Phosphate Layers Grown on Polycaprolactone for Tissue Engineering Purposes, Compos. Sci. Technol., 2010, 70, p 1796–1804
H.W. Kim, J.C. Knowles, and H.E. Kim, Hydroxyapatite/Poly(ε-caprolactone) Composite Coatings on Hydroxyapatite Porous Bone Scaffold for Drug Delivery, Biomaterials, 2004, 25, p 1279–1287
M. Kheradmandfard and M.H. Fathi, Fabrication and Characterization of Nanocrystalline Mg-Substituted Fluorapatite by High Energy Ball Milling, Ceram. Int., 2013, 39, p 1651–1658
S. Rössler, A. Sewing, M. Stölzel, R. Born, D. Scharnweber, M. Dard, and H. Worch, Electrochemically Assisted Deposition of Thin Calcium Phosphate Coatings at Near-Physiological pH and Temperature, J Biomed Mater Res A, 2003, 64, p 655–663
Y. Chen and X. Miao, Effect of Fluorine Addition on the Corrosion Resistance of Hydroxyapatite Ceramics, Ceram. Int., 2004, 30, p 1961–1965
M. Tomozawa and S. Hiromoto, Microstructure of Hydroxyapatite- and Octacalcium Phosphate-Coatings Formed on Magnesium by a Hydrothermal Treatment at Various pH Values, Acta Mater., 2011, 59, p 355–363
M.F.M. Yusoff, M.R. AbdulKadir, N. Iqbal, M.A. Hassan, and R. Hussain, Dipcoating of Poly(ε-caprolactone)/Hydroxyapatite Composite Coating on Ti6Al4 V for Enhanced Corrosion Protection, Surf. Coat. Technol., 2014, 245, p 102–107
D.W. Hong, Z.T. Lai, T.S. Fu, T.T. Tsai, I.M. Chu, and P.L. Lai, The Influences of Polycaprolactone-Grafted Nanoparticles on the Properties of Polycaprolactone Composites with Enhanced Osteoconductivity, Compos. Sci. Technol., 2013, 83, p 64–71
M. Razavi, M. Fathi, O. Savabi, B. Hashemi Beni, D. Vashaee, and L. Tayebi, Surface Microstructure and In Vitro Analysis of Nanostructured akermanite (Ca2MgSi2O7) Coating on Biodegradable Magnesium Alloy for Biomedical Applications, Colloids Surf. B, 2014, 117, p 432–440
M. Burke, B. Clarke, Y. Rochev, A. Gorelov, and W. Carroll, Estimation of the Strength of Adhesion Between a Thermoresponsive Polymer Coating and Nitinol Wire, J. Mater. Sci. Mater. Med., 2008, 19, p 1971–1979
L. Xu and A. Yamamoto, Characteristics and Cytocompatibility of Biodegradable Polymer Film on Magnesium by Spin Coating, Colloids Surf. B, 2012, 93, p 67–74
L. Xu and A. Yamamoto, In Vitro Degradation of Biodegradable Polymer-Coated Magnesium Under Cell Culture Condition, Appl. Surf. Sci., 2012, 258, p 6353–6358
A. Abdal-hay, M. Dewidar, and J.K. Lim, Biocorrosion Behavior and Cell Viability of Adhesive Polymer Coated Magnesium Based Alloys for Medical Implants, Appl. Surf. Sci., 2012, 261, p 536–546
H.R. Bakhsheshi-Rad, E. Hamzah, M. Daroonparvar, M.A.M. Yajid, M. Kasiri-Asgarani, M.R. Abdul-Kadir, and M. Medraj, In-Vitro Degradation Behavior of Mg Alloy Coated by Fluorine Doped Hydroxyapatite and Calcium Deficient Hydroxyapatite, Trans. Nonferrous Met. Soc. China, 2014, 24, p 2516–2528
A. Srinivasan, P. Ranjani, and N. Rajendran, Electrochemical Polymerization of Pyrrole Over AZ31 Mg Alloy for Biomedical Applications, Electrochim. Acta, 2013, 88, p 310–321
G. Wu, Fabrication of Al and Al/Ti Coatings on Magnesium Alloy by Sputtering, Mater. Lett., 2007, 61, p 3815–3817
X. Ye, S. Cai, Y. Dou, G. Xu, K. Huang, M. Ren, and X. Wang, Bioactive Glass-Ceramic Coating for Enhancing the In Vitro Corrosion Resistance of Biodegradable Mg Alloy, Appl. Surf. Sci., 2012, 259, p 799–805
C. Zhou, X. Lu, Z. Xin, J. Liu, and Y. Zhang, Polybenzoxazine/SiO2 Nanocomposite Coatings for Corrosion Protection of Mild Steel, Corros. Sci., 2014, 80, p 269–275
X. Lu, Y. Zuo, X. Zhao, Y. Tang, and X. Feng, The Study of a Mg-rich Epoxy Primer for Protection of AZ91D Magnesium Alloy, Corros. Sci., 2011, 53, p 153–160
H.R. Bakhsheshi-Rad, E. Hamzah, M. Daroonparvar, M. Kasiri-Asgarani, and M. Medraj, Synthesis and Biodegradation Evaluation of Nano-Si and Nano-Si/TiO2 Coatings on Biodegradable Mg-Ca alloy in Simulated Body Fluid, Ceram. Int., 2014, 40, p 14009–14018
M. Liu, X. Mao, H. Zhu, A. Lin, and D. Wang, Water and Corrosion Resistance of Epoxy-Acrylic-Amine Waterborne Coatings: Effects of Resin Molecular Weight, Polar Group and Hydrophobic Segment, Corros. Sci., 2013, 75, p 106–113
G.Y. Liu, S. Tang, D. Li, and J. Hu, Self-adjustment of Calcium Phosphate Coating on Micro-arc Oxidized Magnesium and Its Influence on the Corrosion Behaviour in Simulated Body Fluids, Corros. Sci., 2014, 79, p 206–214
M.I. Jamesh, G. Wu, Y. Zhao, D.R. McKenzie, M.M.M. Bilek, P.K. Chu, and B.S. Necula, Effects of Zirconium and Oxygen Plasma Ion Implantation on the Corrosion Behavior of ZK60 Mg Alloy in Simulated Body Fluids, Corros. Sci., 2014, 82, p 7–26 55. 56.
H.R. Bakhsheshi-Rad, E. Hamzah, M.R. Abdul-Kadir, M. Daroonparvar, and M. Medraj, Corrosion and Mechanical Performance of Double-Layered Nano-Al/PCL Coating on Mg-Ca-Bi Alloy, Vacuum, 2015, 119, p 95–98
S.N. Saud, E. Hamzah, T. Abubakar, H.R. Bakhsheshi-Rad, M. Zamri, and M. Tanemura, Effects of Mn Additions on the Structure, Mechanical Properties, and Corrosion Behavior of Cu-Al-Ni Shape Memory Alloys, J. Mater. Eng. Perform., 2014, 23, p 3620–3629
Acknowledgments
The authors would like to acknowledge the Universiti Teknologi Malaysia (UTM) and Nippon Sheet Glass Foundation for providing research facilities and financial support under Grant No. R.J.130000.7324.4B136.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bakhsheshi-Rad, H.R., Hamzah, E., Abdul-Kadir, M.R. et al. The Mechanical Properties and Corrosion Behavior of Double-Layered Nano Hydroxyapatite-Polymer Coating on Mg-Ca Alloy. J. of Materi Eng and Perform 24, 4010–4021 (2015). https://doi.org/10.1007/s11665-015-1661-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-015-1661-4