Abstract
Al-AlCr was coated on Mg-Ca and Mg-Zn-Ce-La alloys using physical vapor deposition method. The surface morphology of the specimens was characterized by x-ray diffraction, scanning electron microscopy equipped with energy-dispersive x-ray spectroscopy, and atomic force microscopy (AFM). The AFM results indicated that the average surface roughness of Al-AlCr coating on the Mg-Ca alloy is much lower than that of Al-AlCr coating on the Mg-Zn-Ce-La alloy. However, Al-AlCr coating on the Mg-Ca alloy presented a more compact structure with fewer pores, pinholes, and cracks than Al-AlCr coating on the Mg-Zn-Ce-La alloy. Electrochemical studies revealed that the novel coating (Al-AlCr) can remarkably reduce the corrosion rate of the Mg-Ca alloy in 3.5 wt.% NaCl solution. It was seen that the anodic current density of the Al-AlCr-coated Mg-Ca alloy was very small when compared to the Al-AlCr-coated Mg-Zn-Ce-La and uncoated alloys. Impedance modulus (Z) of the Al-AlCr-coated samples was higher than that of the bare Mg alloys. Z of Al-AlCr-coated Mg-Ca alloy was higher than that of the Al-AlCr-coated Mg-Zn-Ce-La alloy at low frequency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnesium alloys are very important structural materials due to their attractive performance (low density, high specific stiffness, good machinability, high damping capacity, castability, weldability, and recyclability), but the factors which restrict their wider structural applications (such as aerospace, electronics, and automobile industries) are their low corrosion and wear resistances (Ref 1-6). Corrosion resistance of the magnesium was improved by adding alloying elements to the pure magnesium (Ref 4, 5). In this regard, rare earth elements could remarkably enhance the corrosion resistance of the magnesium alloys, which was primarily ascribed to the formation of metastable RE (rare earth)-containing phases along the grain boundaries and other reasons such as the melt purity and decreasing activity of the alloy surface (increment of standard electrochemical potential of the alloy surface in comparison with standard hydrogen electrode (Ref 7-9). It was found that the corrosion resistance of the Mg alloy including a small content of RE elements can be increased compared to that of the Mg alloy including a large content of RE elements which showed a low corrosion resistance (Ref 7, 8). In fact, α-Mg matrix (as anode) has surrounded the second phases which can act as cathode and can reduce the corrosion rate of the Mg-RE alloys (Ref 7, 8). This phenomenon depends on the distribution of the RE-phases in the a-Mg matrix. Rare earth phases can act as a galvanic cathode (at a low volume fraction) which is able to increase the corrosion rate of the Mg alloy. In contrary, rare earth phases (at a large volume fraction) can act as an anodic barrier and can also reduce the corrosion rate (Ref 7, 8). Sometimes, reaching the optimum condition is very hard and uneconomical (Ref 5, 7, 8). On the other hand, rare earth elements are more expensive than the conventional elements, because the extraction of rare earth elements from the earth's crust is a highly complex process (Ref 10). So, alternative ways should be found to improve considerably the corrosion resistance of the Mg alloys. Therefore, it is anticipated that Mg (as matrix) activity could be significantly suppressed using coating process. Wear, corrosion, and oxidation resistances of the Mg alloys were considerably improved using the thin and thick coatings (Ref 11-15). In fact, the surface modification technology is a very important method to make up for the disadvantage and improve the mechanical properties of the magnesium alloys significantly (Ref 16, 17).This surface modification was carried out by the prevalent techniques to coat the desired materials on the surface of the Mg alloys (Ref 11-17). Dense coatings are able to protect the substrate. This phenomenon is mainly related to the disconnection between the substrate and its environment (Ref 18-23). Thus, the lifetime of the magnesium alloys which are exposed to aggressive environments can be enhanced using surface treatments (Ref 11-15).
Plasma spraying was used to improve the corrosion, oxidation, and wear resistances of the magnesium alloys. However, the adhesion between the coating and the substrate was considerably reduced due to the formation of a thermally grown oxide layer on the magnesium during spraying (Ref 24, 25). In contrast, physical vapor deposition (PVD) method is a green technique for applying a variety of coatings, such as TiN, Cr, Ti, Ti3Al, and Al/Al2O3 onto the magnesium alloys (Ref 1, 21, 26, 27). Wastes or considerable atmospheric emissions have not been observed in the coatings produced by this clean, environmentally friendly technique (PVD) (Ref 21). Protective coatings can be more easily deposited on the magnesium alloys using PVD than the electrochemical methods (Ref 24).
Al, Al/Ti, and Al/Cr coatings were applied by PVD method on the magnesium alloys, aluminum alloys, and steels, respectively. Aluminum coating was also considered as one of the latent candidates for the protection of the sintered NdFeB products, because of its friendly price and good corrosion resistance in aggressive media (Ref 28-30). On the other hand, it was reported that the corrosion potential of the Al/Ti-, AlN/TiN-, AlN-, Cr-, and CrN-coated samples was positively shifted in comparison to that of the bare samples. According to the mixed potential theory (Ref 21, 31), that happening was related to the higher standard electrochemical potential of the coatings than that of the bare Mg alloys (Ref 14, 21, 32-34). It means that the coatings were nobler than the Mg alloys and could protect the substrate effectively in the NaCl aqueous solution (Ref 14, 21, 32-34). Thus, it is hypothesized that the compact Al and AlCr coatings would be good candidates for the surface modification of the Mg alloys.
So, a coating (multi-layered) with the higher standard electrochemical potential (Ref 28, 35) than the substrate can be applied on the surface of the Mg alloys. It is anticipated that this coating could reduce the Mg dissolution (Mg surface activity) and preserve the formed protective oxide films on the Mg alloys (with or without RE elements) during corrosion. Furthermore, to date, there has been no study on the effect of the multi-layered PVD coatings on the corrosion behavior of Mg-Zn-RE alloys in the aggressive solution. Therefore, in this research, magnetron sputtering machine was used to deposit the double-layered Al-AlCr coating on the Mg-Zn-Ce-La and Mg-Ca alloys, in order to increase their corrosion resistance in an aggressive solution.
Experimental Procedures
Preparation of Samples Surface and PVD Parameters
Magnesium alloys were prepared by melting 99.9% pure magnesium ingots, pure zinc (99.99%), Mg-32 wt.% Ca, and Mg-30 wt.% RE. The materials were melted by electrical resistance furnace under the protection of argon gas in a mild steel crucible coated with boron nitride at 760 °C. The molten metal was maintained for around 45 min at the melting condition for stabilization. After stabilizing, molten metal were poured into mild steel molds which had been preheated at 400 °C accompanied with 30-s stirring process. The melts, with a constant concentration of RE (3 wt.%) and Zn (2 wt.%), were then cast in a 300 °C preheated stainless steel mold to produce an ingot. The chemical compositions of the Mg-1Ca and Mg-2Zn-2Ce-1La alloys are as follows:
Mg-2Zn-2Ce-1La specimens with composition of 0.049% Si, 0.038% Mn, 2.231% Zn, 0.028% Al, 0.014% Fe, 2.189% Ce, 0.938 La, and 94.501% Mg (balanced was Mg).
Mg-1Ca specimens with composition of 0.038% Si, 0.031% Mn, 0.028% Al, 0.011% Fe, 1.128% Ca, and 94.501% Mg (balanced was Mg).
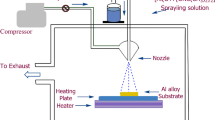
Mg-Zn-Ce-La and Mg-Ca alloys (15 × 15 × 6 mm) were employed as substrates. These substrates were ground with SiC emery paper up to #2400 and then polished with Al2O3 paste (average size of 1 μm), to obtain a mirror surface. A hybrid ion beam deposition system consisting of a linear ion source and a magnetron sputtering source was selected to deposit the coatings on the substrates. The Mg alloys were ultrasonically washed in pure alcohol for 5 min before placement inside the vacuum chamber. An ion source with Ar gas was used to clean the surface of the Mg alloys for 40 min. This pre-treatment was performed when the base pressure of the chamber was below 2.55 × 10−3 Pa. PVD was performed at room temperature with argon as the sputtering gas. The PVD parameters are listed in Table 1.
Electrochemical Measurements
An electrochemical polarization test was carried out using a PARSTAT 2263 advanced electrochemical system. The electrochemical cell consisted of the sample as the working electrode, a saturated calomel electrode (SCE) as the reference electrode, and a platinum rod as the counter electrode. Each sample was masked by paraffin wax such that a surface area of 1.0 × 1.0 cm2 was exposed in 3.5% NaCl solution. The test was run at 1.0 mV/s at room temperature, after allowing a steady-state potential to develop (for 10 min before commencing the potentiodynamic polarization and electrochemical impedance spectra (EIS) tests). The EIS were measured over a frequency range from 0.01 Hz to 100 kHz. EIS test was also performed using VersaSTAT 3 machine. It should be mentioned that the potentiodynamic polarization and EIS tests were accomplished three times to ensure reproducibility of the results.
Characterization of the Coatings
The surface morphologies and cross-sectional area of the coatings were characterized by field-emission scanning electron microscope (FE-SEM, Hitachi S4160, Japan) equipped with energy-dispersive spectrum (EDS, 7401 Oxford) (for chemical composition investigation). The phase analysis of the coatings was accomplished by low-angle x-ray diffraction (XRD, Rigaku D, Japan). The diffraction patterns were collected with Cu Ka radiation over a 2θ range of 5°-90°.
Results and Discussion
Coatings Characterization
Figure 1 exhibits the surface morphology of the coated and uncoated parts of the Mg alloys. A typical cast structure with the presence of inter-dendritic phases surrounding the α-Mg matrix can be easily seen in Fig. 1(a) (uncoated part). EDX analysis of this surface showed that the percentage of Zn, La, and Ce elements in the gray phases (see Fig. 1a(A)) is higher than that in the black phases (see Fig. 1a(B)). It shows that black phases are mainly composed of α-Mg. It can be hypothesized that the α-Mg phases are softer than the second phases (gray phases). So, gray phases would protrude from the matrix (α-Mg) during grinding and polishing. Hence, it is anticipated that the average surface roughness of this alloy would be increased, which could considerably influence the surface morphology of the sputtered coatings on the alloy surface. It was reported that the surface quality of the substrates, e.g., surface morphology and defects can determine the sputtered coating quality by PVD (Ref 14, 34). EDX analysis of the coated part (Fig. 1a) depicted that this coating is mainly composed of Al and Cr elements (Fig. 1a(C)). It should be mentioned that the atomic concentration of the Al and Cr elements was 26.25 and 73.75%, respectively. Hence, it can be postulated that the film may be stoichiometric Al3Cr7.
Figure 1(b) exhibits that the uncoated part surface of the Mg-Ca alloy is much more smoother than the Mg-Zn-RE alloy surface and is mainly composed of α-Mg phase (Fig. 1b(D)). So, it is expected that this smooth surface could affect the sputtered coating quality and could minimize the coating defects. EDX analysis of the coated part (Fig. 1b) depicted that this coating also is mainly composed of Al and Cr elements (Fig. 1b(E)). The atomic concentration of Al and Cr elements was 26.31 and 73.69%, respectively. Therefore, the film may be stoichiometric Al3Cr7. AFM results showed that the average surface roughness of the Mg-Ca alloy is lower than that of the Mg-Zn-RE alloy after grinding and polishing (see Fig. 2).
Figure 3 demonstrates the surface morphology of the double-layered Al-AlCr coating on the Mg alloys. Figure 3(a), (c), and (e) shows the surface morphology of Al-AlCr coating on the Mg-Zn-RE alloy at different magnifications. Figure 3(b), (d), and (f) exhibits the surface morphology of the Al-AlCr coating on the Mg-Ca alloy at different magnifications. The micro-cracks and pinholes are widely seen in the AlCr coating (on the Mg-Zn-RE alloy) (Fig. 3c and e). It is speculated that these pinholes could act as a location for setting up the local galvanic cells between the coating and substrate at the defect sites. It is interesting to note that the possible source of defects extending through the coating can be macro-defects (such as droplet peripheries and craters) and inherent porosity of the coating itself (Ref 32, 34), as shown in Fig. 3(c) and (e). Inherent porosity can be related to the columnar structure of the coatings. Some parts of the boundaries between the columns are sources of porosity extending through the coatings (Ref 32, 34). The formation of pinholes in PVD coatings can be ascribed to the fact that the coated surfaces are always non-uniform and the coating (AlCr) would tend to commence to grow in a non-uniform manner. In fact, after the original nucleation stage, the growth process occurs in the isolated islands. These islands grow together, leaving voids between them (Ref 24). But, a compact coating with finer grains and homogeneous structure (Fig. 3d and f) can be observed in the AlCr coating (on the Mg-Ca alloy). This observation can originate from surface morphology of the Mg-Ca alloy which is able to reduce the defects of deposited coating on its surface (Fig. 3f).
Figure 4a and b exhibit the fracture morphology of the double-layered Al-AlCr coating on the Mg-Zn-RE and Mg-Ca alloys, respectively. Some pinholes can be seen between the columns (Fig. 4a). It was reported that if one of these pores is a through-pore, the corrosive media will penetrate into the coating via the pore and attack the coating/substrate interface (by galvanic cell formation) (Ref 25).
Less and smaller defects were found within the AlCr coating deposited on the Mg-Ca alloy (Fig. 3f) in comparison with the other coating (Fig. 3e). This observation may be due to the fact that the Mg-Ca alloy surface used was smoother in comparison with the Mg-Zn-RE alloy surface.
Figure 5 shows the cross section of double-layered Al-AlCr coating on the Mg alloys. Figure 5 also depicts Al-AlCr coating which has homogeneously covered the surface of the Mg alloys. Figure 5(a) shows the double-layered Al-AlCr coating on the Mg-Zn-RE alloy, the layers of which can be easily distinguished in the x-ray diffractogram. These layers can also be observed on the Mg-Ca alloy (Fig. 5b). Although the samples were severely ground and polished prior to FE-SEM observation, the coating was still bonded to the substrate alloy. Thus, it can be postulated that the adhesion between the coatings and the substrate is appropriate. Based on the cross section and Fig. 4, the double-layered Al-AlCr coating thickness was estimated to be 2.541 μm.
Figure 6 shows the AFM images of the double-layered Al-AlCr coating on the Mg alloys. The average surface roughness of the double-layered Al-AlCr coating on the Mg-Ca alloy (Fig. 6a) is much lower than that of the double-layered Al-AlCr coating on the Mg-Zn-RE alloy (Fig. 6b). The smooth surface of the coating with higher densification is essential for hindering the infiltration of aggressive solutions into the coating and to lessen the local galvanic corrosion of the substrate. Double-layered Al-AlCr coating on the Mg alloys exhibited a nano-structure (see Fig. 3e and f), as can also be confirmed by the AFM images (see Fig. 6c and d). It should be explained that the surface roughness of the part to be coated also significantly influences the corrosion behavior of the system. A higher surface roughness results in a less complete coverage of the substrate with the coating material because of shadowing effects during PVD film growth (Ref 36).
AFM images of the double-layered Al/AlCr coating on the Mg alloys: (a) AFM 3D image of Al/AlCr coating on the Mg-Ca alloy, (b) AFM 3D image of Al/AlCr coating on Mg-Zn-Ce-La alloy, (c) surface morphology of Al/AlCr coating on the Mg-Ca alloy, (d) surface morphology of Al/AlCr coating on Mg-Zn-Ce-La alloy
Mg-Zn-RE alloy showed rough surface morphology which can arise from the difference between the hardness of different phases. As a result, there is a change in the roughness of the sputtered coatings. This surface roughness (including pores, pinholes, cracks) was considerably expanded in the second layer (AlCr) as outer layer of Al-AlCr coating.
Most of the PVD coatings could improve the corrosion resistance of the substrate, but some of them imposed a severe local galvanic corrosion of the substrate. This occurrence was due to high porosity of the coating. In fact, a large amount of pores formed between the columns can be considered as the biggest disadvantage of PVD coatings (Ref 34).
Figure 7 shows the XRD patterns of the double-layered Al-AlCr coatings deposited on the Mg alloys. XRD spectrum (Fig. 7a) shows that the main phase in Mg-Ca alloy is α-Mg. But, Mg2Ca phase was not detected. This observation is mainly related to the crystal structure of Mg2Ca phase which has a hexagonal crystal structure with the space group P63/mmc and the lattice parameters a = 0.623 nm and c = 1.012 nm. This structure is similar to α-Mg but with almost twice the size of the lattice parameter (Ref 37). XRD spectrum (Fig. 7b) shows that the main phases in Mg-Zn-Ce-La alloy are α-Mg and β-Mg12Ce. For the Al-AlCr coatings (Fig. 7c and d), Al3Cr7 (110), Al3Cr7 (200), and Al3Cr7 (211) planes are clearly observed in the XRD patterns based on the Powder Diffraction File (PDF) records 03-065-6108. On the other hand, Al0.3Cr0.7 phase was also considered for the aforementioned planes based on the powder diffraction file records 01-074-5156. Based on a comparison of the peak intensities, Al3Cr7(110), a densely packed plane, is the preferred orientation in the aluminum-chromium coatings. This phase also showed excellent crystallinity.
Electrochemical Measurements
Potentiodynamic Polarization Studies
Figure 8 demonstrates the polarization curves of the Al-AlCr-coated specimens as well as the uncoated specimens in the NaCl solution. The curves show that the corrosion potential (E corr) of the Al-AlCr-coated Mg-Ca alloy is −1390 mVSCE which is approximately 288 mVSCE higher than that of the uncoated sample (Mg-Ca). In this regard, the corrosion potential of the Al-AlCr-coated Mg-Zn-RE alloy was −1379 mVSCE which is approximately 171 mVSCE higher than that of the uncoated sample (Mg-Zn-RE). So, the corrosion potential of the coated samples was positively shifted in comparison to that of the uncoated samples. According to the mixed standard electrode potential theory (Ref 31), it was found that the Al-AlCr coating was nobler than the bare Mg alloys. So, a compact Al-AlCr coating (with lower defects) is able to reduce the Mg dissolution (Mg surface activity) during corrosion.
The cathodic polarization curves usually show the cathodic hydrogen evolution (cathodic Reaction 1), while anodic dissolution of the magnesium can be attributed to the anodic curves (anodic Reaction 2) (Ref 27, 34).
So, the anodic branches can considerably determine the corrosion resistance of the samples. The anodic current density was sharply increased, as shown in Fig. 8(a) and (b). This phenomenon can be attributed to the low compactness and thickness of the formed oxide films (with loose structure) on the Mg alloys, which are not able to protect the electrode surface (alloy surface) during corrosion (Ref 7, 8).
But, the coatings reduced the anodic current density of the magnesium alloys, which is mainly related to the more compactness, homogeneity, and thickness of the coatings compared to those of the formed oxide films on the Mg alloys. These oxide films do not have enough stability in the solution and cannot protect the alloy adequately against the corrosion.
It is interesting to note that it is almost impossible to avoid the formation of defects (pores, pinholes, and cracks) in the coatings. So, some small structural defects inside the coating can act as channels for conducting corrosive materials toward the substrate and can easily form galvanic cells at the defects near the interface. Hence, these defects should be minimized during and/or after the coating process (Ref 36), as shown in Fig. 3(e) and 4(a). So, it is postulated that the coated sample with the lower average surface roughness includes the lower anodic current density and higher corrosion resistance (Fig. 8d).
Uncoated Mg alloys demonstrated an activation-controlled anodic behavior (in the anodic region) while Al-AlCr-coated Mg-Ca alloy showed a passive-like behavior in the aggressive solution (see Fig. 8d). The existence of passivation tendency at the anodic side implies the presence of protective film on the surface of the Mg alloy. It can be observed that the passivation part below the breakdown potential (E b) formed in the coated Mg-Ca alloy. E b is shown with an arrow present in the curve at the anodic side. It can be clearly seen that Al-AlCr coating can considerably lessen the anodic dissolution of the Mg-Ca alloy in the corrosive solution. The electrochemical parameters of the uncoated and the coated specimens are listed in Table 2. The corrosion current density (i corr) of the uncoated Mg-Ca, Mg-Zn-Ce-La, Al-AlCr-coated Mg-Zn-Ce-La, and Al-AlCr-coated Mg-Ca alloys was 250.5, 200, 25, and 0.25 µA/cm2, respectively. The lowest i corr value was considered for Al-AlCr-coated Mg-Ca alloy which showed the lowest corrosion rate in a 3.5% NaCl solution. This observed behavior can be attributed to the less number of defects (lower average surface roughness) in the coating (Fig. 8d) than Al-AlCr coating on the Mg-Zn-Ce-La alloy (Fig. 8c).
The corrosion current density value (i corr) of the uncoated Mg-Zn-RE alloy was nearly close to that of the Al-AlCr-coated Mg-Zn-RE alloy (one order of magnitude) (Fig. 8b and c). It can be explained by the presence of defects in the coating layers and higher average surface roughness of this coating in comparison with Al-AlCr coating on the Mg-Ca alloy. However, it is seen that the anodic current density of the Al-AlCr-coated Mg-Ca alloy was very small when compared to the other coating and the uncoated alloys. Lower corrosion resistance of Al-AlCr-coated Mg-Zn-RE alloy can originate from the surface morphology of the Mg-Zn-RE alloy which could substantially influence the surface morphology of the deposited coatings on the alloy surface. So, the existence of through-thickness pores in the Al-AlCr coating can provide the sites to set up galvanic cells in the solutions. This phenomenon would cause the production of hydrogen gas by the cathodic reactions and followed by rupturing the coating (Ref 36). But, the porosity (surface roughness) was further reduced in the Al-AlCr coating on the Mg-Ca alloy and the corrosion resistance of the Al-AlCr-coated Mg-Ca alloy was enhanced much more greatly compared to that of the other samples. In fact, lower average surface roughness and compact structure of Al-AlCr coating on the Mg-Ca alloy would cause the reduction of infiltration of aggressive ions such as Cl− toward the substrate. In contrast, this coating did not show a compact structure (with lower defects) on Mg-Zn-RE alloy, which caused the reduction of corrosion protection of this coating on the alloy surface effectively. This phenomenon can originate from the rough surface morphology of Mg-Zn-RE alloy which can enhance the average surface roughness of the coating deposited by PVD on the Mg-Zn-RE alloy surface.
Bode Phase and Bode Magnitude Plots of Electrochemical Impedance Spectroscopy (EIS) Data
Bode plots of EIS data for uncoated and coated specimens in a 3.5 wt.% NaCl solution are depicted in Fig. 9 and 10. Clear changes in the bode plots can be easily seen upon coating of the Mg-Ca and Mg-Zn-Ce-La substrates. In general, better corrosion resistance of the metal substrate can be related to the higher Z modulus at lower frequency (in bode magnitude plot) (Ref 16, 17). It is clearly observed that the impedance modulus (Z) of Al-AlCr-coated samples is higher than that of the bare Mg alloys. On the other hand, Z of Al-AlCr-coated Mg-Ca alloy is higher than that of the Al-AlCr-coated Mg-Zn-Ce-La alloy at low frequency. In bode phase plot (Fig. 10), it can be seen that the aperture of the phase angle increased after applying a compact PVD coating. This can be attributed to the formation of a passive film (Ref 38). However, both the bare Mg alloys presented small phase angles and the aperture of the phase angles decreased as the surface film cannot protect the substrate effectively (Ref 38, 39). These results showed that Al/AlCr coating (as a strong barrier layer) can protect the Mg-Ca substrate against aggressive solution. This observation may be related to the higher anodic current density of the Al-AlCr-coated Mg-Zn-Ce-La alloy in comparison to that of the Al-AlCr-coated Mg-Ca alloy. So, it can be said that the compact Al-AlCr coating (with lower defects) can substantially enhance the corrosion resistance of the Mg-Ca alloy. Lower corrosion resistance of the bare Mg alloys can be ascribed to the lower Z and higher anodic current densities in comparison with the coated samples (Fig. 8 and 9). This observation can be ascribed to the fact that the loose and thin formed oxide films on the uncoated Mg alloys (only 5-10 nm) are not able to protect the alloys adequately against the corrosion in the chloride solutions (Ref 7, 8, 40). In fact, the artificial compact and continuous passive film with the thickness of not merely several nanometers but several microns can act as a protective layer on the Mg-Ca alloys during corrosion.
Corrosion Mechanisms
Figure 11 indicates the surface morphology of the coated and bare alloys after the polarization test in 3.5% NaCl solution. The Al-AlCr-coated Mg-Ca specimen exhibited a uniform and compact film containing only a few pits (Fig. 11a). But, the Al-AlCr-coated Mg-Zn-RE sample exhibited a less uniform film with more pits (Fig. 11b) compared to the Al-AlCr-coated Mg-Ca specimen. However, the uncoated alloys are severely corroded in the chloride solution (Fig. 11c and d).
In Al-AlCr-coated Mg-Zn-RE alloy (Fig. 11b), second phases (as long-term cathodic phases) were still kept intact and more protruded from the matrix (α-Mg as anodic phase) surface after corrosion test. This observation may be related to the corrosion (anodic dissolution) of α-Mg matrix phase of the Al-AlCr-coated Mg-Zn-RE alloy. A severe local galvanic corrosion can be expected at the coating defects (through-thickness pores)/substrate interface. This local galvanic corrosion can considerably enhance the anodic dissolution of the α-Mg phase and can rapidly reach the anodic potential to the pitting potential. In fact, once chloride ions diffused into coating through the small defects, such as through-voids or micro-cracks in the coating layer, they formed small channels that spread over the interface, leading to the formation of the pits. These pits then became interconnected, accompanied by increasing gas pressure underneath the coating film. As a result, the coating experienced delamination, and the corrosion rate (anodic current density) of the coated alloy increased (see Fig. 8c and 12).
It should be noted that Cl− in the solution is able to transform Mg(OH)2 (as thin heterogenous porous layer with K SP = 9.628 × 10−4) into a more soluble MgCl2 with K SP = 54.6 (Reactions 3, 4). It was found that the Cl− ion can easily penetrate into the oxide layer. This finding was mainly related to the small radius of the Cl− ion which would cause the preferential adsorption and the OH− replacement by the Cl− (Ref 37) (Reaction 5).
MgCl2 was then dissolved into Mg2+ and 2Cl−, which caused an increase in the concentration of hydroxide (OH−) ions near the surface of the Mg alloy. The pit nucleation can be easily done on this surface and can considerably increase the anodic current density of the sample during corrosion.
The results of the polarization test showed that the coating had a more noble electrochemical potential than the substrate (Fig. 8). Therefore, the coating was cathodically protected during the galvanic corrosion. Figure 11(b) depicts the corrosion pits that occurred at the center of the sample, which indicates that there were through-thickness defects prior to corrosion test (Ref 27, 32, 41). So, the smooth surface of the coating with higher densification was essential for preventing greater infiltration of aggressive solutions into the coating and lessening local galvanic corrosion of the substrate, which were fulfilled by applying the Al-AlCr coating on the Mg-Ca alloy (with lower average surface roughness). Galvanic corrosion process at the substrate/coating interface can be facilitated by the high level of porosity in the coatings (Ref 36, 42). In fact, Al-AlCr coating on Mg-Zn-RE alloy (with higher average surface roughness) has more defects than the Al-AlCr coating on Mg-Ca alloy. On the other hand, Al-AlCr coating could increase the corrosion potential of the bare magnesium alloys, but Al-AlCr coating on the Mg-Ca alloy could significantly improve the corrosion resistance of the uncoated Mg-Ca alloy which is less expensive than the Mg-RE alloys.
Conclusions
-
1.
Based on a comparison of the peak intensities, Al3Cr7(110), a densely packed plane, was the preferred orientation in the aluminum-chromium as outer layer of Al-AlCr coating on the Mg alloys.
-
2.
Less and smaller defects were found within the AlCr coating deposited on the Mg-Ca alloy compared to the other coating. This observation may be due to the fact that the Mg-Ca alloy surface used was smoother in comparison with the Mg-Zn-RE alloy surface.
-
3.
Mg-Zn-RE alloy showed rough surface morphology which can arise from the difference between the hardness of different phases in this alloy. As a result, there is a change in the roughness of the sputtered coatings. This surface roughness (including pores, pinholes, and cracks) was considerably expanded in the second layer ((AlCr) as outer layer) of the Al-AlCr coating.
-
4.
Uncoated Mg alloys demonstrated an activation-controlled anodic behavior (in the anodic region) while Al-AlCr-coated Mg-Ca alloy showed a passive-like behavior in the aggressive solution, indicating that Al-AlCr coating can considerably lessen the anodic dissolution of the Mg-Ca alloy in the corrosive solution.
-
5.
Novel coating (Al-AlCr) could remarkably reduce the corrosion rate of the Mg-Ca alloy in a 3.5 wt.% NaCl solution. In fact, lower average surface roughness and compact structure of Al-AlCr coating on the Mg-Ca alloy would cause the reduction of infiltration of aggressive ions such as Cl− toward the substrate. In contrast, this coating did not show a compact structure (with lower defects) on the Mg-Zn-RE alloy, which caused the reduction of corrosion protection of this coating on this alloy surface.
-
6.
Impedance modulus (Z) of the Al-AlCr-coated samples was higher than that of the bare Mg alloys. On the other hand, Z of the Al-AlCr-coated Mg-Ca alloy was higher than that of the Al-AlCr-coated Mg-Zn-Ce-La alloy at low frequency. This observation was related to the higher anodic current density of the Al-AlCr-coated Mg-Zn-Ce-La alloy in comparison to that of the Al-AlCr-coated Mg-Ca alloy. So, it can be said that the compact Al-AlCr coating (with lower defects) can substantially enhance the corrosion resistance of the Mg-Ca alloys.
References
P.C. Wang, T.C. Cheng et al., Effects of Pre-sputtered Al Interlayer Films on the Atomic Layer Deposition of Al2O3 on Mg-10Li-0.5Zn Alloy, Appl. Surf. Sci., 2013, 270, p 452–456
E.E. Demirci, E. Arslan, K.V. Ezirmik et al., Investigation of Wear, Corrosion and Tribocorrosion Properties of AZ91Mg Alloy Coated by Micro arc Oxidation Process in the Different Electrolyte Solutions, Thin Solid Films, 2013, 528, p 116–122
Y. Ge, W. Wang et al., Study on Laser Surface Remelting of Plasma-Sprayed Al-Si/1wt% Nano-Si3N4 Coating on AZ31B Magnesium Alloy, Appl. Surf. Sci., 2013, 273, p 122–127
G. Wu, X. Ziu, G. Li, S. Yiuy, and X. Wug, Preparation and Characterization of Ceramic/Metal Duplex Coatings Deposited on AZ31 Magnesium Alloy by Multi-magnetron Sputtering, Mater. Lett., 2006, 60, p 674–678
H.R. Bakhseshi-rad, M.H. Idris, M.R. Abdulkadi, A. Ourdjini, M. Medraj, and M. Daroonparvar, Mechanical and Bio-corrosion Properties of Quaternary Mg-Ca-Mn-Zn Alloys Compared with Binary Mg-Ca Alloys, Mater. Des., 2014, 53, p 283–292
S. Dejiu, M. Haojia et al., Effect of Cerium and Lanthanum Additives on Plasma Electrolytic Oxidation of AZ31 Magnesium Alloy, J. Rare Earths, 2013, 31, p 1208–1213
X. Zhang, Y. Li, K. Zhang, C. Wang, H. Li, M. Ma, and B. Zhang, Corrosion and Electrochemical Behavior of Mg-Y Alloys in 3.5% NaCl Solution, Trans. Nonferrous Met. Soc. China, 2013, 23, p 1226–1236
Z. Xin, Z. Kui, L. Gang, D. Xia, L. Wei, Z.B. Wang, and C. Shun, Comparative Study on Corrosion Behavior of As-Cast and Extruded Mg5Y7Gd1Nd0Zr alloy in 5% NaCl Aqueous Solution, Trans. Nonferrous Met. Soc. China, 2012, 22, p 1018–1027
M. Yang, H. Li et al., Effects of Minor Ti Addition on As-Cast Microstructure and Mechanical Properties of Mg-3Sn-2Sr (wt%) Magnesium Alloy, J. Alloys Compd., 2013, 579, p 92–99
M.A. Alam, L. Zuga, and M.G. Pecht, Economics of Rare Earth Elements in Ceramic Capacitors, Ceram. Int., 2012, 38, p 6091–6098
J.E. Gray and B. Luan, Protective Coatings on Magnesium and Its Alloys—A Critical Review, J. Alloys Compd., 2002, 336, p 88–113
X. Fan, B. Zou, L. Gu, C. Wang, Y. Wang et al., Investigation of Bond Coats for Thermal Barrier Coatings on Mg Alloy, Appl. Surf. Sci., 2013, 265, p 264–273
H. Wang, B. Yu et al., Improved Corrosion Resistance of AZ91D Magnesium Alloy by a Zinc-Yttrium Coating, Alloys Compd., 2014, 582, p 457–460
H. Tang, D. Yu et al., Preparation and Characterization of HA Microflowers Coating AZ31 Magnesium Alloy by Micro-arc Oxidation and a Solution Treatment, Appl. Surf. Sci., 2013, 264, p 816–822
H.R. Bakhsheshi-Rad, E. Hamzah, M. Daroonparvar, R. Ebrahimi-Kahrizsangi, and M. Medraj, In-Vitro Corrosion Inhibition Mechanism of Fluorine-Doped Hydroxyapatite and Brushite Coated Mg-Ca Alloys for Biomedical Applications, Ceram. Int., 2014, 40, p 7971–7982
Y. Wang, X. Wang et al., Role of b Phase During Micro arc Oxidation of Mg Alloy AZ91D and Corrosion Resistance of the Oxidation Coating, J. Mater. Sci. Technol., 2013, 29, p 1129–1133
M.F. Yousof, M.R. Abdul-Kadir et al., Dipcoating of Poly(e-caprolactone)/Hydroxyapatite Composite Coating on Ti6Al4V for Enhanced Corrosion Protection, Surf. Coat. Technol., 2014, 245, p 102–107
S. Mao, H. Yang, F. Huang et al., Corrosion Behaviour of Sintered NdFeB Coated with Al/Al2O3 Multilayers by Magnetron Sputtering, Appl. Surf. Sci., 2011, 257, p 3980–3984
A. Rahman, R. Jayaganthan et al., High Temperature Degradation Behavior of Sputtered Nanostructured Co-Al Coatings on Super-Alloy, Appl. Surf. Sci., 2013, 265, p 10–23
T. Xie, S. Mao, and C. Yu, Structure, Corrosion, and Hardness Properties of Ti/Al Multilayers Coated on NdFeB by Magnetron Sputtering, Vacuum, 2012, 86, p 1583–1588
S. Wu and Y. Xu, Fabrication of Cr Coating on AZ31 Magnesium Alloy by Magnetron Sputtering, Trans. Nonferrous Met. Soc. China, 2008, 18, p 329–333
L. Yangjia, F. Xizhi et al., Corrosion Behavior of Coating with Plasma Sprayed 8YSZ on the Surface of Carbon Steel, J. Rare Earths, 2012, 30, p 592–598
Y. Jianjun, M. Wenhii et al., Preparation and Characterization of La0.9Sr0.1Ga0.8Mg0.2O Thin Film Deposited by Radio Frequency Magnetic Sputtering, J. Rare Earths, 2013, 31, p 582–588
X. Fan, L. Gu, S. Zeng et al., Improving Stability of Thermal Barrier Coatings on Magnesium Alloy with Electroless Plated Ni-P Interlayer, Surf. Coat. Technol., 2012, 206, p 4471–4480
X. Fan, Y. Liu et al., Preparation and Characterization of 8YSZ Thermal Barrier Coatings on Rare Earth-Magnesium Alloy, J. Therm. Spray Technol., 2010, 20, p 948–957
G. Wu, X. Zeng, and S. Yao, Formation of a Novel Nanocrystalline Coating on AZ31 Magnesium Alloy by Bias Sputtering, Mater. Lett., 2007, 61, p 4019–4022
H. Altun and S. Sen, The Effect of PVD Coatings on the Corrosion Behavior of AZ91 Magnesium Alloy, Mater. Des., 2006, 27, p 1174–1179
S. Mao, H. Yang et al., The Properties of Aluminum Coating on Sintered NdFeB by DC Magnetron Sputtering, Vacuum, 2011, 85, p 772–775
L.H. Chiu, C.C. Chen, and C.F. Yang, Improvement of Corrosion Properties in an Aluminum-Sprayed AZ31 Magnesium Alloy by a Post-hot Pressing and Anodizing Treatment, Surf. Coat. Technol., 2005, 191, p 181–187
S. Mao, H. Yang et al., Corrosion Behavior of Sintered NdFeB Deposited with an Aluminum Coating, Corros. Sci., 2011, 53, p 1887–1894
J. Crues, H. Mazille, and H. Idrissi, Porosity Evaluation of Protective Coatings onto Steel, Through Electrochemical Techniques, Surf. Coat. Technol., 2000, 130, p 224–232
G. Wu and X. Wang, Corrosion Behavior of Ti-Al-N/Ti-Al Duplex Coating on AZ31 Magnesium Alloy in NaCl Aqueous Solution, Mater. Charact., 2009, 60, p 803–807
G. Wu, Fabrication of Al and Al/Ti Coatings on Magnesium Alloy by Sputtering, Mater. Lett., 2007, 61, p 3815–3817
H. Altun and S. Sen, The Effect of DC Magnetron Sputtering AlN Coatings on the Corrosion Behavior of Magnesium Alloys, Surf. Coat. Technol., 2005, 197, p 193–200
A. Pardo, M.C. Merino et al., Corrosion Behavior of Mg/Al Alloys with Composite Coatings, Surf. Coat. Technol., 2009, 203, p 1252–1263
M. Flores, O. Blanco, S. Muhl, C. Pina, and J. Heiras, Corrosion of a Zn-Al-Cu Alloy Coated with TiN/Ti Films, Surf. Coat. Technol., 1998, 108–109, p 449–453
H.R. Bakhsheshi-Rad, M.R. Abdul-Kadir, M.H. Idris, and S. Farahany, Relationship Between the Corrosion Behavior and the Thermal Characteristics and Microstructure of Mg-0.5Ca-xZn Alloys, Corros. Sci., 2012, 64, p 184–197
M. Mandal, A.P. Moon, G. Deo, C.L. Mendis, and K. Mondal, Corrosion Behavior of Mg-2.4Zn Alloy Micro-alloyed with Ag and Ca, Corros. Sci., 2014, 78, p 172–182
Y. Song, E.H. Han, D. Shan, and C.D. Yim, The Effect of Zn Concentration on the Corrosion Behavior of Mg-xZn Alloys, Corros. Sci., 2012, 65, p 322–330
Y. Niu, R. Cui, Y. He, and Z. Yu, Wear and Corrosion Behavior of Mg-Gd-Y-Zr Alloy Treated by Mixed Molten-Salt Bath, Alloys Compd., 2014, 610, p 294–300
M. Daroonparvar, M.A.M. Yajid, H.R. Bakhsheshi-Rad, N.M. Yusof, S. Izman, E. Hamzah, and M.R. Abdul-Kadir, Corrosion Resistance Investigation of Nanostructured Si-and Si/TiO2-Coated Mg Alloy in 3.5% NaCl Solution, Vacuum, 2014, 108, p 61–65
H.R. Bakhsheshi-Rad, E. Hamzah, M. Daroonparvar, M.A.M. Yajid, and M. Medraj, Fabrication and Corrosion Behavior of Si/HA Nano-composite Coatings on Biodegradable Mg-Zn-Mn-Ca Alloy, Surf. Coat. Technol., 2014, 258, p 1090–1099
Acknowledgments
The authors would like to acknowledge the Universiti Teknologi Malaysia (UTM) for providing research facilities and financial support under Grants No: (1) UTM-Research University Grant (RUG) (Q.J130000.2524.04H78), (2) Fundamental Research Grant Scheme (FRGS), Ministry of Education (R.J130000.7824.4F340) and (3) UTM – Post Doctoral Research University Grant (R.J130000.7113.4N001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daroonparvar, M., Yajid, M.A.M., Yusof, N.M. et al. Improvement of Corrosion Resistance of Binary Mg-Ca Alloys Using Duplex Aluminum-Chromium Coatings. J. of Materi Eng and Perform 24, 2614–2627 (2015). https://doi.org/10.1007/s11665-015-1543-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-015-1543-9